Abstract
Objective
Incident acute kidney injury (AKI) and prevalent chronic kidney disease (CKD) are commonly encountered in septic patients. We examined the differential effect of AKI and CKD on the association between cumulative fluid balance (CFB) and hospital mortality in critically ill septic patients.
Design
Retrospective cohort study.
Setting
Urban academic medical center ICU.
Patients
ICU adult patients with severe sepsis or septic shock and serum creatinine measured within 3 months prior to and 72 h of ICU admission. Patients with estimated glomerular filtration rate <15 mL/min/1.73m2 or receiving chronic dialysis were excluded.
Interventions
None.
Measurements and Main Results
2632 patients, 1211 with CKD, were followed until hospital death or discharge. AKI occurred in 1525 (57.9%), of whom 679 (44.5%) had CKD. Hospital mortality occurred in 603 (22.9%) patients. Every 1 L increase in CFB at 72 h of ICU admission was independently associated with hospital mortality in all patients, adjusted odds ratio (aOR) 1.06, 95% CI (1.04–1.08), p <0.001, and in each AKI/CKD subgroup: aOR 1.06 (1.03–1.09) for AKI+/CKD+; 1.09 (1.05–1.13) for AKI−/CKD+; 1.05 (1.03–1.08) for AKI+/CKD−; and 1.07 (1.02–1.11) for AKI−/CKD−. There was a significant interaction between AKI and CKD on CFB, p =0.005, such that different CFB cut-offs with the best prognostic accuracy for hospital mortality were identified: 5.9 L for AKI+/CKD+; 3.8 L for AKI−/CKD+; 4.3 L for AKI+/CKD−; and 1.5 L for AKI−/CKD−. The addition of CFB to the admission SOFA score had increased prognostic utility for hospital mortality when compared to SOFA alone, particularly in patients with AKI.
Conclusions
Higher CFB at 72 h of ICU admission was independently associated with hospital mortality regardless of AKI or CKD presence. We characterized CFB cut-offs associated with hospital mortality based on AKI/CKD status, underpinning the heterogeneity of fluid regulation in sepsis and kidney disease.
Keywords: cumulative fluid balance, sepsis, acute kidney injury, chronic kidney disease, mortality
Introduction
Sepsis is the most common cause of intensive care unit (ICU) admissions and is associated with significant morbidity and mortality (1, 2). Acute kidney injury (AKI) is a frequent complication in critically ill patients and occurs in nearly 45% of septic patients and 60% of those with septic shock (3–5). The combination of sepsis and AKI may synergistically increase mortality rates to up to 50% (5–7). Most patients with sepsis have pre-existing comorbidities, including chronic kidney disease (CKD) (1). As compared to those without CKD, those with CKD have a higher incidence and severity of sepsis, as well as increased mortality from sepsis (8–10). CKD is now recognized as a relevant poor prognostic factor in patients with sepsis (11, 12).
Despite the known benefits of fluid therapy in sepsis (13–15), the recognition of potential deleterious effects of excessive fluid administration is alarming. Humphrey et al (16) demonstrated a significant decrease in mortality with a fluid-conservative resuscitation strategy in a small sample with acute respiratory distress syndrome. More recently, Wiedemann et al (17) showed that a fluid-conservative approach shortened the duration of mechanical ventilation in patients with acute lung injury. Subsequent studies have proposed “fluid accumulation” or “positive fluid balance” as a marker of adverse outcomes in patients with septic shock (2, 18, 19). Importantly, fluid overload (defined as fluid accumulation >10% above baseline weight) and mean daily fluid balance were independently associated with mortality in critically ill patients with AKI (20, 21).
Previous studies have not investigated the impact of cumulative fluid balance (CFB) on adverse outcomes based on incident AKI and/or prevalent CKD stratification. The purpose of the present study was to determine whether CFB was independently associated with hospital mortality in critically ill septic patients with or without incident AKI and prevalent CKD, and whether a differential effect of AKI or CKD on this association could be identified. We also investigated whether the addition of CFB to the admission SOFA score would improve the prognostic accuracy for hospital mortality.
Materials and Methods
Study Design and Participants
We conducted a single-center, retrospective cohort study utilizing a database of patients with severe sepsis or septic shock admitted to the ICU in an urban, tertiary care hospital. Study participants were identified using administrative-linked electronic databases for ICU admissions from May 2007 through April 2012. Severe sepsis or septic shock was defined by Angus (1) criteria, using International Classifications of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (22) for both a bacterial or fungal infection and a diagnosis of acute organ dysfunction excluding gastrointestinal failure. We included adult patients admitted from the emergency department (ED) to the ICU with a diagnosis of severe sepsis or septic shock who had at least one serum creatinine (SCr) measured and documented in the electronic medical records (EMR) at two different time points: within 3 months prior to and within the first 72 h of admission. Patients with absent or incomplete recorded daily fluid balance within the first 72 h of ICU stay, and those with estimated glomerular filtration rate (eGFR) <15 mL/min/1.73m2 or receiving chronic dialysis were excluded. The protocol was approved by the Institutional Review Board (IRB #7044).
Study Variables
Baseline SCr was defined as the most recent SCr within the 3-month period before ICU admission, which was used to calculate the baseline eGFR using the 4-variable Modification of Diet in Renal Disease (MDRD) study equation (23). Patients were categorized as having AKI if the baseline SCr increased by ≥0.3 mg/dl or by ≥150%, or required acute dialysis as defined by KDIGO SCr-based criteria (24). The highest SCr within 72 h of admission was used to determine the occurrence of AKI. Pre-existing CKD was defined as baseline eGFR of 15–59 mL/min/1.73m2 in the absence of chronic dialysis or end-stage renal disease.
Cumulative fluid balance was calculated as follows: total fluid input minus total fluid output within the first 72 h of ICU stay. Subject-specific variables were obtained from EMR. Acute Physiology and Chronic Health Evaluation II (APACHE-II) (25) and Sequential Organ Failure Assessment (SOFA) (26) scores were calculated integrating clinical and laboratory data from the first day of ICU admission. Oliguria was defined as urine output <500 mL within 24 h. Prevalent comorbidity was identified using ICD-9-CM codes, except for anemia that was defined as admission hematocrit <39% for men and <36% for women. Data pertaining to drug exposure, red blood cell transfusion, mechanical ventilation, and acute dialysis were based on hospital billing codes for the indexed admission. All collected data were validated through comprehensive individual review of 10% of EMR by data management personnel blinded to the study.
Study Outcome
The observation period was from admission to the ICU until the time of hospital death or discharge. The primary outcome measure was all-cause hospital mortality, adjudicated based on EMR review by data management personnel blinded to the study.
Statistical Analysis
The study sample was analyzed as a whole group and stratified into 4 subgroups by the occurrence of AKI (incident AKI) and pre-existing CKD (prevalent CKD) as follows: AKI+/CKD+; AKI−/CKD+; AKI+/CKD−; AKI−/CKD−. Categorical data were reported as percentages and continuous data as means ± SD or median [25th – 75th percentile]. Comparisons between groups for categorical variables were made using the Fisher Exact test. For continuous variables, analysis of variance was used for Gaussian and Wilcoxon Rank-Sum test for non-Gaussian distributed data.
Multivariable logistic regression models were constructed for hospital mortality as the dependent variable and to evaluate CFB as an independent variable. The two-way interaction between incident AKI and prevalent CKD (AKI*CKD) on CFB and on hospital mortality was first evaluated in the entire cohort to validate subgroup stratification if significant (p <0.1). CFB was modeled as a continuous variable (per 1 L increase) and categorical variable (≥ vs < cut-off value). Optimal predicted probability cut-offs were determined by Youden’s index from receiver-operating characteristic (ROC) analysis. Candidate variables for the multivariable models included demographic data (age, gender, and race); comorbidities (diabetes, hypertension, heart failure, and anemia); indicators of critical illness (SOFA and APACHE-II scores, oliguria, mechanical ventilation, red blood cell transfusion, and length of hospital stay); and drug exposure (vasoactive drug and diuretic). Length of hospital stay was dichotomized as ≥ vs < median value of 12 days. Inclusion into the final model was based upon significance of univariable results and clinical relevance. Only 1 of 2 variables was included in the event of collinearity between variables.
To test the model performance of CFB + admission SOFA score vs SOFA alone for the prediction of hospital mortality, ROC-areas under the curve (AUCs) were compared and continuous net reclassification index (NRI) and absolute integrated discrimination improvement (IDI) were calculated (27). NRI quantifies the hospital mortality events correctly reclassified with the addition of CFB to the model that included SOFA alone. IDI measures the increment in the predicted probabilities for the hospital mortality subset and the decrement for the subset without hospital mortality. The 95% confidence intervals (CI) reported for the logistic regression odds ratios (ORs) were based on Wald estimation. Two-sided P-values <0.05 indicated statistical significance. Spreadsheet software and SAS 9.4 (SAS Institute, Cary, NC) were used in data acquisition and analysis.
Sensitivity Analyses
CFB Adjustment by Body Weight
We adjusted CFB by ICU admission body weight (W) in order to quantify fluid overload percentage (FO) using the following formula: FO = [(W+CFB/W) − 1] × 100%. FO was similarly evaluated as an independent variable in multivariable logistic regression models for hospital mortality.
Multiple Imputation Method for Missing Baseline SCr Values (28)
A total of 3070 patients had to be excluded from the primary analysis because of absent baseline SCr within 3 months prior to ICU admission. As part of a sensitivity analysis, these missing SCr values were imputed using a linear regression model derived from subject-specific characteristics of the primary study cohort (2632 patients). Log-transformed SCr was the dependent variable and independent predictors included age, gender, race, diabetes, hypertension, APACHE-II score, and their interactions. The association between CFB and hospital mortality was further evaluated in this secondary cohort of 5688 patients (2632 with known baseline SCr + 3056 with imputed baseline SCr, after exclusion of 14 patients with imputed baseline eGFR <15 ml/min/1.73 m2).
Propensity-regression analysis
The primary cohort logistic regression model of CFB (independent variable) and hospital mortality (dependent variable) included a continuous propensity score as a covariate for statistical adjustment. This propensity score was generated from all available study covariates that influenced the occurrence of AKI and/or CKD.
Standardized Mortality Ratio (SMR) determination to examine the relationship between CFB and hospital mortality
SMR for each AKI/CKD subgroup by CFB quintiles was calculated as follows: SMR =observed/predicted mortality; where predicted mortality was determined by the multivariable logistic regression estimate for each AKI/CKD subgroup.
Results
Clinical Characteristics
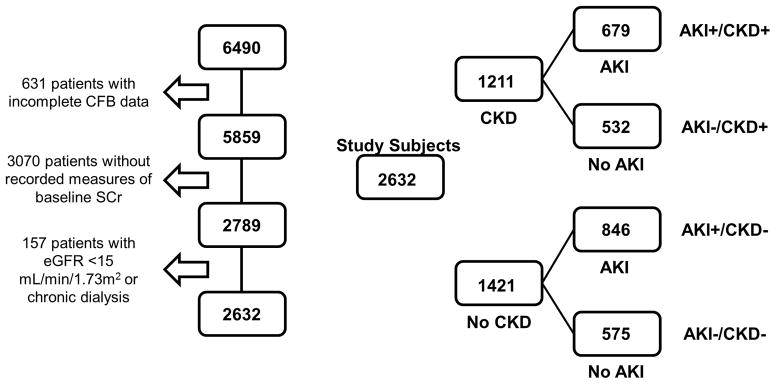
Of 6490 patients admitted from the ED to the ICU with the diagnosis of severe sepsis or septic shock, 3858 were excluded due to the following reasons: no recorded measures of baseline SCr within 3 months before admission; incomplete CFB data at 72 h; or receiving chronic dialysis (Figure 1). The primary study cohort included 2632 patients: 1211 (46%) with pre-existing CKD defined as an eGFR of 15–59 mL/min/1.73m2 and 1421 (54%) without CKD (eGFR ≥60 mL/min/1.73m2). AKI occurred in 1525 (57.9%) patients, 679 (44.5%) with pre-existing CKD and 846 (55.5%) without CKD (Figure 1). A total of 238 (9.0%) patients required acute dialysis for AKI.
Figure 1.
Cohort derivation and study scheme. AKI =occurrence of acute kidney injury; CFB =cumulative fluid balance; CKD =pre-existing chronic kidney disease; eGFR =estimated glomerular filtration rate; SCr =serum creatinine
Clinical characteristics of the cohort are reported in Table 1. Patients that suffered from AKI, independently of CKD status, had a higher frequency of pressor or inotrope requirement, higher APACHE-II and SOFA scores, and more frequent use of mechanical ventilation (Table 1). The median length of hospital stay (LOS) [25th – 75th percentile] was 12 [7 – 21] days in the entire cohort. In the CKD group, LOS was not different based on the presence of AKI, whereas in the non-CKD group, those with AKI had a LOS of 12 [7 – 20] days as compared to those without AKI, 13 [8 – 22], p =0.01 (Table 1). Importantly, this difference was influenced by the observation that AKI patients that died had shorter LOS when compared to their non-AKI counterparts: 9 [4 – 19] vs 14 [7 – 25] days, p =0.007 (data not shown).
Table 1.
Patient characteristics
| AKI+/CKD+ (n =679) | AKI−/CKD+ (n =532) | AKI+/CKD− (n =846) | AKI−/CKD− (n =575) | AKI * CKD interaction | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years, mean ± SD | 69.4 ± 14.2f | 70.0 ± 14.8f | 62.8 ± 15.8 | 61.5 ± 16.4 | 0.13 |
| Women, (%) | 336 (49.5)e | 284 (53.4)e | 355 (42) | 254 (44.2) | 0.001 |
| African-American, (%) | 261 (38.4)e | 178 (33.5)e | 401 (47.4)b | 238 (41.4) | <0.001 |
| Chronic conditions | |||||
| * Baseline SCr, mg/dl, mean ± SD | 1.80 ± 0.66f | 1.86 ± 0.73f | 0.93 ± 0.24 | 0.92 ± 0.23 | 0.12 |
| * Baseline eGFR, mL/min/1.73m2, mean ± SD | 41.7 ± 11.7bf | 40.0 ± 12.5f | 93.4 ± 36.2 | 92.3 ± 29.7 | 0.04 |
| Diabetes, (%) | 176 (25.9)e | 136 (25.6)e | 170 (20.1) | 99 (17.2) | 0.001 |
| Hypertension, (%) | 181 (26.7)cf | 213 (40.0)e | 432 (51.1) | 297 (51.7) | <0.001 |
| Systolic heart failure, (%) | 30 (4.4) | 15 (2.8) | 25 (3) | 18 (3.1) | 0.36 |
| Anemia, (%) | 600 (89) | 455 (86.7) | 722 (86.4) | 488 (85.6) | 0.26 |
| Drug exposure | |||||
| Diuretic, (%) | 47 (6.9) | 45 (8.5) | 66 (7.8) | 55 (9.6) | 0.12 |
| Statin, (%) | 257 (37.9)f | 185 (34.8)e | 211 (25) | 151 (26.3) | <0.001 |
| Iodine contrast, (%) | 103 (15.2)bf | 115 (21.6)f | 222 (26.2)b | 199 (34.6) | <0.001 |
| Aminoglycoside, (%) | 44 (6.5)e | 33 (6.2)e | 101 (11.9) | 66 (11.5) | <0.001 |
| Critical indicators | |||||
| Oliguria, (%) | 112 (20.2)cd | 24 (5.6) | 105 (14.7)c | 16 (3.3) | 0.002 |
| CFB 72 h, liters, mean ± SD | 4.16 ± 7.34bf | 2.85 ± 5.91 | 5.55 ± 7.50c | 2.74 ± 5.31 | 0.005 |
| FO 72 h, %, mean ± SD | 5.7 ± 9.7e | 4.7 ± 9.1 | 8.0 ± 11.1c | 4.0 ± 7.7 | 0.001 |
| * LOS, days, median [25th–75th percentile] | 12.0 [6.0 – 21.0] | 12.0 [7.0 – 20.0]e | 12.0 [7.0 – 20.0]b | 13.0 [8.0 – 22.0] | 0.07 |
| Pressor or inotrope, (%) | 290 (42.7)c | 144 (27.1) | 366 (43.3)c | 167 (29.0) | <0.001 |
| Mechanical ventilation, (%) | 297 (43.7)b | 190 (35.7) | 407 (48.1)b | 219 (38.1) | <0.001 |
| Blood transfusion, (%) | 19 (2.8) | 19 (3.6) | 29 (3.4) | 18 (3.1) | 0.77 |
| APACHE-II score, mean ± SD | 14.5 ± 7.3c | 12.2 ± 5.8e | 13.4 ± 6.9c | 11.1 ± 6.1 | 0.91 |
| * SOFA score, median [25th–75th percentile] | 5.0 [3.0 – 9.0]c | 4.0 [2.0 – 6.0]e | 5.0 [3.0 – 8.0]c | 3.0 [1.0 – 6.0] | 0.002 |
AKI =occurrence of acute kidney injury; anemia =hematocrit <39% for men and <36% for women; APACHE-II =Acute Physiology and Chronic Health Evaluation II score; CFB 72 h =cumulative fluid balance (total fluid input minus output within the first 72 h of ICU admission); CKD =pre-existing CKD; eGFR =estimated glomerular filtration rate based on Modification of Diet in Renal Disease (MDRD) Study equation, mL/min/1.73m2; FO =fluid overload percentage [(W+CFB/W) − 1] × 100%, W is ICU admission body weight; iodine contrast =only if intravenous or intra-arterial; LOS =length of hospital stay; oliguria =urine output less than 500 mL in 24 h; SCr =serum creatinine; SOFA =Sequential Organ Failure Assessment score.
Data were log-transformed before analysis. Comparisons for categorical variables were made using the Fisher Exact test. For continuous variables, analysis of variance was used for Gaussian and Wilcoxon Rank-Sum test for non-Gaussian distributed data.
p <0.05;
p <0.01;
p <0.0001; AKI vs no AKI for the same CKD status
p <0.05;
p <0.01;
p <0.0001; CKD vs no CKD for the same AKI status
Study Outcomes
A total of 603 (22.9%) patients died during the observation period, median LOS 10 [4 – 20] days. A higher proportion of patients with AKI (28.1%) vs without AKI (15.8%) died, p <0.001. There was significant interaction between AKI and CKD (AKI*CKD) on hospital mortality (p =0.04): 173 (25.5%) patients with AKI vs 91 (17.1%) without AKI died in the CKD group, p <0.001; and 255 (30.1%) with AKI vs 84 (14.6%) without AKI died in the non-CKD group, p <0.001.
CFB (mean ± SD) at 72 h was higher in those who died: 7.67 ± 7.94 vs 2.95 ± 6.05 L in survivors, p <0.001. CFB was also higher in those with AKI requiring dialysis (9.16 ± 8.91 L) when compared to those with AKI not requiring dialysis (4.61 ± 7.24 L) or those that did not suffer from AKI (2.80 ± 5.60 L), p for trend <0.001. CFB was independently associated with hospital mortality in the entire cohort (adjusted OR per 1 L increase [95% CI] 1.06 [1.04 – 1.08], p <0.001). The occurrence of AKI was an independent predictor of hospital mortality (adjusted OR 1.28 [1.01 – 1.62], p =0.04) but pre-existing CKD was not (p =0.22).
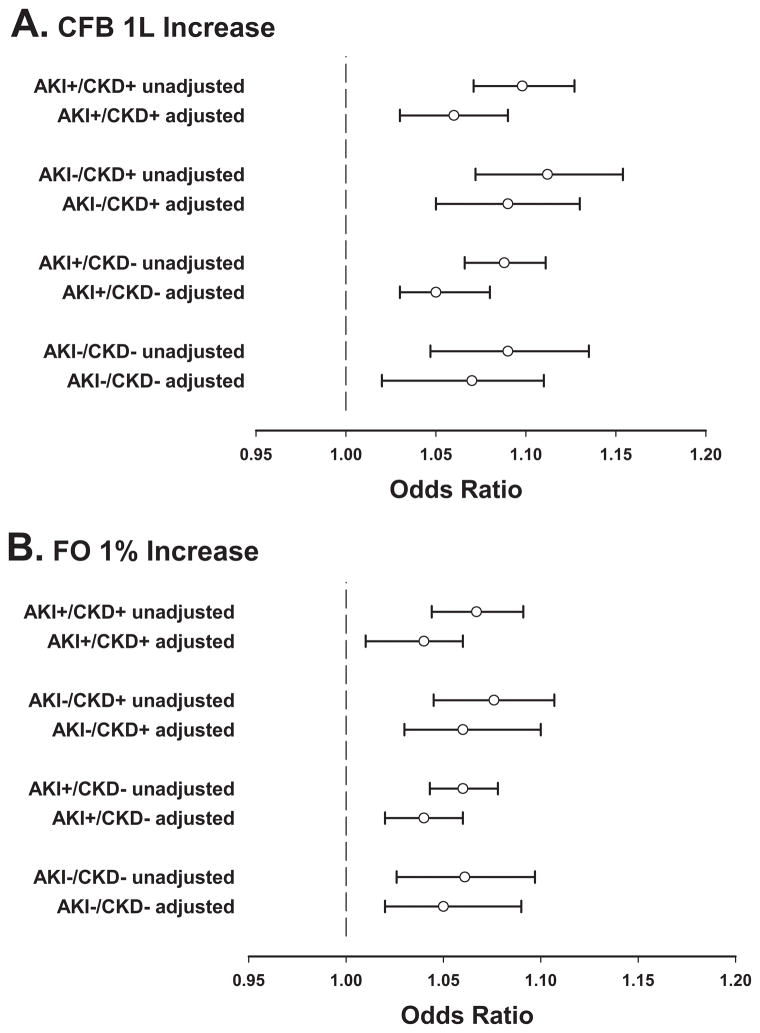
There was significant interaction between AKI and CKD (AKI*CKD) on CFB, p =0.005 (Supplemental Figure 1). After subgroup stratification by incident AKI and prevalent CKD, univariable analyses revealed a significant association between CFB and hospital mortality in all subgroups (Figure 2A). After multivariable adjustment, every 1 L increase of CFB at 72 h was independently associated with hospital mortality, with adjusted ORs (95% CI) of 1.06 (1.03 – 1.09), p <0.001, for AKI+/CKD+; 1.09 (1.05 – 1.13), p <0.001, for AKI−/CKD+; 1.05 (1.03 – 1.08), p <0.001, for AKI+/CKD−; and 1.07 (1.02 – 1.11), p =0.002, for AKI−/CKD− (Figure 2A and Table 2). A similar association with hospital mortality was found when CFB was adjusted by ICU admission body weight (FO per 1% increase). The adjusted ORs (95% CI) were: 1.04 (1.01 – 1.06), p =0.005, for AKI+/CKD+; 1.06 (1.03 – 1.10), p <0.001, for AKI−/CKD+; 1.04 (1.02 – 1.06), p <0.001, for AKI+/CKD−; 1.05 (1.02 – 1.09), p =0.003, for AKI−/CKD− (Figure 2B).
Figure 2.
Forest plots of unadjusted and adjusted odds ratios for hospital mortality in the primary cohort (n =2632). A) CFB per 1 L increase at 72 h of ICU admission. Adjusted odds ratio (95% CI) for hospital mortality in the entire cohort 1.06 (1.04 – 1.08); B) FO per 1% increase at 72 h of ICU admission. Adjusted odds ratio (95% CI) for hospital mortality in the entire cohort 1.04 (1.03 – 1.06). AKI =occurrence of acute kidney injury; CFB =cumulative fluid balance; CKD =pre-existing chronic kidney disease; FO =fluid overload percentage
Table 2.
Multivariable analysis of hospital mortality as the dependent variable and CFB as the study independent variable in all AKI/CKD subgroups
| Clinical variables | AKI+/CKD+ aOR (95%CI) | p | AKI−/CKD+ aOR (95%CI) | p | AKI+/CKD− aOR (95%CI) | p | AKI−/CKD−aOR (95%CI) | p |
|---|---|---|---|---|---|---|---|---|
| CFB, per 1 L increase | 1.06 (1.03 – 1.09) | <0.001 | 1.09 (1.05 – 1.13) | <0.001 | 1.05 (1.03 – 1.08) | <0.001 | 1.07 (1.02 – 1.11) | 0.002 |
| Age, per 10-year increase | 1.05 (0.91 – 1.22) | 0.52 | 1.40 (1.16 – 1.68) | <0.001 | 1.08 (0.96 – 1.21) | 0.21 | 1.12 (0.96 – 1.30) | 0.15 |
| SOFA, per 1-unit score | 1.07 (1.01 – 1.13) | 0.02 | 1.08 (1.00 – 1.17) | 0.04 | 1.05 (1.00 – 1.11) | 0.05 | 1.08 (1.00 – 1.17) | 0.07 |
| LOS, <12 vs ≥12 days | 0.70 (0.45 – 1.07) | 0.10 | 0.74 (0.44 – 1.24) | 0.26 | 0.57 (0.39 – 0.83) | 0.003 | 1.02 (0.63 – 1.66) | 0.94 |
| Mechanical ventilation, yes vs no | 1.65 (1.02 – 2.65) | 0.04 | 2.81 (1.58 – 4.99) | <0.001 | 2.78 (1.82 – 4.24) | <0.001 | 1.57 (0.90 – 2.73) | 0.11 |
| Oliguria, yes vs no | 1.85 (1.11 – 3.08) | 0.02 | -- | -- | 2.31 (1.42 – 3.75) | <0.001 | -- | -- |
Candidate variables for the multivariable models included demographic data (age, gender, and race); comorbidities (diabetes, hypertension, heart failure, and anemia); indicators of critical illness (SOFA and APACHE-II scores, oliguria, mechanical ventilation, red blood cell transfusion, and length of hospital stay); and drug exposure (vasoactive drug and diuretic). Inclusion into the final model (depicted in the above Table) was based upon significance of univariable results (p <0.10) and clinical relevance. APACHE-II was not included in the multivariable model because of collinearity with the SOFA score. No collinearity between CFB and oliguria was detected in all subgroups (VIF =1.0). Model C-statistic (95% CI): 0.71 (0.65 – 0.76) for AKI+/CKD+, 0.75 (0.69 – 0.81) for AKI−/CKD+, 0.74 (0.69 – 0.78) for AKI+/CKD−, and 0.67 (0.61 – 0.74) for AKI−/CKD−. aOR =adjusted odds ratio; CFB =cumulative fluid balance; CI =confidence interval; LOS =length of hospital stay, oliguria =urine output less than 500 mL in 24 h
CFB Cut-offs
For each of the 4 AKI/CKD subgroups, different CFB cut-offs with the best prognostic accuracy for hospital mortality were identified: 5.9 L for AKI+/CKD+; 3.8 L for AKI−/CKD+; 4.3 L for AKI+/CKD−; and 1.5 L for AKI−/CKD−. The CFB cut-off was lowest if both AKI and CKD were absent (Supplemental Table 1). A stronger association with hospital mortality was found when CFB was tested as a dichotomized variable (≥ vs < cut-off value). The adjusted ORs (95% CI) were: 2.65 (1.70 – 4.12), p <0.001, for AKI+/CKD+; 2.34 (1.41 – 3.89), p =0.001, for AKI−/CKD+; 2.37 (1.60 – 3.50), p <0.001, for AKI+/CKD−; 2.61 (1.53 – 4.45), p <0.001, for AKI−/CKD− (Supplemental Figure 2).
Utility of CFB and SOFA Score for the Prediction of Hospital Mortality
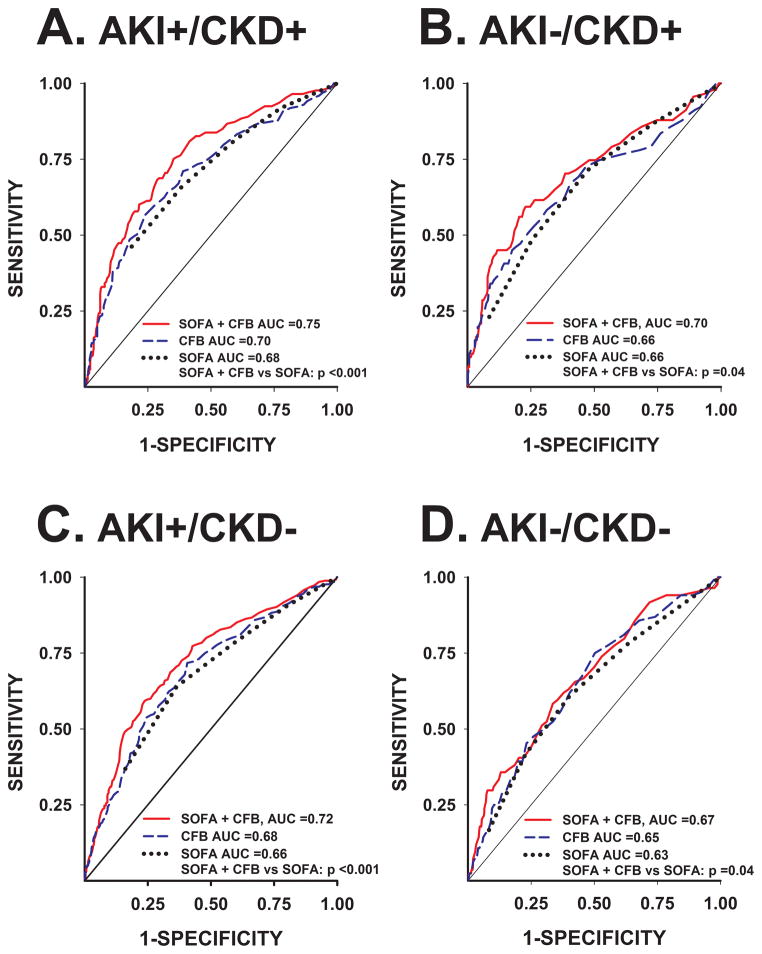
CFB at 72 h was combined with the admission SOFA score in ROC plots for the prediction of hospital mortality in each of the 4 AKI/CKD subgroups (Figure 3). In all subgroups, the model (SOFA + CFB) significantly improved the predictive value for hospital mortality when compared to SOFA alone. This observation was more pronounced in those patients who suffered from AKI regardless of whether CKD was present or absent. The model (SOFA + CFB) significantly improved the risk reclassification of hospital mortality over admission SOFA score alone, as evident by NRI and IDI metrics (Table 3).
Figure 3.
Receiver-Operating Characteristic plots representing the area under the curve (AUC) for the prediction of hospital mortality by the model of admission SOFA score + CFB at 72 h (red), CFB at 72 h (blue), and SOFA score (black). Comparison P-values of SOFA + CFB vs SOFA alone for each AKI/CKD subgroup were calculated. AKI =occurrence of acute kidney injury; CFB =cumulative fluid balance; CKD =pre-existing chronic kidney disease; SOFA =Sequential Organ Failure Assessment score
Table 3.
Improvement in the discrimination of admission SOFA score for the prediction of hospital mortality by combining SOFA score + CFB at 72 h in all AKI/CKD subgroups
| Model performance metric | AKI+/CKD+ (n =679) | AKI−/CKD+ (n =532) | AKI+/CKD− (n =846) | AKI−/CKD− (n =575) | ||||
|---|---|---|---|---|---|---|---|---|
| ROC AUC (95% CI) | ||||||||
| CFB | 0.70 | (0.65, 0.75) | 0.66 | (0.60, 0.73) | 0.68 | (0.64, 0.72) | 0.65 | (0.58, 0.71) |
| SOFA | 0.69 | (0.64, 0.73) | 0.66 | (0.59, 0.72) | 0.66 | (0.62, 0.70) | 0.63 | (0.56, 0.70) |
| SOFA + CFB | 0.74 | (0.70, 0.79) | 0.71 | (0.64, 0.77) | 0.72 | (0.68, 0.75) | 0.67 | (0.60, 0.73) |
|
| ||||||||
|
P-value# SOFA vs SOFA + CFB |
<0.001 | 0.04 | <0.001 | 0.08 | ||||
|
| ||||||||
| Continuous NRIa (95% CI) | 0.53 | (0.37, 0.70) | 0.38 | (0.16, 0.60) | 0.46 | (0.32, 0.61) | 0.35 | (0.13, 0.58) |
| NRI events correctly reclassified | 0.21 | (0.15, 0.27) | 0.01 | (0.002, 0.06) | 0.14 | (0.10, 0.19) | 0.07 | (0.03, 0.15) |
| NRI non-events correctly reclassified | 0.32 | (0.28, 0.36) | 0.37 | (0.33, 0.42) | 0.33 | (0.29, 0.37) | 0.28 | (0.24, 0.32) |
|
| ||||||||
| Absolute IDIb (95% CI) | 0.052 | (0.032, 0.072) | 0.058 | (0.028, 0.087) | 0.053 | (0.036, 0.070) | 0.023 | (0.008, 0.037) |
| IDI events | 0.039 | (0.019, 0.079) | 0.047 | (0.019, 0.113) | 0.037 | (0.020, 0.068) | 0.019 | (0.005, 0.076) |
| IDI non-events | −0.013 | (−0.028, −0.006) | −0.010 | (−0.024, −0.004) | −0.016 | (−0.03, −0.009) | −0.003 | (−0.014, −0.001) |
NRI evaluates the incremental effect of adding CFB to SOFA score for the prediction of hospital mortality and quantifies the net number of individuals reclassified correctly using the model of SOFA + CFB compared to SOFA alone with upward reclassification considered beneficial.
IDI measures the increment in the predicted probabilities for the hospital mortality subset and the decrement for the subset without hospital mortality between the model of SOFA + CFB vs SOFA alone.
P-value compares model ROC AUC for SOFA + CFB vs SOFA alone.
AKI =occurrence of acute kidney injury; AUC =area under the curve; CFB =cumulative fluid balance (total fluid input minus output within the first 72 h of ICU admission); CI =confidence interval; CKD =pre-existing CKD; IDI =absolute integrated discrimination improvement; NRI =net reclassification index; ROC =receiver-operating characteristic; SOFA =Sequential Organ Failure Assessment score. NRI and IDI are presented as proportions.
Sensitivity Analyses
After multiple imputation of missing baseline SCr values, a secondary cohort of 5688 patients was generated (2632 with known baseline SCr + 3056 with imputed baseline SCr). In this secondary cohort, results were essentially the same: CFB was also independently associated with hospital mortality in all patients, adjusted OR per 1 L increase (95% CI) 1.07 (1.06 – 1.08), p <0.001. After subgroup stratification by incident AKI and prevalent CKD, CFB at 72 h was also independently associated with hospital mortality, adjusted ORs (95% CI) of 1.07 (1.05 – 1.09), p <0.001, for AKI+/CKD+; 1.07 (1.04 – 1.09), p <0.001, for AKI−/CKD+; 1.05 (1.04 – 1.07), p <0.001, for AKI+/CKD−; and 1.07 (1.03 – 1.10), p <0.001, for AKI−/CKD− (Supplemental Figure 3A). Furthermore, this independent association persisted after adjustment by ICU admission body weight or FO (Supplemental Figure 3B).
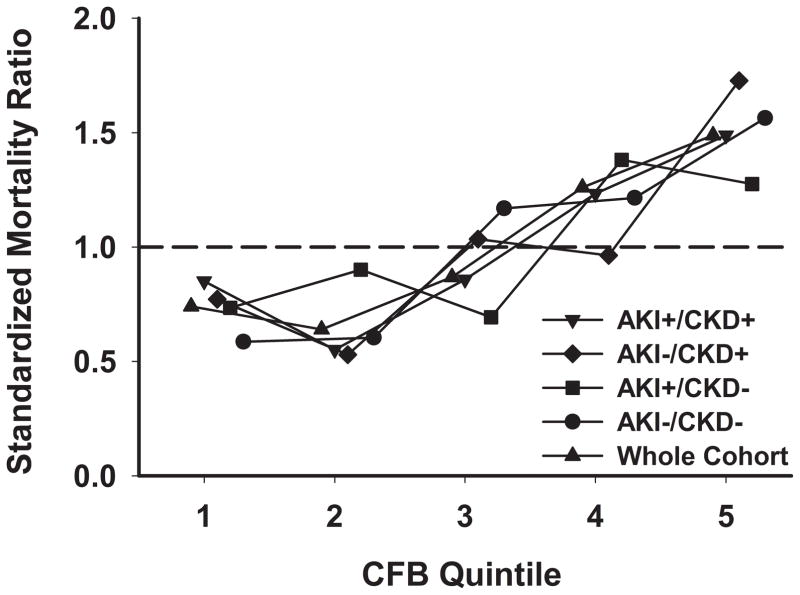
The propensity-regression adjusted odds ratio for hospital mortality in the primary cohort was 1.09 (95% CI, 1.07 – 1.11, p <0.001) for every 1 L increase in CFB at 72 h. In addition, there was a stepwise increase in SMR across CFB quintiles, evident in the entire cohort and in each AKI/CKD subgroup (Figure 4).
Figure 4.
Association between cumulative fluid balance relative to baseline and risk-adjusted hospital mortality. AKI =occurrence of acute kidney injury; CFB =cumulative fluid balance expressed as quintiles; CKD =pre-existing chronic kidney disease; SMR =Standardized Mortality Ratio. SMR for each AKI/CKD subgroup by CFB quintiles was calculated as follows: SMR =observed/predicted mortality; where predicted mortality was determined by the multivariable logistic regression estimate for each AKI/CKD subgroup
Discussion
The principle new finding in our study is that the association of higher CFB with hospital mortality is evident in all critically ill septic patients, regardless of the occurrence of AKI and/or pre-existing CKD. However, we found a significant interaction between AKI/CKD categories and CFB such that for the first time, to our knowledge, we characterized different CFB cut-offs associated with hospital mortality based on whether AKI and/or CKD were present. Finally, we showed that combining CFB at 72 h and admission SOFA score improves the predictive value of the universally-accepted SOFA score for hospital mortality.
Fluid therapy in septic shock consists of initial fluid resuscitation followed by conservative fluid management and regulation (29–32). The inflammatory cascade of sepsis is thought to disrupt the endothelial surface, alter the microvascular system, and cause capillary leakage (33–35). Fluid therapy may enhance filling pressures and improve microcirculation in early sepsis but not in late sepsis (15, 36). In this context, detrimental consequences of fluid accumulation in critically ill patients, including mortality, have been previously reported in acute lung injury (17, 37), in sepsis (2, 38), and in patients with AKI with or without requirement for dialysis (20, 21, 39–41).
An observational study of 198 ICUs in 24 European countries revealed that CFB within the first 72 h of sepsis onset was directly associated with higher mortality, with an OR per 1 L increase of 1.1 (1.0 – 1.1), p =0.001 (2). A secondary analysis of this study later reported that CFB was associated with increased mortality specifically in the subgroup of patients with AKI (29). One limitation of this study was that AKI was defined as a SCr of >3.5 mg/dL (310 μmol/L) or urine output of <500 mL/day, and baseline SCr was not taken into consideration for AKI definition. Our investigation extends these findings by using a more contemporary and accepted AKI definition taking the baseline SCr into account. Later, Bouchard et al (20) reported that fluid overload defined as >10% increase in body weight was associated with 60-day mortality in critically ill patients with AKI, with or without requirement for dialysis. Although this analysis did consider the baseline SCr in the definition of AKI, only patients for whom a nephrology consultation for AKI was obtained were included which could have led to selection bias. In addition, a non-AKI control group was not included for comparison, and the influence of pre-existing CKD was not examined. Furthermore, fluid overload was defined arbitrarily as the accumulation of fluid from 3 days prior to nephrology consultation until hospital discharge, which may not represent a uniform CFB estimate in patients who develop AKI later in the course of ICU stay. In contrast, we used a widely accepted definition for CFB as net fluid accumulated over the first 72 h of ICU stay. This strategy has been previously tested (2) and provides clinically useful information to more uniformly risk-stratify critically ill septic patients using CFB as an additional clinical parameter.
More recently, Texeira et al (41) confirmed the association of higher fluid balance with mortality in ICU patients with AKI and demonstrated higher CFB in non-survivors vs. survivors in the first 7 days of ICU stay. However, this study included only 132 participants with AKI, and the adjudication of AKI occurrence for the primary analysis was based on SCr ≥3.5 mg/dL (310 μmol/L) or urine output <500 mL/day, without the use of baseline SCr to assess absolute or relative changes in SCr. Moreover, recent studies have shown that higher fluid overload at the time of acute dialysis initiation for AKI was associated with 90-day mortality (39) and worse renal recovery at 1 year (40).
Another important finding in our study was that patients without pre-existing CKD that did not develop AKI had the lowest CFB and FO cut-offs associated with hospital mortality. A possible explanation for this observation may be that although in patients without kidney disease excess fluid is usually self-regulated and excreted by preserved renal function, this subgroup may be more susceptible to the negative consequences of acute fluid accumulation than those with pre-existing CKD. Patients with CKD, particularly those with edema, may have greater interstitial system adaptation to fluid overload than patients with preserved kidney function (42). The adaptive response and compliance of the interstitial system (43) can tolerate up to 4.5 L of excess total body fluid before edema becomes evident on physical examination (44). Ebah et al (45) demonstrated in patients with CKD stages 3 to 5 and obvious edema that both interstitial volume and pressure were significantly increased in comparison to healthy volunteers. This observation may illustrate chronic fluid overload adaptation (42). An additional observation was that FO cut-offs were all lower than the >10% FO cut-off associated with mortality previously reported in literature (20, 46, 47) (Supplemental Table 1). The heterogeneity of these different cut-offs for adverse hospital outcomes in the context of critical illness, sepsis, and kidney disease may be prognostically important but needs further investigation for validation. The purpose of our study was to characterize this heterogeneity rather than determine specific cut-offs that are readily available for implementation in clinical practice.
Our study has important strengths that need to be delineated. First, we utilized universally-accepted AKI and CKD definitions, taking into consideration the baseline SCr. Second, we adjusted the analyses for appropriate confounders, including objective and comprehensive critical illness indicators. Third, we demonstrated a significant interaction between AKI/CKD categories and CFB and therefore justified our subgroup stratification. Fourth, we characterize CFB cut-offs associated with hospital mortality in each AKI/CKD subgroup. Fifth, we performed rigorous sensitivity analyses: 1) CFB adjustment by ICU admission body weight (FO); 2) imputation method of missing values of baseline SCr to overcome the selection bias inherent to the lack of these data in all participants; 3) propensity-regression analysis; and 4) SMR determination to further examine the association between CFB relative to baseline and risk-adjusted hospital mortality. Sixth, the accuracy of CFB data collection was validated by individual EMR review of 10% of data. Finally, unique to our study is the stratification of participants based on kidney disease status (e.g., the occurrence of AKI and pre-existing CKD), and the use of CFB both as continuous and categorical independent predictors.
Our study also has important limitations. First, we did not have hourly urine output data for all participants and therefore did not use urine output criteria for AKI adjudication. Nonetheless, we included oliguria (urine output <500 mL/day) as a potential confounder in the multivariable models. Second, data pertaining to fluid administration prior to ICU admission were not available for inclusion in the study. However, given that the study subjects are from an institution where standardized goal-directed fluid resuscitation is generally practiced, we can assume similar patterns of pre-ICU fluid therapy for most, if not all participants. Third, the determination of eGFR by using the MDRD equation may have led to over-classification of CKD status in a small number of patients, although this would have affected only <10% of the cohort. Fourth, although we adjusted for confounding by rigorous multivariable regression analyses, residual confounding by unmeasured covariates may not have been completely eliminated. Howbeit, different sensitivity analyses, including propensity-regression analysis, confirmed our results.
Conclusions
Higher CFB at 72 h of ICU admission was independently associated with hospital mortality in adult patients with severe sepsis or septic shock, regardless of AKI or CKD presence. The combination of CFB at 72 h and admission SOFA score improved the predictive value of SOFA score for hospital mortality. Stratification of patients by the occurrence of AKI and pre-existing CKD identified different CFB cut-offs associated with hospital mortality, with the lowest CFB cut-off in those without incident AKI or prevalent CKD. The characterization of different CFB cut-offs underpins the heterogeneity of fluid regulation in critical illness, sepsis, and kidney disease. These differences should be further investigated in future prospective studies in which measurements of interstitial volume and microcirculatory dynamics, in addition to intravascular volume, can be used for guiding fluid therapy in critically ill patients with or without kidney disease.
Supplementary Material
Two-way interaction between incident acute kidney injury and prevalent chronic kidney disease (AKI * CKD) on cumulative fluid balance. AKI =occurrence of acute kidney injury; CFB =cumulative fluid balance; CKD =pre-existing chronic kidney disease. Two-way interaction p <0.1 was considered significant, warranting AKI/CKD subgroup stratification
Forest plots of unadjusted and adjusted odds ratios for hospital mortality in the primary cohort (n =2632). CFB is dichotomized as ≥ vs. < cut-off value of highest predictive accuracy. AKI =occurrence of acute kidney injury; CFB =cumulative fluid balance; CKD =pre-existing chronic kidney disease
Forest plots of unadjusted and adjusted odds ratios for hospital mortality in the secondary cohort (n =5688, 2632 with known baseline SCr + 3056 with imputed baseline SCr). A) CFB per 1 L increase at 72 h of ICU admission. Adjusted odds ratio (95% CI) for hospital mortality in the entire cohort 1.07 (1.06 – 1.08); B) FO per 1% increase at 72 h of ICU admission. Adjusted odds ratio (95% CI) for hospital mortality in the entire cohort 1.05 (1.04 – 1.06). AKI =occurrence of acute kidney injury; CFB =cumulative fluid balance; CKD =pre-existing chronic kidney disease; FO =fluid overload percentage
Cumulative fluid balance and fluid overload percentage cut-offs for the prediction of hospital mortality in primary and secondary study cohorts
Acknowledgments
Source of Funding: Research reported in this publication was supported by the University of Texas Southwestern Medical Center O’Brien Kidney Research Core Center (NIH, P30 DK079328-06), the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH, UL1TR001105), and the Division of Nephrology and Hypertension of Henry Ford Hospital. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health, the University of Texas Southwestern, Henry Ford Hospital, or the Veterans Affairs North Texas Health Care System. JAN is supported by the Ben J. Lipps Research Fellowship Program of American Society of Nephrology Foundation for Kidney Research and the Truelson Fellowship Fund at UT Southwestern Charles and Jane Pak Center of Mineral Metabolism and Clinical Research.
The authors express their gratitude to Roberta Mooney and Wendy Koscierzynski for expert data extraction and validation. We thank Song Zhang, Ph.D. for helpful interactions and critical review of the manuscript.
Footnotes
Conflicts of Interest: The authors declare that they have no relevant financial interests.
Author Contributions
Study concept and design: J.A.N. and S.S.H.; analysis and interpretation of data: J.A.N., X.L., F.C-E., B.A.H. and S.S.H.; drafting of the manuscript: J.A.N. and S.S.H.; critical revision of the manuscript for important intellectual content: J.A.N., R.D.T., J.Y. and S.S.H.; statistical analysis: X.L. and B.A-H. Administrative, technical, and material support: J.A.N., F.C-E., R.D.T., and J.Y. Study supervision: J.A.N.
Copyright form disclosures: Dr. Toto received support for article research from the National Institutes of Health (NIH) and received funding from Boehringer Ingelheim Abbvie Relypsa ZS Pharma, Reata Pharmaceuticals, and Celgene. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. Jama. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 4.Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2(3):431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 5.Bagshaw SM, Lapinsky S, Dial S, et al. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009;35(5):871–881. doi: 10.1007/s00134-008-1367-2. [DOI] [PubMed] [Google Scholar]
- 6.Bagshaw SM, George C, Bellomo R. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12(2):R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccinni P, Cruz DN, Gramaticopolo S, et al. Prospective multicenter study on epidemiology of acute kidney injury in the ICU: a critical care nephrology Italian collaborative effort (NEFROINT) Minerva Anestesiol. 2011;77(11):1072–1083. [PubMed] [Google Scholar]
- 8.Dalrymple LS, Go AS. Epidemiology of acute infections among patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3(5):1487–1493. doi: 10.2215/CJN.01290308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naqvi SB, Collins AJ. Infectious complications in chronic kidney disease. Adv Chronic Kidney Dis. 2006;13(3):199–204. doi: 10.1053/j.ackd.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 10.James MT, Laupland KB, Tonelli M, et al. Risk of bloodstream infection in patients with chronic kidney disease not treated with dialysis. Arch Intern Med. 2008;168(21):2333–2339. doi: 10.1001/archinte.168.21.2333. [DOI] [PubMed] [Google Scholar]
- 11.Leelahavanichkul A, Huang Y, Hu X, et al. Chronic kidney disease worsens sepsis and sepsis-induced acute kidney injury by releasing High Mobility Group Box Protein-1. Kidney Int. 2011;80(11):1198–1211. doi: 10.1038/ki.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maizel J, Deransy R, Dehedin B, et al. Impact of non-dialysis chronic kidney disease on survival in patients with septic shock. BMC Nephrol. 2013;14:77. doi: 10.1186/1471-2369-14-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 15.Rivers EP, Jaehne AK, Eichhorn-Wharry L, et al. Fluid therapy in septic shock. Curr Opin Crit Care. 2010;16(4):297–308. doi: 10.1097/MCC.0b013e32833be8b3. [DOI] [PubMed] [Google Scholar]
- 16.Humphrey H, Hall J, Sznajder I, et al. Improved survival in ARDS patients associated with a reduction in pulmonary capillary wedge pressure. Chest. 1990;97(5):1176–1180. doi: 10.1378/chest.97.5.1176. [DOI] [PubMed] [Google Scholar]
- 17.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 18.Alsous F, Khamiees M, DeGirolamo A, et al. Negative fluid balance predicts survival in patients with septic shock: a retrospective pilot study. Chest. 2000;117(6):1749–1754. doi: 10.1378/chest.117.6.1749. [DOI] [PubMed] [Google Scholar]
- 19.Sadaka F, Juarez M, Naydenov S, et al. Fluid Resuscitation in Septic Shock: The Effect of Increasing Fluid Balance on Mortality. J Intensive Care Med. 2013 doi: 10.1177/0885066613478899. [DOI] [PubMed] [Google Scholar]
- 20.Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76(4):422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 21.Payen D, de Pont AC, Sakr Y, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12(3):R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification Codes for Acute Renal Failure. J Am Soc Nephrol. 2006;17(6):1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 24.Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17(1):204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 26.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 27.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008;27(2):157–172. doi: 10.1002/sim.2929. discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 28.Siew ED, Peterson JF, Eden SK, et al. Use of multiple imputation method to improve estimation of missing baseline serum creatinine in acute kidney injury research. Clin J Am Soc Nephrol. 2013;8(1):10–18. doi: 10.2215/CJN.00200112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shum HP, Lee FM, Chan KC, et al. Interaction between fluid balance and disease severity on patient outcome in the critically ill. J Crit Care. 2011;26(6):613–619. doi: 10.1016/j.jcrc.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Murphy CV, Schramm GE, Doherty JA, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136(1):102–109. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
- 31.A Randomized Trial of Protocol-Based Care for Early Septic Shock. N Engl J Med. 2014 doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 33.Rubio-Gayosso I, Platts SH, Duling BR. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;290(6):H2247–2256. doi: 10.1152/ajpheart.00796.2005. [DOI] [PubMed] [Google Scholar]
- 34.Ince C. The microcirculation is the motor of sepsis. Crit Care. 2005;9(Suppl 4):S13–19. doi: 10.1186/cc3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundy DJ, Trzeciak S. Microcirculatory dysfunction in sepsis. Crit Care Nurs Clin North Am. 2011;23(1):67–77. doi: 10.1016/j.ccell.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Boldt J, Ince C. The impact of fluid therapy on microcirculation and tissue oxygenation in hypovolemic patients: a review. Intensive Care Med. 2010;36(8):1299–1308. doi: 10.1007/s00134-010-1912-7. [DOI] [PubMed] [Google Scholar]
- 37.Flori HR, Church G, Liu KD, et al. Positive fluid balance is associated with higher mortality and prolonged mechanical ventilation in pediatric patients with acute lung injury. Crit Care Res Pract. 2011;2011:854142. doi: 10.1155/2011/854142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyd JH, Forbes J, Nakada TA, et al. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 39.Vaara ST, Korhonen AM, Kaukonen KM, et al. Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: data from the prospective FINNAKI study. Crit Care. 2012;16(5):R197. doi: 10.1186/cc11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heung M, Wolfgram DF, Kommareddi M, et al. Fluid overload at initiation of renal replacement therapy is associated with lack of renal recovery in patients with acute kidney injury. Nephrol Dial Transplant. 2012;27(3):956–961. doi: 10.1093/ndt/gfr470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teixeira C, Garzotto F, Piccinni P, et al. Fluid balance and urine volume are independent predictors of mortality in acute kidney injury. Crit Care. 2013;17(1):R14. doi: 10.1186/cc12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Titze J. Interstitial fluid homeostasis and pressure: news from the black box. Kidney Int. 2013;84(5):869–871. doi: 10.1038/ki.2013.287. [DOI] [PubMed] [Google Scholar]
- 43.Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73(1):1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Diskin CJ, Stokes TJ, Dansby LM, et al. Towards an understanding of oedema. Bmj. 1999;318(7198):1610–1613. doi: 10.1136/bmj.318.7198.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ebah LM, Wiig H, Dawidowska I, et al. Subcutaneous interstitial pressure and volume characteristics in renal impairment associated with edema. Kidney Int. 2013 doi: 10.1038/ki.2013.208. [DOI] [PubMed] [Google Scholar]
- 46.Gillespie RS, Seidel K, Symons JM. Effect of fluid overload and dose of replacement fluid on survival in hemofiltration. Pediatr Nephrol. 2004;19(12):1394–1399. doi: 10.1007/s00467-004-1655-1. [DOI] [PubMed] [Google Scholar]
- 47.Fulop T, Pathak MB, Schmidt DW, et al. Volume-related weight gain and subsequent mortality in acute renal failure patients treated with continuous renal replacement therapy. Asaio J. 2010;56(4):333–337. doi: 10.1097/MAT.0b013e3181de35e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-way interaction between incident acute kidney injury and prevalent chronic kidney disease (AKI * CKD) on cumulative fluid balance. AKI =occurrence of acute kidney injury; CFB =cumulative fluid balance; CKD =pre-existing chronic kidney disease. Two-way interaction p <0.1 was considered significant, warranting AKI/CKD subgroup stratification
Forest plots of unadjusted and adjusted odds ratios for hospital mortality in the primary cohort (n =2632). CFB is dichotomized as ≥ vs. < cut-off value of highest predictive accuracy. AKI =occurrence of acute kidney injury; CFB =cumulative fluid balance; CKD =pre-existing chronic kidney disease
Forest plots of unadjusted and adjusted odds ratios for hospital mortality in the secondary cohort (n =5688, 2632 with known baseline SCr + 3056 with imputed baseline SCr). A) CFB per 1 L increase at 72 h of ICU admission. Adjusted odds ratio (95% CI) for hospital mortality in the entire cohort 1.07 (1.06 – 1.08); B) FO per 1% increase at 72 h of ICU admission. Adjusted odds ratio (95% CI) for hospital mortality in the entire cohort 1.05 (1.04 – 1.06). AKI =occurrence of acute kidney injury; CFB =cumulative fluid balance; CKD =pre-existing chronic kidney disease; FO =fluid overload percentage
Cumulative fluid balance and fluid overload percentage cut-offs for the prediction of hospital mortality in primary and secondary study cohorts