Summary
Primary hyperparathyroidism (PHPT) is one of the most frequent endocrine disease in developed countries. It mainly occurs as sporadic cases (about 90–95% of cases), while only the remaining 5–10% is represented by familial inherited parathyroid disorders due to causative mutations in specific target genes. Clinical variability among the different familial parathyroid syndromes is generally linked to the specific mutated gene and it can predispose subjects to different manifestations of parathyroid pathology, various degrees of PHPT severity, persistence and/or after-surgery recurrences. Genetic tests is helpful in differential diagnosis favouring the recognition of the specific familial PHPT syndrome and, subsequently, in planning the most suitable surgical procedures and/or pharmacological interventions. Moreover, genetic test is important to recognise mutation carriers, within PHPT familial forms, even before the appearance of biochemical and/or clinical symptoms.
This review resumes general concepts about genetic diagnosis of PHPT in familial hereditary syndromes, specifically describing why, when, and which genetic screenings should be performed in every specific PHPT-associated parathyroid disease.
Keywords: primary hyperparathyroidism, parathyroid adenoma, genetic test, MEN, FHH, FIHP
Introduction
Primary hyperparathyroidism (PHPT) is one of the most frequent endocrine diseases in developed countries (1), characterised by excessive hypersecretion of parathyroid hormone (PTH) by parathyroid glands, resulting in a constant high serum calcium level. PHPT is due to the presence of a benign single parathyroid adenoma in 80% of the cases, to multiple parathyroid adenoma and/or multiglandular hyperplasia in 15–20% of the cases or to parathyroid carcinoma in only 1% of the cases (2).
Sporadic PHPT cases represent about 90–95% of all PHPT, mostly sustained by a single parathyroid adenoma. The remaining 5–10% of cases occurs within familial inherited parathyroid disorders such as multiple endocrine neoplasia type 1 (MEN1), multiple endocrine neoplasia type 2A (MEN2A), multiple endocrine neoplasia type 4 (MEN4), familial hypocalciuric hypercalcemia syndrome (FHH), neonatal severe hyperparathyroidism (NSHPT), HPT-jaw tumour (HPT-JT) and familial isolated PHPT (FIPH) (Table 1). They are mainly sustained by multiglandular disease and often characterized by an earlier age of onset with respect to sporadic PHPT.
Table 1.
Main clinical characteristics of familial hereditary forms of PHPT.
| Syndrome | Mean age of onset | Parathyroid pathology | Clinical treatment |
|---|---|---|---|
| MEN1 | 20–25 years | Multiglandular hyperplasia and/or adenoma | Subtotal parathyroidectomy of hyperplastic and/or adenomatous parathyroids; or total parathyroidectomy with healthy tissue reimplantation in the non-dominant forearm, in case of pathological involvement of all four parathyroids |
| MEN2A | Over 30 years | Single or multiglandular hyperplasia and/or adenoma | Specific resection on only the hyperplastic and/or adenomatous parathyroids |
| MEN4 | Over 45 years | Single or multiglandular hyperplasia and/or adenoma | Specific resection on only the hyperplastic and/or adenomatous parathyroids |
| FHH | All ages | Multiglandular mildly hyperplastic parathyroids | Parathyroidectomy is not only unnecessary but also inappropriate, since it does not cure FHH-associated hypercalcemia |
| NSHPT | At birth or within the first six months of life | Multiglandular markedly hyperplastic parathyroids | Total parathyroidectomy |
| HPT-JT | Over 30 years | Single or multiglandular (usually two glands) cystic adenoma. Carcinomas in 10–15% of cases |
Subtotal parathyroidectomy of adenomatous and/or carcinomatous parathyroids, or total parathyroidectomy, with healthy tissue reimplantation in the non-dominant forearm, in case of adenoma involvement of all four parathyroids |
| FIPH | N.R. | Single or multiglandular adenoma | Subtotal parathyroidectomy of adenomatous parathyroids, or total parathyroidectomy, with healthy tissue reimplantation in the non-dominant forearm, in case of adenoma involvement of all four parathyroids |
N.R. = not reported. Footnotes: N.R. = not reported
PHPT is mostly asymptomatic. In the symptomatic forms it can be associated with increased morbidity because of secondary osteoporosis, nephrolithiasis, kidney stones, neuro-muscular effects and cognitive changes (3), due to the chronic increased level of circulating PTH and calcium. Since PHPT can present a longstanding asymptomatic course, particularly in case of familial forms and hereditary multiple cancer syndromes, the systematic assessment of the genetic risk is very important to early recognise at risk individuals even before clinical symptoms. The diagnosis of PHPT is biochemical. Indeed, biochemical analyses are able to recognise signs of PHPT about 10 years earlier than instrumental evidence of gland involvement. Genetic testing permits the identification of at risk individuals even before any biological, biochemical and clinical manifestation, providing the opportunity for routine preventive screenings and early treatments, particularly in preclinical, asymptomatic disease in relatives of index cases, allowing the recognition of familial PHPT associated syndrome. Although a specific phenotype-to-genotype correlation does not exist, there is a certain degree of clinical variability due to the specific causative genetic alteration that can predispose subjects to different manifestations of parathyroid pathology, various degrees of PHPT severity, persistence and/or after-surgery recurrences, and, subsequently, the management of PHPT differs among the different familial syndromes (Table 1). Therefore, it is useful to identify the specific syndrome before planning surgical procedures and/or any pharmacological intervention.
This review resumes general concepts about genetic diagnosis of PHPT in familial hereditary syndromes and in sporadic cases, specifically describing why, when, and which genetic screenings should be performed in every specific PHPT-associated parathyroid disease and which are the benefits of genetic test application in differential diagnosis and clinical management of PHPT.
Genetic testing in PHPT in multiple endocrine neoplasia type 1 (MEN1)
Multiple endocrine neoplasia type 1 (MEN1; OMIM phenotype #131100) is a rare endocrine disorder presenting with varying combinations of PHPT, endocrine pancreatic tumours, and pituitary adenomas, but it can include combinations of more than 20 endocrine and non-endocrine tumours such as carcinoids, lipomas, and skin tumours. PHPT, mostly caused by parathyroid multiple adenomas and/or multi-glandular hyperplasia, represents the first clinical manifestation in about 90% of patients (4) with a typical onset between 20 and 25 years, three decades earlier than its sporadic counterpart and is the most common clinical feature of this syndrome, with a penetrance reaching the 100% after 50 years of age.
This disease shows an autosomal dominant pattern of inheritance. The responsible gene, the oncosuppressor gene MEN1 (OMIM gene 613733), encoding menin (a nuclear protein involved in the negative regulation of cell cycle progression, in the control of DNA integrity, in the regulation of apoptosis, etc.), has been identified in 1997 in the long arm of chromosome 11 (locus 11q13). Since then a specific mutational screening test is available for this syndrome. One inactivating mutation of MEN1 gene can be inherited by one of the parent (familial form) or developed de novo at embryo level (sporadic form), giving subjects the predisposition to develop the syndrome. The second wild type copy of the gene is lost during lifetime at tissue somatic level, generating specific endocrine and non-endocrine MEN1-associated tumours, according to Knudson’s two hits hypothesis for tumour suppressor genes. The exact molecular role of MEN1’s protein, menin, in MEN1 tumorigenesis is not yet well understood, but it is surely related to the important functions that this protein has in regulation of cell cycle, DNA replication and integrity maintenance, gene transcription, signal transduction, etc. An extensive review in 2008 described over 1,300 different germinal (1,133) or somatic (203) mutations and 24 benign polymorphisms that have been identified, by mutation screening of the MEN1 gene, all along the entire gene without any mutational hot spot (i.e. a DNA sequence and/or region of high mutation susceptibility) (5). A 2016 update reported 208 novel further germline MEN1 mutations, identified in patients with MEN1 syndrome between 2007 and 2015 (6). Unfortunately, none of the described MEN1 mutations has been associated to a peculiar clinical phenotype, even within members of the same family, thus a genotype-to-phenotype correlation does not exist. Therefore, unrelated individuals with different mutations may present with the same clinical phenotype, making impossible to foresee the course of the disease by the result of genetic test and to design a personal predictive and therapeutic plan. Despite this limitation, the use of genetic test in MEN1 has been shown to reduce the morbidity and mortality of the syndrome. Indeed, a positive test in asymptomatic subjects within a family with a known mutation of the MEN1 gene, even if it does not lead to immediate surgical and medical interventions, is an indication for earlier and more frequent preventive instrumental and biochemical screenings for MEN1-associated lesions (Table 2). Conversely, a negative test in individuals from a mutation-bearing family indicates that they are not at risk of develop the disease in the future, and leads to the decision for no further routine invasive medical analyses. The MEN1 genetic test can also direct the type of surgical intervention in subjects with parathyroid adenoma. A positive test strongly suggests to perform a total parathyroidectomy (with an intra-operative PTH dosage to individuate and remove also possible supra-numerary and/or ectopic parathyroid glands), to prevent future recurrences of parathyroid tumours. This surgical approach is often associated with re-implantation of healthy, fresh or cryo-preserved parathyroid tissue in the non-dominant forearm, to avoid the necessity of a second intervention at neck site in case of adenoma recurrence. In case of a positive MEN1 test, it is also recommended to perform a prophylactic thymectomy, at the same time of parathyroid intervention, to prevent the occurrence of thymic carcinoids that are one of the main cause of morbidity and mortality of MEN1 syndrome, and/or to remove intrathymic ectopic/supra-numerary parathyroids. Conversely, a negative MEN1 test leads to surgical ablation of only the adenomatous or hyperplastic parathyroid gland.
Table 2.
Suggested guidelines for biochemical and instrumental screenings in familial PHPT individuals bearing a genetic mutation.
| Syndrome | Responsible gene | Gene mutation | Recommended age of begin screenings | Type of screenings | Frequency |
|---|---|---|---|---|---|
| MEN1 | MEN1 | Any inactivating MEN1 mutation | Age of 5 | Serum concentration of prolactin | Yearly |
| Age of 8 | Fasting total serum calcium concentration (corrected for albumin) and/or ionized-serum calcium concentration. Fasting serum concentration of full-length PTH | Yearly | |||
| Age of 20 | Fasting serum gastrin concentration | Yearly | |||
| Age of 5 | Head MRI | Every 3–5 years (depending on results of biochemical screenings) | |||
| Age of 20 | Abdominal CT or MRI | Every 3–5 years (depending on results of biochemical screenings) | |||
| MEN2A | RET | C634R/G/F/S/W/Y | Age of 8 | Fasting total serum calcium concentration (corrected for albumin) and/or ionized-serum calcium concentration. Fasting serum concentration of full-length PTH | Yearly |
| Age of 8 | Biochemical screenings for pheochromocytoma | Yearly | |||
| C609/F/R/G/S/Y; C611R/G/F/S/W/Y; C618R/G/F/S/Y; C620R/G/F/S/W/Y; C630R/F/S/Y; D631Y | Age of 8 for individuals with a codon 630 mutation, age of 20 for all the others | Fasting total serum calcium concentration (corrected for albumin) and/or ionized-serum calcium concentration. Fasting serum concentration of full-length PTH | Yearly | ||
| Age of 8 for individuals with a codon 630 mutation, age of 20 for all the others | Biochemical screenings for pheochromocytoma | Yearly | |||
| C515S; G533C; R600Q; K603E; Y606C; T636P; K666E; E768D | Age of 20 | Fasting total serum calcium concentration (corrected for albumin) and/or ionized-serum calcium concentration. Fasting serum concentration of full-length PTH | Yearly | ||
| Age of 20 | Biochemical screenings for pheochromocytoma | Yearly | |||
| MEN4 | CDKN1B | ATG-7G>C | N.A. | N.A. | N.A. |
| G9R | N.A. | N.A. | N.A. | ||
| c.59_77dup19 (K25fs) | N.A. | N.A. | N.A. | ||
| P69L | N.A. | N.A. | N.A. | ||
| W76X | N.A. | N.A. | N.A. | ||
| P95S | N.A. | N.A. | N.A. | ||
| P133T | N.A. | N.A. | N.A. | ||
| Stop1>Q | N.A. | N.A. | N.A. | ||
| FHH1 | CaSR | Any inactivating CaSR mutations | N.A. | N.A. | N.A. |
| FHH2 | GNA11 | Ile199Del | N.A. | N.A. | N.A. |
| FHH3 | AP2S1 | R15C; R15L; R15H | N.A. | N.A. | N.A. |
| NSHPT | CaSR | Homozygote inactivating CaSR mutations | N.A. | N.A. | N.A. |
| HPT-JT | CDC73 (HRPT2) | Any inactivating CDC73 mutation | N.A | Fasting total serum calcium concentration (corrected for albumin) and/or ionized-serum calcium concentration. Fasting serum concentration of full-length PTH | Every 6–12 months |
| N.A | Panoramic jaw X-ray with neck shielding | Every 5 years | |||
| N.A. | Abdominal MRI | Every 5 years | |||
| N.A. | Transvaginal or transabdominal ultrasound (only in women) | Yearly |
N.A. = not available. Footnotes: N.A. = not available
Currently, the worldwide most common used genetic testing for MEN1 encompasses the sequencing of encoding regions (exons 2–10) and intronexon junctions of MEN1 gene. Gene sequencing fails to find mutations of MEN1 coding region and splice sites in about 10% of MEN1 families and MEN1 sporadic cases (5). It has been estimated that 1–3% of all MEN1 germline mutations are large deletions within the gene that cannot be detected by sequencing method. In case of a sequencing negative test in a MEN1 family and/or in a sporadic case presenting the clinical signs of MEN1 syndrome, MEN1 gene should be screened by multiple ligation-dependent probe amplification (MPLA), a PCR-based gene dosage procedure that allows to detect large nucleotide deletions within the gene (7). If also MPLA test fails to find mutations, it is possible to perform a haplotypes linkage analysis (using microsatellite markers flanking the 11q13 locus) in families with at least two generations of affected individuals, to individuate a haplotypes, which segregates with the disease. A negative test may also indicate the presence of a phenocopy, due to a different genetic alteration. Less than 2% of MEN1 patients without any MEN1 mutation may bear a mutation in CDKN1A (OMIM gene 116899), CDKN1B (OMIM gene 600778), CDKN2B (OMIM gene 600431), CDKN2C (OMIM gene 600927) genes, encoding respectively p21Cip1, p27Kip1, p15Ink4b, and p18Ink4c, cyclin-dependent kinase inhibitors (CDKI) that negatively regulate cell cycle progression and cell growth (8). Loss-of-function mutations of one of these genes have been described to contribute to tumorigenesis (9, 10). Interestingly, it has also been demonstrated that menin directly targets CDKN1B and CDKN2C genes (11). In particular, inactivating mutations of the CDKN1B gene have been associated to the MEN4 syndrome (as further described below). Patients presenting an inactivating mutation of one of these CDKI genes are at risk of developing all the MEN1-associated main tumours, including parathyroid multiple adenomas, in a similar manner of subjects bearing a MEN1 mutation. A genetic screening of these genes should be considered in patients presenting a clinical MEN1-like phenotype but with a MEN1 gene negative test.
In addition, recently, Circelli et al. (12) exerted the prognostic role of the Val109Gly (V109G) polymorphism (rs2066827) in the exon 1 of CDKN1B gene, in patients affected by MEN1 syndrome. Authors found that, in MEN1 patients bearing a MEN1 mutation, the biallelic presence of the genetic variant guanine at nucleotide 326, encoding for the glycine, was significantly associated to a more aggressive course of the disease and a worse prognosis. The presence of glycine at position 109 presumably alters the interaction with Jun activation domain-binding protein 1, enhancing the nuclear export and degradation of p27Kip1, and it has been correlated with low nuclear level of this protein. The hypothesis is that the simultaneous presence of a MEN1 mutation and of heterozygote 326G variations may exert synergic oncogenetic effects, in which MEN1 acts as “driver” gene and CDKN1B polymorphism as a “modifier” factor of tumorigenesis. Further study are needed to verify the utility of V109G polymorphism genetic analysis, in MEN1 mutated patients, as a marker for the guidance of diagnostic and prognostic management of PHPT and other MEN1-associated tumours.
Genetic testing in PHPT in multiple endocrine neoplasia type 2A (MEN2A)
Multiple endocrine neoplasia type 2 (MEN2; OMIM phenotype #171400) is a rare heritable endocrine syndrome characterised by medullary thyroid carcinoma (MTC), unilateral or bilateral pheochromocytoma and, only in the MEN2A variant, PHPT (resulting from hyperplasia or adenoma of the parathyroid glands). MEN2A accounts for about 70–80% of all MEN2 syndromes. PHTP occurs in 20 to 30% of MEN2A patients, usually after the third decade of life and in most cases (>80%) it is asymptomatic (13). Usually, MEN2A-related PHPT is mild and may range from a single parathyroid adenoma to extended multiglandular hyperplasia.
MEN2 syndrome presents an autosomal dominant pattern of inheritance. The only identified susceptibility gene associated with MEN2 is the proto-oncogene RET (REarranged during Transfection; locus 10q12.2, OMIM gene 164761), that encodes a membrane tyrosine kinase receptor protein consisting of: A) an extracellular portion composed by four cadherine-like domains, a calcium-binding site and a cysteine-rich domain; B) a single-pass transmembrane domain and C) an intracellular portion containing two distinct tyrosine kinase domains. Mutation in one of the allele of RET gene (inherited by parent in familial form or developed at embryo stage in sporadic form) is sufficient to develop MEN2 syndrome, according to dominant mutation of oncogenes. Mutations of RET in the cysteine-rich domain and in the tyrosine kinase domains result all in a constitutive tyrosine kinase activation of the mutant receptor.
RET germline high-penetrance gain-of-function mutations at one of six cysteines in the extracellular cysteine-rich domain of RET (codons 609, 611, 618, 620, 630 and 631 in exon 10 and codon 634 in exon 11) have been found in about 98% of MEN2A families (14). Therefore, these loci have been identified as disease-associated hot spots. The most common mutation is the amino acid substitution at codon 634, particularly C634R, that has been found in about 85% of MEN2A patients, and strongly associated to PHPT (15). A strong correlation between RET specific mutation and MEN2 clinical phenotype is well assessed. Thus, the results of genetic test on RET allow a very specific preventive clinical intervention for mutation carriers. The presence of mutations in exons 10 and 11 of RET gene is indicative of a MEN2A variant with a 15–30% risk to develop PHPT. Mutations in other RET exons (i.e. mutations at codon 883 in exon 15 and at codon 918 in exon 16) have been associated to the MEN2B variant of the syndrome that does not develop PHPT, thus, a genetic test indicating mutations in these two exons permits to exclude the possibility to develop PHPT in the future.
Due to the strong genotype-phenotype correlation, RET genetic test largely directs the planning of medical interventions in mutation carriers, with a major focus on the prophylactic thyroidectomy, to prevent MTC. Anyway, even if MEN2A-associated PHPT is usually mild and asymptomatic, a genetic test indicating a MEN2A variant is helping in leading to specific PHPT biochemical screenings and in early treating this associated disorder, to prevent morbidity due to chronic hypercalciuria, renal calculi, secondary osteoporosis, etc. In MEN2A carriers of mutations at codons 630 and 634, annual biochemical analyses for serum calcium concentration and serum PTH level are recommended since the age of 8 (15) (Table 2). For carriers of other mutations associated to MEN2A, annual PHPT biochemical screening should begin since 20 years of age (16) (Table 2). Since PHPT in MEN2A is usually mild and asymptomatic, prophylactic parathyroidectomy is not usually recommended, even at the time of neck surgery for medullary thyroid carcinoma.
Genetic testing in PHPT in multiple endocrine neoplasia type 4 (MEN4)
Multiple endocrine neoplasia type 4 (MEN4; OMIM phenotype #610755) is an extremely rare inherited multiglandular tumoural syndrome presenting a MEN1-like clinical phenotype with a penetrance of 100% of PHPT, caused by parathyroid hyperplasia or adenomas, and reported cases of associated pituitary adenomas (GH- or ACTH-secreting), and duodenal, pancreatic and stomach lesions. Human MEN4 cases do not usually harbor MEN2-like tumours, unlike in the murine MENX model, which develops tumours that overlap both MEN1 and MEN2 syndromes (i.e. parathyroid adenomas, bilateral pheochromocytomas, thyroid C cell hyperplasia, endocrine pancreas hyperplasia and paragangliomas). The spectrum of MEN4-associated tumours is highly variable between affected subjects, like in the MEN1 syndrome. MEN4 affected individuals may present as a MEN1 clinical phenotype, but without MEN1 mutations (phenocopy). However, all the MEN4 patients, described to date, have shown a relatively late age of clinical manifestation with respect to MEN1 counterpart. Also in MEN4, the PHPT has a high penetrance, and it is the first diagnosed endocrinopathy in most cases, but with an age at onset more than two decades later than in MEN1 patients (40–50 years in MEN4 versus 20–25 years in MEN1).
Today is well assessed that MEN4 is a novel form of multiple endocrine neoplasia caused by inactivating mutations in the CDKN1B gene (12p13 locus, OMIM 600778) that encodes the p27kip1 cyclin-dependent kinase inhibitor (17). p27kip1 is a negative key regulator of cell cycle progression by acting as kinase inhibitor protein for cyclin-dependent kinase, thus, preventing their association with cell cycle-regulating cyclin and inducing cell cycle arrest at G1-to-S-phase transition. The functional disruption of p27kip1 (presumably due to the reduction of nuclear localization of the protein in association with an increased degradation at cytoplasmatic level) is responsible for uncontrolled cell cycle progression in neuroendocrine cells, in which is presumable that the lack of p27kip1 activity cannot be compensated by other cyclin-CDK inhibitors. Therefore, the p27kip1 absence plays a crucial role in induction and progression of neuroendocrine tumorigenesis.
CDKN1B mutations might explain part of the suspected MEN1 clinical cases lacking MEN1 gene mutations. Sequencing of CDKN1B gene is a recommended analysis for differential diagnosis between MEN1 and MEN4 syndromes, and it is strongly suggested in all MEN1 phenotypes in which the MEN1 gene is negative for mutations.
To date, 9 different germline CDKN1B mutations, affecting the coding region and the 5′ untranslated region (5′-UTR) of the gene, have been identified in patients with MEN1-like clinical phenotype (MEN1 phenocopies) but negative for MEN1 mutations (18, 19). However, given the limited number of described MEN4 patients, the clinical penetrance of the disease, the precise tumour spectrum of the syndrome and a clear association between specific mutation and clinical phenotype is still not well defined, and no suggested guidelines for biochemical and instrumental preventive screenings in mutation carriers are not yet available.
Recently, a case of apparently sporadic early onset PHPT (no family history of calcium or other endocrine disorders has been reported and both parents had a normal serum calcium level) has been described in a 15-year-old girl presenting recurrent renal calculi, persistent elevated serum calcium level, and a single gland parathyroid adenoma in the anterior mediastinum, associated with a novel heterozygote missense mutation in exon 1 of the CDKN1B gene (c.378G>C), resulting in an amino acid substitution at position 126 (Glu126Asp) (20). Interestingly, both her mother (46 years) and her maternal grandfather (74 years) carried the same heterozygote mutation but they presented normal calcium and PTH serum levels and no signs or symptoms of PHPT.
Genetic testing in PHPT in familial hypocalciuric hypercalcaemia (FHH)
Familial hypocalciuric hypercalcaemia (FHH) comprises a group of rare hereditary disorders of calcium homeostasis, characterised by lifelong hypercalcaemia, usually asymptomatic, and by inappropriately low urinary calcium excretion. FHH type 1 variant (FHH1; OMIM phenotype #145980), that accounts for approximately 65% of all FHH cases, and FHH type 2 variant (FHH2; OMIM phenotype 145981) are usually associated with high-normal PTH and mild hyper-magnesemia. FHH type 3 variant (FHH3; OMIM phenotype 600740) presents different clinical characteristics that include increased serum PTH, hypophosphatemia and osteomalacia. These disorders can be easily misdiagnosed as PHPT because of the numerous their clinical overlaps, especially considering mild, asymptomatic forms of PHPT. However, differentiating between FHH syndromes and sporadic PHPT is fundamental since these two disorders require radically different therapies (i.e. almost all of FHH 100% of cases do not require parathyroidectomy, as opposed to PHPT).
All the three variants of FHH are autosomal dominant hereditary disorders. Specific genetic tests can be very useful for the differential diagnosis between FHH and PHPT and also to discriminate between the three FHH variants.
FHH1 is caused by a heterogeneous group of heterozygote loss-of-function mutations in calcium sensing receptor (CaSR) gene, at chromosome 3q21.1 (OMIM gene 601199). This gene encodes for a G-protein trans-membrane receptor, widely expressed in parathyroids and kidney tubule and responsible for the maintaining of stable extracellular calcium ion level. Inactivating mutations of CaSR result in a receptor relatively insensitive to serum calcium level. At parathyroid level, a less sensitive CaSR results in a continuous release of PTH even when serum calcium is high, resulting in a mild, usually asymptomatic, hypercalcaemia, hypocalciuria and high normal to elevated serum magnesium levels. Biochemical variations in FHH are supposed to be mutation dependent (21). A study by Ward et al. (22) evidenced that CaSR truncating mutations, presumably not exhibiting a dominant-negative effect (i.e. a mutation whose gene product adversely affects the wild type gene product function), are associated to a less severe hypercalcaemia and a minor increasing of PTH level, than CaSR missense mutations responsible for a protein dominant-negative effect. However, different types of inactivating mutations do not correlate with urinary calcium excretion, calcium/creatinine clearance nor with clinical symptoms. Over 200 different CaSR mutations, mostly missense mutations, have been described and reported in the CaSR database (http://www.casrdb.mcgill.ca).
Conversely, heterozygous activating mutations of CaSR gene are responsible for the development of autosomal dominant hypocalcemia (HYPOC1; OMIM phenotype #601198), a rare hereditary disorder of calcium homeostasis characterised by persistent mild, usually asymptomatic hypocalcemia, inappropriately normal PTH level and high urinary calcium excretion. Activating CaSR mutations induce an increased sensitisation of receptor to extracellular calcium that, at parathyroid level, is responsible for the induction of a chronic hypocalcemia, and, at kidney level, determines a minor reabsorption of calcium with subsequent constant hypercalciuria and risk of renal stones and nephrocalcinosis.
Twenty-seven polymorphisms have been identified within the CaSR gene locus, but no definitive results are available about their possible role in susceptibility to FHH1 and/or sporadic form of PHPT or ADH (23).
FHH2 has been originally mapped to chromosome 19p. Recently, inactivating mutations in G-protein alpha 11 (GNA11, OMIM gene 139313) have been identified as cause of the disease. Normally, elevated concentrations of extracellular calcium ion activate CaSR, and in turn GNA11, resulting in a reduction of PTH production and secretion. The presence of an inactivating mutation in GNA11 protein alters the switch from guanosine diphosphate (GDP) to guanosine triphosphate (GTP), thus, reducing CaSR-induced signal transduction, inducing a constitutive secretion of PTH and leading to FHH2 syndrome. Loss-of-function heterozygous 3-bp (CAT) deletion, leading to an in-frame deletion of Ile200 (Ile200del) has been identified in a family affected by FHH2, co-segregating only with affected members. This genetic variation was not found in 120 alleles from 60 unrelated normocalcemic individuals, thereby indicating that it was not a common benign polymorphism but a real disease-predisposing mutation. Also a missense Leu135Gln mutation has been identified in a patients affected by FHH2. Both these two loss-of-function mutations of GNA11 gene disrupt the protein structure leading to a decreased sensitivity of cells expressing CaSR to the extracellular changes in calcium level. Conversely, gain-of-function mutations of GNA11 increase cell sensitivity to extracellular calcium variations and cause the autosomal dominant hypocalcemia type 2 syndrome (ADH2, OMIM phenotype #615361) (24).
FHH3 has been associated to missense heterozygote loss-of-function mutations at codon 15 off the adaptor protein 2 sigma 1 (AP2S1; locus 19q13.3, OMIM 602242) which is a protein necessary for the membrane trafficking of the CaSR. Inactivating mutations of AP2S1 protein decrease the expression of CaSR at cell membrane level, reducing cell sensitivity to extracellular calcium variations. All AP2S1 mutations identified to date affect the Arg15 residue (Arg15Cys, Arg15His or Arg15Leu), indicating this amino acid as a probable mutational hotspot for FHH3. No correlation between different mutations and a specific clinical phenotype has been found. Mutations at Arg15 in AP2S1 gene are suspected to be the genetic cause of FHH in more than 20% of patients without CaSR mutations (25). Therefore, the genetic screening of AP2S1 codon 15 is strongly suggested in all FHH-suspected patients proven negative for CaSR mutations, given the clinical benefits (i.e. avoiding unnecessary parathyroidectomy) that have already been demonstrated for CASR screening in FHH1. Conversely to CaSR and GNA11, gain-of-function AP2S1 mutations have not been identified to date.
Genetic testing in neonatal severe primary hyperparathyroidism (NSHPT)
Neonatal severe primary hyperparathyroidism (NSHPT; OMIM phenotype #239200) is a very rare autosomal recessive disorder and the most severe form of FHH. It consists of PHPT occurring at birth or within the first six months of life, causing life-threatening severe hypercalcaemia, hypotonia, bone demineralization, fragility fractures and respiratory distress. It is a very severe hereditary disease for which the survival of children depends on early diagnosis and total parathyroidectomy. NSHPT is the homozygous form of FHH, caused by inactivating mutations of both the allele of CaSR gene.
Prenatal genetic test or genetic test at birth are recommended when both parents have been resulted to be carriers of CaSR mutations and/or present clinical signs of FHH.
Genetic testing in hereditary hyperparathyroidism-jaw tumour (HPT-JT) syndrome
Hereditary hyperparathyroidism-jaw tumour (HPT-JT; OMIM phenotype #145001) syndrome is a rare autosomal dominant disorder encompassing PHPT, ossifying fibromas of jaw bones (mandible and/or maxilla), polycystic bilateral kidney lesions, papillary renal carcinoma, renal hamartomas, Wilms’ tumour, and uterine tumours (adenofibromas, leyomiomas or adenosarcomas) in about 75% of HPT-JT affected women. Parathyroid tumours occur in about 95% of patients and they are typically the first clinical manifestation of the syndrome. PHPT in HPT-JT is characterised by aggressive behaviour (severe hypercalcaemia with likelihood of hypercalcaemic crisis) and it is usually associated with parathyroid carcinoma in about 10–15% of cases (23).
Inactivating germline mutations of the tumour suppressor CDC73 gene (cell division cycle 73 gene, known also as HRPT2 gene; locus 1q31.2, OMIM gene 607393, encoding parafibromin) have been described in over 50% of HPT-JT families and in about 20% of apparently sporadic parathyroid carcinoma (26). As all tumour suppressor genes, the first mutation is usually inherited by one of the parent or, in very rare cases, developed at embryo level, and the second allele inactivating mechanism is a loss of heterozygosity (LOH) at somatic level in HPT-JT tumour-related tissues. To date, over 100 different CDC73 mutations have been described all along the entire coding region of the gene. The CDC73 protein, parafibromin, is a nuclear protein acting as a transcriptional regulator within the RNA polymerase II associated complex, that regulates key transcriptional and post-transcriptional events such as initiation of transcription, transcript elongation, mRNA maturation and maintenance of poly(A) length at 3′UTR for mRNA stability.
The great majority of CDC73 mutations (>80%) is frameshift or nonsense mutations that determine the functional loss of parafibromin by causing a premature truncation of this protein or cause a rapid loosing of the translated protein via nonsense-mediated mRNA decay. Therefore, the expression of parafibromin is completely lost in HPT-JT-associated tumour tissues. Immunohistochemical staining of parafibromin in parathyroid tumours and other HPT-JT-associated tumours is a direct method to recognize HPT-JT syndrome in patients with already developed tumours. For an early diagnosis in asymptomatic subjects from HPT-JT families a sequencing screening of CDC73 coding region and exon-intron junctions is strongly recommended. Moreover, it is estimated that gross deletions within the gene may represent about 1% of all CDC73 mutations in HPT-JT pedigrees (27), thus genetic test should encompass the research of CDC73 gross deletions in HPT-JT families that are negative for point mutations or small frameshift mutations. Even in presence of a CDC73 mutation, the penetrance of PHPT is incomplete in HPT-JT patients, and no specific genotype-to-phenotype correlation has been clearly described for each of HPT-JT-associated clinical feature. Thus, a positive CDC73 mutational analysis is likely to lead not to immediate surgical or pharmacological therapies, even if it suggests to perform an earlier and more frequent instrumental and biochemical screening. A genetic test identifying an individual as a mutant gene carrier is indicative for a specific HPT-JT screening program which comprises dosing of serum calcium and PTH every 6–12 months for the monitoring of PHPT, panoramic jaw X-ray with neck shielding every 5 years for the prevention of ossifying jaw fibroma, abdominal MRI every 5 years for the checking of kidney cystis or tumour and, only in women, annual transvaginal or transabdominal ultrasound screening for the prevention of uterine tumours and for early identification of uterine lesions also to improve reproductive fitness (Table 2). In families bearing a CDC73 mutation the genetic analysis should be performed in children before the age of 10, since tumours have been also described at that very early age.
Genetic testing in familial isolated primary hyperparathyroidism (FIPH)
Familial isolated primary hyperparathyroidism (FIPH; OMIM phenotype #145000) is a rare autosomal dominant disorder characterised by PHPT in various members of the same family in the absence of other endocrine clinical manifestations and/or tumoral lesions.
A specific genetic cause for FIPH has not been assessed, and the great majority of FIHP does not have any identifiable mutation. In families with FIHP, genetic screening for MEN1 and CaSR mutations is suggested when individuals present mild hypercalcaemia, parathyroid multiglandular involvement at a relatively young age (before the third decade of life) for the differential diagnosis and appropriate diagnostic and therapeutic planning. In FIPH families with parathyroid carcinoma, cystic parathyroid tumour or jaw tumours the CDC73 genetic screening is mandatory to diagnose/exclude the HPT-JT syndrome. In up to 14% of FIPH cases mutations of CDC73 gene have been found (26), suggesting FIPH as a possible variant of HPT-JT syndrome. FIPH families with a CDC73 mutations should be enrolled in the biochemical and instrumental screening program of HPT-JT syndrome.
Sporadic PHPT
Sporadic PHPT represents about 90–95% of all PHPT cases, but the causative genetic alterations of PHPT sporadic forms remain poorly understood. Two main genes have been associated to parathyroid adenomas in sporadic PHPT: oncosuppressor MEN1 and cyclin D1 gene (CCND1, OMIM gene 168461), both located in chromosome 11. Homozygous loss of MEN1 locus (caused by two distinct inactivating events at parathyroid somatic level) has been observed in 25–40% of sporadic parathyroid tumours (28, 29) and it represents the most common genetic somatic alteration in sporadic PHPT. Pericentromeric inversions of chromosome 11, involving the PTH promoter and the CCND1 gene have been found in 8% of parathyroid adenomas (30). Cyclin D1 overexpression has been associated to 20–40% of sporadic parathyroid adenomas (31) and in up to 90% of sporadic parathyroid carcinomas (32).
Some studies (27, 33) have found about 4% of somatic CDC73 mutations in sporadic parathyroid adenomas. Conversely, no mutation of RET and CaSR genes has been detected in sporadic PHPT (34).
Very recently, a study (35) evaluated the involvement of the AIP gene in a large series of sporadic parathyroid adenomas by sequencing and MPLA analyses of the AIP gene itself and by LOH analysis of the AIP locus. In two of 132 tumours has been identified the R304Q germline mutation, and heterozygous AIP locus large deletions have been detected in 29 cases, including one of the two mutated adenomas.
Sanger DNA sequencing on large cohorts of sporadic parathyroid adenomas or whole exome next generation sequencing analysis identified low-frequency germline and somatic mutations of other and novel genes, such as inactivating mutations in the CDKI genes (i.e two CDKN1B mutations in a total of 86 cases) (36), stabilizing mutations of the beta catenin (CTNNB1) gene (37–39), an activating mutation (Y641N) in the histone 3 lysine 27 methyltransferase (EZH2) gene (found in two of 193 analysed adenomas) (40), and somatic mutations (at codons 786 and 787) in the zinc finger X-linked (ZFX) gene (41). However, a recent work on an Italian cohort of 12 atypical parathyroid adenomas, 45 typical parathyroid adenomas and 23 sporadic parathyroid carcinomas failed to confirm these results and to detect any mutation of EZH2 and ZFX genes (42).
For parathyroid carcinomas, inactivating somatic mutations of the CDC73 gene are the most frequent genetic alteration ranging from 66 to 100% of cases (43).
No preventive routinely genetic screening is available for sporadic PHPT since mutations occur only a somatic level and can be detected only when parathyroid hyperplasia, adenoma or carcinoma have developed and been surgically removed.
Given the relatively high incidence of familial forms of PHPT (5–10% of cases), a determination of calcaemia/ionized serum calcium in first-degree relatives of affected individuals with apparently sporadic parathyroid adenoma is mandatory. Then, if a familial form is suspected, a genetic testing (CaSR, MEN1, etc.) is strongly suggested for differential diagnosis of PHPT.
Diagnostic genetic workup in PHPT
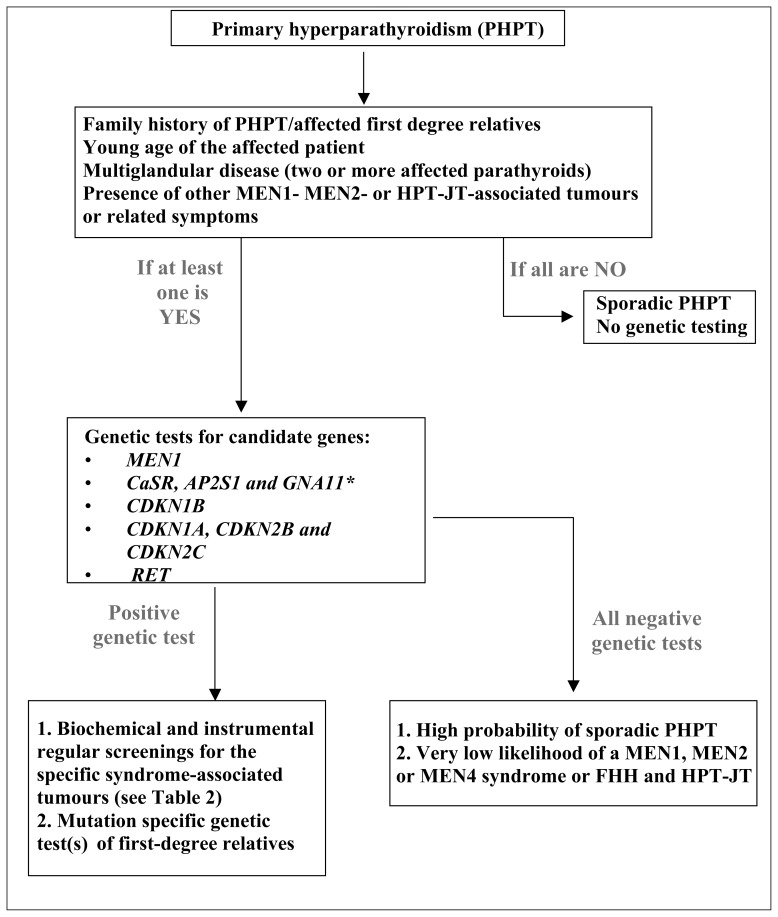
Since PHPT can be genetically determined in up to 10–15% of cases, genetic testing has to be considered in the diagnostic classification of this disease. In Figure 1 a clinical and genetic workup is proposed. Even if the proband does not display a clear positive family for the disease, serum calcium should always be determined in first-degree relatives. In the case of positive family history and/or if the patient is young, genetic testing is advisable even before surgery. If a multiglandular involvement is detected at the time of parathyroidectomy and/or suspicious lesions such as atypical parathyroid adenoma or carcinoma are diagnosed at histological examination, genetic testing is mandatory, along with proper assessment for possible MEN, HPT-JT or FHH associated clinical manifestations.
Figure 1.
Proposed genetic testing workup in patients with primary hyperparathyroidism (PHPT) as derived from the Consensus Statement of the Fourth International Workshop on Primary Hyperparathyroidism (modified from ref. 33). *CaSR, AP2S1 and GNA11 are examined in the case of familial hypercalciuric hypercalcemia (FHH).
If MEN1-associated manifestations such as neuroendocrine tumours and/or pituitary adenomas are detected, mutational analysis of MEN1 gene has to be undertaken. If a MEN1 phenotype is present in the absence of MEN1 mutations, the exclusion of deletions of 11q13.1 region must be excluded or confirmed by specific analyses such as multiplex ligation-dependent probe amplification (MLPA). In the case these analyses are negative, sequencing of other genes such as genes of the CDKI family is advisable.
If a MEN2 is suspected because of high values of serum calcitonin, RET mutation analysis is indicated. In addition, the identification of specific RET mutations associated with a more aggressive medullary thyroid cancer can induce to perform prophylactic thyroidectomy, thus having a deep rebound on clinical management in these patients.
If a relative hypocalciuria for the degree of hypercalcaemia is detected, in an otherwise asymptomatic patient, sequencing of the CaSR and other genes encoding for proteins associated with the CaSR signal transduction pathway (AP2S1, GNA11) is required.
In the case of jaw tumours associated with PHPT, or suspicious findings at histology (atypical parathyroid adenoma, parathyroid carcinoma), mutational analysis of CDC73 has to be performed.
Since no specific and unique genetic alterations have been identified in FIHP, sequencing of the genes responsible for the other syndromes, in particular MEN1 and CaSR, can be carried out. If a MEN1 mutation is detected, a proper follow-up to unveil possible MEN1-related manifestations other than PHPT can be planned (Table 2).
Once a mutation has been identified in the proband, first degree family members have to be screened for that particular genetic defect, even if asymptomatic, and a proper baseline assessment and follow-up has to be planned according to clinical guidelines for the management of that particular syndrome (Table 2). If a phenotype-to-genotype correlation exists, the identification of a particular mutation may influence the management of the disease itself.
A positive test could also enable the performing of prenatal tests in the foetus of a mutated individual in order to assess the genetic status of the foetus before birth, giving parents the change of termination of affected pregnancies or, alternatively, giving clinicians the opportunity to intervene with prevention protocols and therapies just after birth. In a mutation-positive pedigree also pre-implantation tests could be performed on embryos derived from in vitro fertilization, in a couple with one parent bearing a PHPT-associated genetic mutation, in order to positively select non-mutated embryos before uterus implantation.
Recently, the proceedings of the fourth International workshop on PHPT have been published (44). They resume the Consensus achieved by the group meeting and the Statement for the diagnosis of asymptomatic primary hyperparathyroidism. A detailed section about the genetic testing for the differential diagnosis of familial PHPT and hypercalcaemia is reported. In the presence of an isolated PHPT case (i.e in the absence of other specific syndromic characteristics) the recommended guidelines for mutation screening in order of likely frequency are MEN1, CASR, AP2S1, GNA11, CDC73, CDKN1A/1B/2C, RET, and PTH genes.
Conclusions
Genetic and molecular alterations underlying the different familial forms of PHPT are highly variable. The increasing knowledge on molecular pathophysiology of hereditary PH-PT, together with application of specific genetic tests have contributed to the availability of more effective and individualised prevention and treatments, granting patients a reduction of PHPT-associated morbidity and a better quality of life. Some points should be highlighted: 1) genetic test in all family members of mutation-bearing pedigrees is fundamental because of the autosomal dominant pattern of inheritance; 2) the identification of mutation carriers, even the asymptomatic ones, is important for the prevention and therapeutic choices; 3) the recognition of the specific mutated gene, and in some cases of the specific mutation, is pivotal for the planning of future preventive screenings and for clinical decisions; 4) a positive test can lead to a decision for an in vitro fertilisation with pre-implantation genetic selection of non-mutated embryos or to a decision for a pre-natal genetic test; 5) a negative test allows individuals to avoid future periodic syndrome-related screenings.
Acknowledgements
This work was supported by unrestricted grants from Fondazione Ente Cassa di Risparmio di Firenze and from Fondazione F.I.R.M.O. Raffaella Becagli to M.L.B.
References
- 1.Nilsson IL, Yin L, Lundgren E, Rastad J, Ekbom A. Clinical presentation of primary hyperparathyroidism in Europe-nationwide cohort analysis on mortality from nonmalignant causes. J Bone Miner Res. 2000;17:N68–N74. [PubMed] [Google Scholar]
- 2.Carlson D. Parathyroid pathology: hyperparathyroidism and parathyroid tumours. Arch Pathol Lab Med. 2010;134:1639–1644. doi: 10.5858/2009-0578-CCR.1. [DOI] [PubMed] [Google Scholar]
- 3.Sharretts JM, Simonds WF. Clinical and molecular genetics of parathyroid neoplasms. Best Pract Res Clin Endocrinol Metab. 2010;24:491–502. doi: 10.1016/j.beem.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, et al. Guidelines for the diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658–5671. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- 5.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mut. 2008;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- 6.Concolino P, Costella A, Capoluongo E. Multiple endocrine neoplasia type 1 (MEN1): An update of 208 new germline variants reported in the last nine years. Cancer Genet. 2007;209:36–41. doi: 10.1016/j.cancergen.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Tham E, Grandell U, Lindgren E, Toss G, Skogseid B, Nordenskjöld M. Clinical testing for mutations in the MEN1 gene in Sweden: a report on 200 unrelated cases. J Clin Endocrinol Metab. 2007;92:3389–3395. doi: 10.1210/jc.2007-0476. [DOI] [PubMed] [Google Scholar]
- 8.Turner JJO, Christie PT, Pearce SHS, Turnpenny PD, Thakker RV. Diagnostic challenges due to phenocopies: lessons from multiple endocrine neoplasia type 1 (MEN1) Hum Mut. 2010;31:E1089–E1101. doi: 10.1002/humu.21170. [DOI] [PubMed] [Google Scholar]
- 9.Fearon E. The sweet secrets of p27kip1 regulation and function in cell migration. Cell Cycle. 2011;10:3429. doi: 10.4161/cc.10.20.17529. [DOI] [PubMed] [Google Scholar]
- 10.Itamochi H, Yoshida T, Walker CL, Bartholomeusz C, Aoki D, Ishihara H, et al. Novel mechanism of reduced proliferation in ovarian clear cel-cells: cytoplasmic sequestration of CDK2 by p27. Gynecol Oncol. 2011;122:641–647. doi: 10.1016/j.ygyno.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Milne TA, Hughes CM, Lloyd R, Yang Z, Rozenblatt-Rosen O, Dou Y, et al. Menin and MLL cooperatively regulate expression of cyclin-de7pendent kinase inhibitors. Proc Natl Acad Sci USA. 2005;102:749–754. doi: 10.1073/pnas.0408836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Circelli L, Ramundo V, Marotta V, Sciammarella C, Marciello F, Del Prete M, et al. Prognostic role of the CDNK1B V109G polymorphsm in multiple endocrine neoplasia type 1. J Cell Mol Med. 2015;19:1735–1741. doi: 10.1111/jcmm.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raue F, Kraimps JL, Dralle H, Cougard P, Proye C, Frilling A, et al. Primary hyperparathyroidism in multiple endocrine neoplasia type 2A. J Inter Med. 1995;238:369–373. doi: 10.1111/j.1365-2796.1995.tb01212.x. [DOI] [PubMed] [Google Scholar]
- 14.Machens A, Dralle H. Multiple endocrine neoplasia type 2 and the RET proto-oncogene: from bedside to bench to bedside. Mol Cell Endocrinol. 2006;247:34–40. doi: 10.1016/j.mce.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Punales MK, Graf H, Gross JL, Maia AL. RET codon 634 mutations in multiple endocrine neoplasia type 2: variable clinical features and clinical outcome. J Clin Endocrinol Metab. 2003;88:2644–2649. doi: 10.1210/jc.2002-021422. [DOI] [PubMed] [Google Scholar]
- 16.Moline J, Eng C. Multiple endocrine neoplasia type 2: an overview. Genet Med. 2011;13:755–764. doi: 10.1097/GIM.0b013e318216cc6d. [DOI] [PubMed] [Google Scholar]
- 17.Geogitsi M. MEN-4 and other multiple endocrine neoplasias due to cyclin-dependent kinase inhibitors (p27kip1 and p18INK4C) mutations. Best Pract Res Clin Endocrinol Metab. 2010;24:425–437. doi: 10.1016/j.beem.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Pellegata NS. MENX and MEN4. Clinics. 2012;67:13–18. doi: 10.6061/clinics/2012(Sup01)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonelli F, Giudici F, Giusti F, Marini F, Cianferotti L, Nesi G, Brandi ML. A heterozygous frameshift mutation in exon 1 of CDKN1B gene in a patient affected by MEN4 syndrome. Eur J Endocrinol. 2014;171:K7–K17. doi: 10.1530/EJE-14-0080. [DOI] [PubMed] [Google Scholar]
- 20.Elston MS, Meyer-Rochow GY, Dray M, Swarbrick M, Conaglen JV. Early Onset Primary Hyperparathyroidism Associated with a Novel Germline Mutation in CDKN1B. Case Rep Endocrinol. 2015;2015:510985. doi: 10.1155/2015/510985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunn IR, Gaffney D. Clinical and laboratory features of calcium-sensing receptor disorders: a systematic review. Ann Clin Biochem. 2004;41:441–458. doi: 10.1258/0004563042466802. [DOI] [PubMed] [Google Scholar]
- 22.Ward BK, Magno AL, Blitvich BJ, Rea AJ, Stuckey BG, Walsh JP, et al. Novel mutations in the calcium-sensing receptor gene associated with biochemical and functional differences in familial hypocalciuric hypercalcaemia. Clin Endocrinol (Oxf) 2006;64:580–587. doi: 10.1111/j.1365-2265.2006.02512.x. [DOI] [PubMed] [Google Scholar]
- 23.Falchetti A, Marini F, Giusti F, Cavalli L, Cavalli T, Brandi ML. DNA-based test: when and why to apply it to primary hyperparathyroidism clinical phenotypes. J Int Med. 2009;266:69–83. doi: 10.1111/j.1365-2796.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- 24.Nesbit MA, Hannan FM, Howles SA, Babinsky VN, Head RA, Cranston T, et al. Mutations affecting G-protein subunit α11 in hypercalcemia and hypocalcemia. N Engl J Med. 2013;368:2476–2486. doi: 10.1056/NEJMoa1300253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nesbit MA, Hannan FM, Howles SA, Reed AA, Cranston T, Thakker CE, et al. Mutations in AP2S1 cause familial hypocalciuric hypercalcemia type 3. Nat Genet. 2013;45:93–97. doi: 10.1038/ng.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cascón A, Huarte-Mendicoa CV, Javier Leandro-García L, Letón R, Suela J, Santana A, et al. Detection of the first gross CDC73 germline deletion in an HPT-JT syndrome family. Genes Chrom Cancer. 2011;50:922–929. doi: 10.1002/gcc.20911. [DOI] [PubMed] [Google Scholar]
- 27.Newey PJ, Bowl MR, Cranston T, Thakker RV. Cell division cycle protein 73 homolog (CDC73) mutations in the hyperparathyroidism-jaw tumour syndrome (HPT-JT) and parathyroid tumours. Hum Mutat. 2010;31:295–307. doi: 10.1002/humu.21188. [DOI] [PubMed] [Google Scholar]
- 28.Heppner C, Kester MB, Agarwal SK, Debelenko LV, Emmert-Buck MR, Guru SC, et al. Somatic mutation of the MEN1 gene in parathyroid tumours. Nat Genet. 1997;16:375–378. doi: 10.1038/ng0897-375. [DOI] [PubMed] [Google Scholar]
- 29.Miedlich S, Krohn K, Lamesch P, Müller A, Paschke R. Frequency of somatic MEN1 gene mutations in monoclonal parathyroid tumours in patients with primary hyperparathyroidism. Eur J Endocrinol. 2000;143:47–54. doi: 10.1530/eje.0.1430047. [DOI] [PubMed] [Google Scholar]
- 30.Yi Y, Nowak NJ, Pacchia AL, Morrison C. Chromosome 11 genomic changes in parathyroid adenoma and hyperplasia: array CGH, FISH, and tissue microarrays. Genes Chromosomes Cancer. 2008;47:639–48. doi: 10.1002/gcc.20565. [DOI] [PubMed] [Google Scholar]
- 31.Mallya SM, Arnold A. Cyclin D1 in parathyroid disease. Front Biosci. 2000;5:D367–371. doi: 10.2741/mallya. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L, Sun LH, Liu DM, He XY, Tao B, Ning G, et al. Copy number variation in CCND1 gene is implicated in the pathogenesis of sporadic parathyroid carcinoma. World J Surg. 2014;38:1730–1737. doi: 10.1007/s00268-014-2455-9. [DOI] [PubMed] [Google Scholar]
- 33.Shattuck TM, Välimäki S, Obara T, Gaz RD, Clark OH, Shoback D, et al. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med. 2003;349:1722–1729. doi: 10.1056/NEJMoa031237. [DOI] [PubMed] [Google Scholar]
- 34.Willeke F, Hauer MP, Buchcik R, Gebert JF, Hahn M, Fitze G, et al. Multiple endocrine neoplasia type 2-associated RET proto-oncogene mutations do not contribute to the pathogenesis of sporadic parathyroid tumours. Surgery. 1988;124:484–490. [PubMed] [Google Scholar]
- 35.Pardi E, Marcocci C, Borsari S, Saponaro F, Torregrossa L, Tancredi M, et al. Aryl Hydrocarbon Receptor-Interacting protein (AIP) mutations occur rarely in sporadic parathyroid adenomas. J Clin Endocrinol Metab. 2013;98:2800–2810. doi: 10.1210/jc.2012-4029. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal SK, Mateo CM, Marx SJ. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab. 2009;94:1826–34. doi: 10.1210/jc.2008-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Björklund P, Lindberg D, Akerström G, Westin G. Stabilizing mutation of CTNNB1/beta-catenin and protein accumulation analyzed in a large series of parathyroid tumors of Swedish patients. Mol Cancer. 2008;7:53. doi: 10.1186/1476-4598-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guarnieri V, Baorda F, Battista C, Bisceglia M, Balsamo T, Gruppioni E, et al. A rare S33C mutation of CTNNB1 encoding β-catenin in a parathyroid adenoma found in an Italian primary hyperparathyroid cohort. Endocrine. 2012;41:152–155. doi: 10.1007/s12020-011-9558-y. [DOI] [PubMed] [Google Scholar]
- 39.Starker LF, Fonseca AL, Akerström G, Björklund P, Westin G, Carling T. Evidence of a stabilizing mutation of β-catenin encoded by CTNNB1 exon 3 in a large series of sporadic parathyroid adenomas. Endocrine. 2012;42:612–615. doi: 10.1007/s12020-012-9690-3. [DOI] [PubMed] [Google Scholar]
- 40.Cromer MK, Starker LF, Choi M, Udelsman R, Nelson-Williams C, Lifton RP, et al. Identification of somatic mutations in parathyroid tumors using whole-exome sequencing. J Clin Endocrinol Metab. 2012;97:E1774–E1781. doi: 10.1210/jc.2012-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soong CP, Arnold A. Recurrent ZFX mutations in human sporadic parathyroid adenomas. Oncoscience. 2014;1:360–366. doi: 10.18632/oncoscience.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanpaolo E, Miroballo M, Corbetta S, Verdelli C, Baorda F, Balsamo T, et al. EZH2 and ZFX oncogenes in malignant behaviour of parathyroid neoplasms. Endocrine. 2016;54:55–59. doi: 10.1007/s12020-016-0892-y. [DOI] [PubMed] [Google Scholar]
- 43.Alvelos MI, Vinagre J, Fonseca E, Barbosa E, Teixeira-Gomes J, Sobrinho-Simões M, et al. MEN1 intragenic deletions may represent the most prevalent somatic event in sporadic primary hyperparathyroidism. Eur J Endocrinol. 2012;168:119–128. doi: 10.1530/EJE-12-0327. [DOI] [PubMed] [Google Scholar]
- 44.Eastell R, Brandi ML, Costa AG, D’Amour P, Shoback DM, Thakker RA. Diagnosis of asymptomatic primary hyperparathyroidism: proceeding of the fourth International workshop. J Clin Endocrinol Metab. 2014;99:3570–3579. doi: 10.1210/jc.2014-1414. [DOI] [PubMed] [Google Scholar]