Abstract
Objectives
Cerebrovascular autoregulation can be monitored with a moving linear correlation of blood pressure to cerebral blood flow velocity (mean velocity index, Mx) during cardiopulmonary bypass (CPB). Vascular reactivity can be monitored with a moving linear correlation of blood pressure to cerebral blood volume trended with near-infrared spectroscopy (hemoglobin volume index, HVx). We hypothesized that the lower limits of autoregulation (LLA) and the optimal blood pressure (ABPopt) associated with the most active autoregulation could be determined by HVx in patients undergoing CPB.
Methods
Adult patients (n=109) who underwent CPB for cardiac surgery had monitoring of both autoregulation (Mx) and vascular reactivity (HVx). Individual curves of Mx and HVx were constructed by placing each in 5 mmHg bins. The LLA and ABPopt for each subject were then identified by both methods and compared for agreement by correlation analysis and Bland–Altman.
Results
The average LLA defined by Mx compared to HVx were comparable (66±13 and 66±12 mmHg). Correlation between the LLA defined by Mx and HVx was significant (Pearson r=0.2867; P=0.0068). The average ABPopt with the most robust autoregulation by Mx was comparable to HVx (75±11 and 74±13 mmHg) with significant correlation (Pearson r=0.5915; P≤0.0001).
Discussion
Autoregulation and vascular reactivity monitoring are expected to be distinct, as flow and volume have different phasic relationships to pressure when cerebrovascular autoregulation is active. However, the two metrics have good agreement when identifying the LLA and optimal blood pressure in patients during CPB.
Keywords: Cerebral blood flow autoregulation, Clinical study, Lower limit of autoregulation, Near-infrared spectroscopy, Vascular reactivity monitoring
Introduction
Maintaining blood pressure within the cerebral blood flow (CBF) autoregulation range (termed ‘optimal blood pressure’) is associated with improved outcomes for patients with traumatic brain injury.1,2 Real-time autoregulation monitoring can be accomplished by the continuous correlation between transcranial Doppler (TCD)-measured CBF velocity of the middle cerebral artery and cerebral perfusion pressure (CPP).1,3–10 Alternatively, in the absence of invasive intracranial pressure monitoring to determine CPP, a continuous and comparable correlation of CBF velocity can be made with the mean arterial blood pressure (termed mean velocity index or Mx).11 Individualizing blood pressure targets using Mx to be above a patient’s lower limit of autoregulation (LLA) during cardiopulmonary bypass (CPB) might avoid cerebral hypoperfusion — a vital goal for the growing number of surgical patients with cerebral vascular disease.12,13
Autoregulation of CBF is mediated by changes in the diameter of cerebral resistance vessels. While CBF autoregulation monitoring assesses the relationship between slow fluctuations in CBF and perfusion pressure (or blood pressure), cerebral vascular reactivity can be assessed by quantifying the correlation between changes in cerebral blood volume (CBV) and blood pressure.3,14 The most common method for monitoring dynamic cerebrovascular reactivity is the pressure reactivity index that uses intracranial pressure (ICP) as a surrogate for CBV.3,14 Low-frequency changes in ICP are assumed to indicate CBV changes resulting from the collective increase in the size of arterial resistance vessels. In adults and children with traumatic brain injury, a positive pressure reactivity index indicates a direct correlation between ICP and arterial blood pressure in this low-frequency bandwidth and is associated with both mortality and poor neurological recovery.3,14,15 However, assessing the pressure reactivity index requires invasive ICP monitoring which limits its application in many clinical areas, including CPB.
Alternatively, near-infrared spectroscopy (NIRS) measurements use non-invasive technology to compare different absorption spectra of oxyhemaglobin and deoxyhemaglobin in relation to relative total hemoglobin (rTHb). The rTHb is obtained by measuring light absorption at the isobestic (805 nm) wavelength and is a function of the hematocrit and the blood volume in the tissue. Similar to ICP, changes in cerebral rTHb may serve as a surrogate of CBV as changes in rTHB reflect the collective changes in the size of cerebral resistance vessels. In both experimental and traumatic brain injury studies, continuous correlation between rTHb and arterial blood pressure (termed hemoglobin volume index or HVx) was accurate for detecting LLA and functioned like the pressure reactivity index.16,17
In the present study, our objective was to compare the HVx, a measurement of vascular reactivity with the Mx, a measurement of autoregulation. Measurements of vascular reactivity have good correlation.16 However, when compared across classes, measurements of autoregulation do not have good correlation with measurements of vascular reactivity. In part, this results from differences in the phase relationships between changes in CBF (used to measure autoregulation) and changes in CBV (used to measure vascular reactivity) relative to changes in blood pressure.3
Recognizing these differences, we hypothesized that the HVx method could identify the LLA and would have good agreement with Mx-derived LLA within a specific patient. Further, the HVx method could identify the ‘optimal blood pressure’ (or ABPopt), by allowing the demonstration of mean blood pressure with the lowest value of HVx which should also be similar and well correlated with the Mx-derived ABPopt.
Materials and Methods
The study was approved by The Johns Hopkins Medical Institutions Investigational Review Board. Written informed consent was obtained from all subjects. Patients ≥45 years of age undergoing coronary artery bypass graft surgery and/or valvular surgery requiring CPB were enrolled from 8 December 2008 to 4 October 2010. Those included in the current study were not enrolled in our prior proof of concept study18 but did overlap with a prior investigation of the cerebral effects of patient rewarming.4 In this prior outcome-based study, those patients did not have NIRS-based cerebrovascular reactivity measurements as part of the analysis.
Intraoperative care
Perioperative patient care has been described and included direct radial artery blood pressure monitoring, nasal temperature monitoring, and anesthesia with midazolam, fentanyl, and isoflurane, with pancuronium for skeletal muscle relaxation.4,18 Non-pulsatile CPB flow between 2.0 and 2.4 l/minute/m2 was used with a membrane oxygenator and a 27-μm arterial line filter. Alpha-stat pH management was used. Isoflurane concentrations during CPB were kept between 0.5 and 1.0% on a vaporizer connected to the oxygenator inflow. Hemoglobin level and arterial blood gases were measured after tracheal intubation, 10 min after initiation of CPB, and then hourly. Gas flow to the oxygenator during CPB was manipulated to maintain normocarbia based on arterial PaCO2 results or continuous in-line arterial blood gas monitoring. During CPB, blood pressure targets, transfusion of packed red blood cells, and the rate of rewarming were based on standard clinical practice.
Monitoring autoregulation and vascular reactivity
The Mx and HVx were monitored and recorded using methods previously described.16,19 Reflectance cerebral cortical NIRS-based monitoring was performed bilaterally using the INVOS (Covidien, Inc., Boulder, CO, USA) monitor. Right and left middle cerebral artery flow velocity was measured with TCD monitoring (Doppler Box; DWL, Compumedics, Charlotte, NC, USA) using two 2.5-MHz transducers fitted on a headband. The depth of insonation was varied until representative spectral artery flow was obtained typically between 35 and 55 mm. Arterial blood pressure (ABP) (GE Marquette, Piscataway, NJ, USA) and middle cerebral artery blood flow velocity (CBFV) were recorded from analog outputs using analog to digital conversion (DT9804; Data Translation, Marlboro, MA, USA) sampled at 100 Hz to a bedside computer with ICM+ software (Cambridge Enterprise, Cambridge, UK). Cerebral blood volume was derived from reflectance cortical NIRS. Cerebral blood volume or relative total hemoglobin (rTHb) is inversely proportional to the transmittance of light with a wavelength of 805–810 nm, an optical density that is reported in the RS232 data stream from the INVOS monitor. This was sampled at the monitor refresh rate of 0.25 Hz. All three waveforms, ABP, CBFV, and rTHb, were low pass filtered by 10-second averaging to remove pulse and respiratory frequency waveforms. Slow oscillations (slower than 0.05 Hz) of ABP, CBFV, and rTHb were retained in the resampling of these filtered waves at 0.1 Hz for subsequent correlation analysis. The signals were further filtered to remove noise from arterial line blood sampling, and noise in the analog signals from cautery, by deleting ABP changes greater than 15 mmHg in 300 seconds and Doppler signals that reached the bounds of analog output ranges. A linear (Pearson’s) coefficient of correlation performed between 30 paired samples of ABP and CBFV yields a single Mx. A similar correlation coefficient between 30 paired samples of ABP and rTHb yields a single HVx. The Mx and HVx are refreshed every 10 seconds during monitoring using the precedent 300-second epoch of recordings. An Mx value that approaches zero indicates autoregulated CBF (i.e. CBF and ABP are independent or not correlated); an Mx value approaching +1 indicates dysregulated CBF (i.e. CBF and ABP correlated). Similarly, increasingly positive values for HVx indicate pressure-passive vascular tone, and increasingly negative values of HVx indicate pressure-reactive vascular tone. Right and left TCD recordings and NIRS data were combined for analysis unless only unilateral recordings were available (see Fig. 1).
Figure 1.
Representative graphic of cerebral blood flow (CBF) autoregulation and vascular reactivity monitoring during cardiopulmonary bypass (CPB) in a single patient. (A) The top panel shows mean arterial blood pressure (ABP), the middle panel shows the middle cerebral artery flow velocity (MCA-FV), and the lower panel shows the near-infrared spectroscopy (NIRS)-based measurements of frontal lobe relative total hemoglobin (rTHb), all synchronously measured on the same time scale. (B) Values of mean velocity index (Mx) and (C) values of hemoglobin volume index (HVx) obtained from the time window in A are sorted according to mean ABP, and show a similar putative lower limit of autoregulation at 90 mmHg, where Mx and HVx values remain increased.
Sample size determination
Identification of LLA during monitoring occurs when ABP is low enough for a sufficient period of time (>5 minutes) to result in autoregulatory impairment (indicated by an increasingly positive index value). Based on our prior human studies, the ability to determine the LLA was possible in 62% of adult patients by CBF-related autoregulation methods.20 For adult patients with brain injury using vascular reactivity methods, the ability to detect the LLA or optimal blood pressure ranged from 50 to 60%.17 Given the ability to identify an LLA identification by autoregulation monitoring with both Mx and HVx with a prevalence of 55%, the sample size of 100 patients was needed to observe with 95% confidence at least 50 patients’ autoregulation curves demonstrating an LLA by both monitoring methods.
Determining the LLA and ABPopt by Mx
Autoregulation values by Mx range from 0 to 1. While increasingly positive values suggest worsening CBF autoregulation, the exact threshold value of intact to inactive autoregulation is unknown. While patients with invasive intracranial monitoring can improve the accuracy of the Mx for detecting impaired autoregulation, our current method utilizing ABP remains a good surrogate.11 When determining the impaired autoregulation, threshold of an Mx>0.4 was used in our previous description of Mx monitoring during bypass18 and Mx values above this threshold have been associated with impaired autoregulation and stroke. An individual autoregulation curve was constructed for each subject using methods previously described with Mx recordings.17,20 Following this method, individual autoregulation curves were constructed by: (1) all values of Mx were binned into 5 mmHg ABP values; (2) binned values of Mx were averaged to give a single Mx for each 5 mmHg decrement of ABP, this creates an individual autoregulation curve for the entire operative procedure of the patient. The LLA from Mx was defined as the ABP demarcating this curve such that not more than one in four bins above the Mx autoregulation impairment threshold of 0.4 had an average Mx greater than 0.4 and not more than one in four bins below the demarcation had an average Mx less than 0.4 (see Fig. 2). Each Mx curve was also evaluated for the optimal blood pressure or ABPopt. ABPopt was defined as the blood pressure bin with the lowest mean Mx value (see Fig. 2).
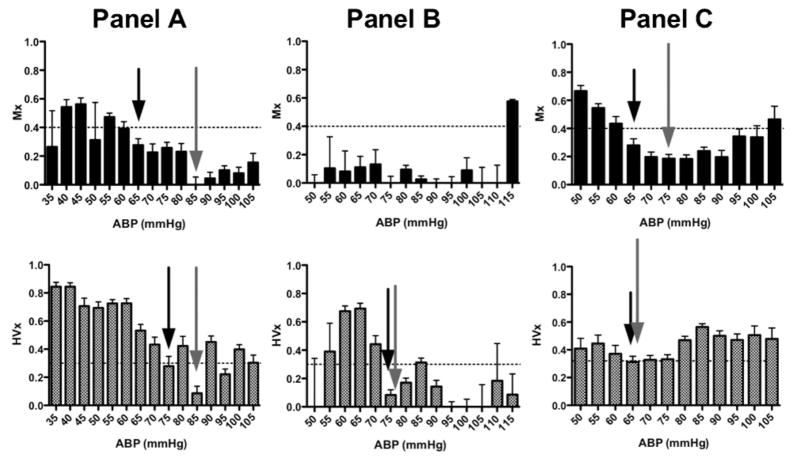
Figure 2.
Examples of individual autoregulation curves for three different patients (one in each panel). In each graph, binned averages of either the mean velocity index (Mx) or hemoglobin volume index (HVx) are plotted across mean arterial blood pressure (ABP) with standard error. The horizontal line at 0.4 for the Mx and 0.3 for the HVx indicates the suspected threshold for loss of autoregulation. Subjects were randomly selected to illustrate the identification of lower limits of autoregulation (LLA) and optimal blood pressure (ABPopt) by both indices. LLA (black arrow) and ABPopt (gray arrow) are indicated on each curve where applicable. Panel (A) demonstrates an individual’s Mx and HVx curves that meet criteria for determining both an LLA and ABPopt. Based on the Mx curve, the subject maintained autoregulation to an ABP of 65 mmHg, but then lost autoregulatory function with a further drop in ABP to 60 mmHg. The lowest Mx value was at 85 mmHg. The HVx curve also met criteria for LLA and ABPopt determination. However, the lowest ABP to maintain autoregulation by HVx was 75 mmHg. The difference between the LLA by the two methods was 10 mmHg and the difference between ABPopt was 0 mmHg. Panel (B) This patient’s Mx curve fails to meet criteria for demonstrating either an LLA or ABPopt since all measurements demonstrate intact autoregulation. However, the HVx curve for this same subject demonstrates both LLA and ABPopt at 75 mmHg. Panel (C) The Mx curve demonstrates an LLA of 65 mmHg and ABPopt at 75 mmHg, while the HVx curve demonstrates both LLA and ABPopt at 65 mmHg.
Determining the LLA and ABPopt by HVx
Vascular reactivity monitoring using HVx results in values ranging from −1 to 1. The exact threshold of impaired HVx monitoring is unknown, but progressively more positive values suggest impaired vascular responsiveness and autoregulatory impairment. The sensitivity and specificity of an HVx threshold of >0.3 to indicate impaired autoregulation and to detect the LLA in a non-bypass piglet model were 77 and 84%, respectively.16 In addition, an HVx >0.3 was used in the adult head trauma patients with invasive and non-invasive vascular reactivity monitoring demonstrating good correlation.17
Individual curves of vascular reactivity as a function of ABP were constructed for each subject using our previously described method, which was adapted from the work of Steiner et al.1 As was done with the Mx recordings, values of HVx were binned and averaged across 5 mmHg decrements of ABP for each individual subject. The LLA from HVx was defined as the ABP demarcating this curve such that not more than one in four bins below the demarcation had an average HVx greater than 0.3 and not more than one in four bins below the demarcation had an average HVx less than 0.3. The ABPopt was defined as the blood pressure bin with the lowest mean HVx measurement as described by Zwiefel et al.17
Data analysis
Patient demographic and physiological variables are reported as mean and range values. The LLA determined by HVx was compared against the LLA determined by Mx using linear correlation (Pearson) and Bland–Altman analysis. In addition, the ABPopt by HVx was compared against the ABPopt by Mx using the same methods. A composite pressure autoregulation curve was constructed by summarizing each subject’s Mx–ABP and HVx–ABP histograms into a mean of means for the entire cohort with equal weight assigned to individual subjects (see Fig. 3). To account for within-subject correlation and inconsistently repeated measures (not every ABP was experienced by every subject), the effects of ABP on Mx and HVx were determined independently by a multiple linear regression model with generalized estimating equations and robust variance estimation.21 Mx and HVx are reported for the cohort for the entire CPB period and by stage of CPB: pre, cooling, rewarming, and post. Comparisons were made between measurements during each stage by ANOVA for repeated measures and Dunnett multiple comparison test. We reported statistical significance levels at P<0.05. All statistical analysis was performed using STATA (Version 8; Stata Corp, College Station, TX, USA) and Prism 5 (GraphPad Software Inc., La Jolla, CA, USA).
Figure 3.
Distribution of cerebral vascular autoregulation during cardiopulmonary bypass (CPB) for the study cohort. (A) Mean velocity index (Mx) (mean±SD) and simultaneous (B) hemoglobin volume index (HVx) (mean±SD) are binned to the corresponding arterial blood pressure (ABP) at the time of their measurement. The horizontal lines at 0.4 for Mx and 0.3 for HVx indicate the suspected threshold for loss of autoregulation. Both Mx and HVx demonstrated increases (impaired autoregulation) with decreasing ABP.
Results
Clinical characteristics of the 109 enrolled patients are listed in Table 1. Mean age of the patients was 65±11 years (median: 64 years; range: 45–89 years). The majority of patients were males (73.4%) and the principle procedure was coronary artery bypass grafting (67%). All patients in the study survived to discharge from the hospital with six new strokes clinically diagnosed in the post-operative period.
Table 1.
Demographic, medical, and physiological data
| Variable | N=109 |
|---|---|
| Age (mean±SD, range) | 65±11 (45–89) |
| Male/female | 80 (73.4%)/29 (26.6%) |
| Prior stroke | 4 (3.7%) |
| Hypertension | 77 (70.6%) |
| Diabetes | 38 (35.2%) |
| Prior myocardial infarction | 37 (33.9%) |
| Congestive heart failure | 11(10.2%) |
| Peripheral vascular disease | 11 (10.2%) |
| Chronic obstructive lung disease | 10 (9.2%) |
| Current smoker | 17 (15.6%) |
| Left ventricular ejection fraction <30% | 14 (12.8%) |
| Preoperative medication | |
| Beta-blockers | 60 (55.0%) |
| Statins | 69 (63.3%) |
| Aspirin | 67 (61.5%) |
| ACE inhibitors | 34 (31.2%) |
| Ca2+ channel blockers | 18 (16.5%) |
| Surgical procedure | |
| CABG only | 73 (67.0%) |
| CABG with aortic valve replacement | 4 (3.7%) |
| CABG with mitral valve replacement/repair | 4 (3.7%) |
| Aortic valve replacement | 20 (18.3%) |
| Mitral valve replacement | 3 (2.7%) |
| Valve sparing aortic root replacment | 5 (4.6%) |
| Duration of CPB (mean±SD, minutes) | 107±44 |
| Duration of aortic cross-clamping (mean±SD, minutes) | 69±26 |
| Intraoperative physiological data | |
| Mean arterial blood pressure (mmHg) | 75±8 |
| pH | 7.4±0.2 |
| PaCO2 (mmHg) | 40±4 |
| PaO2 (mmHg) | 258±49 |
| Hemoglobin (gm/dl) | 9.3±2.3 |
| Glucose (mg/dl, range) | 139±48 (68–231) |
| MCA CBF velocity (cm/second) | |
| Left | 30±12 |
| Right | 32±11 |
| Cerebral saturation (rSO2, %) | |
| Left | 57±10 |
| Right | 56±11 |
| Postoperative stroke | 6 (5.5%) |
| Survival to hospital discharge | 109 (100%) |
Note: Data are listed as the number and percent of patients unless otherwise indicated. CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; ACE, angiotensin converting enzyme; PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen; MCA, middle cerebral artery; CBF, cerebral blood flow.
Clinical laboratory data and monitor measurements are summarized in Table 1. Measured values of mean blood flow velocity and mean cerebral oximetry were similar between the left and right sides of the cranium in our study population. There was no significant difference in these measured values when comparing data from those patients with clinical stroke or other co-morbid condition (i.e. diabetes and hypertension) to the remaining patients (data not shown).
Sixty-eight percent (74/109) of patients had left-and right-sided cranial measurements of TCD and NIRS-based monitoring for all phases of the CPB procedure, but 100% (109/109) had right-sided measurements. Therefore, performance comparison between Mx and HVx was analyzed using simultaneous measurements on the right-brain. Comparison of the two monitoring methods required elimination of signal noise and artifact. Based on abnormalities in the TCD signal, 36% of measurements (or 45±40 minutes per patient) were eliminated primarily from the pre- and post-CPB period of the procedure when electrocautery was most prevalent. Less than 1% of indices measurements were eliminated based on artifact in the ABP signal. This resulted in approximately 480 measurements (or 80±29 minutes per patient) of simultaneous HVx and Mx. No measurements were eliminated secondary to abnormalities in the NIRS-based measurement.
Values for Mx and HVx at different mean ABP values are shown in Fig. 3A and B. Both Mx and HVx increased with decreasing ABP, indicating impairment of autoregulation (P<0.0001). Routine clinical care resulted in a range of mean ABP during CPB from 30 to 105 mmHg. The autoregulatory behavior in patients undergoing CPB was similar to prior reports.18,20
Based on the analysis criteria of the individual autoregulation curves (see Fig. 2), the LLA by Mx could be determined in 74 patients (67.9%), and the LLA by HVx determined in 90 patients (82.5%). The average LLA defined by Mx was 66±13 mmHg [interquartile range (IQR): 56–75 mmHg], and by HVx was 66±12 mmHg (IQR: 60–75 mmHg). There was significant correlation and agreement between LLA determined by the two methods (Pearson r= 0.2867; P=0.0068; Bland–Altman bias: 0.5966; 95% limits of agreement: −28 to 30; see Fig. 4A). The distribution of the difference between the two LLAs is shown in Fig. 5A.
Figure 4.
Correlation of the (A) lower limit of autoregulation (LLA) and (B) optimal blood pressure (ABPopt) detected by mean velocity index (Mx) and hemoglobin volume index (HVx) monitoring. The dashed lines represent the 95% confidence band of each regression line and the 95% limits of agreement (−28 to 30 on LLA and −19 to 21 on ABPopt) for each bias analysis.
Figure 5.
Distribution of differences between the (A) lower limit of autoregulation (LLA) and (B) optimal arterial blood pressure (ABPopt) as defined by two different methods of autoregulation monitoring.
The ABPopt determined from the individual autoregulation curves, was observed in 95 patients (87.2%) by Mx and 109 patients (100%) by HVx. The average ABPopt signifying robust autoregulation was 75±11 mmHg (IQR: 65–80 mmHg) by Mx and 74±13 mmHg (IQR: 65–85 mmHg) by HVx. The distribution of the difference between the two ABPopts is shown in Fig. 5B. Again, a significant correlation and agreement for ABPopt was found between the two methods (Pearson r=0.5915; P<0.0001; Bland–Altman bias: 1.310; 95% limits of agreement: −19 to 22; see Fig. 4B).
Analysis of autoregulation during the stages of CPB resulted in comparable changes in both the Mx and HVx (see Fig. 6). For the cohort, both Mx and HVx demonstrated increasing index values (suggestive of worsening autoregulation) over the course of the CPB (P value: <0.0001 and 0.0084, respectively). The greatest change from the baseline was during the rewarming phase of CPB. The mean difference from baseline CPB autouregulation by Mx was 0.13 (95% CI: 0.205–0.055) and by HVx was 0.10 (95% CI: 0.177–0.016).
Figure 6.
Mean autoregulation values by phase of cardiopulmonary bypass (CPB). (A) Mean velocity index (Mx, in white) and (B) hemoglobin volume index (HVx, in gray) are summarized using Tukey box whisker plots of each phase of CPB. The highest mean Mx and HVx values were during rewarming when compared to pre-CPB values. The change in Mx and HVx by phase was significant by ANOVA for repeated measures (P≤0.0001 and 0.0084, respectively). Solid circles indicate those values that were outliers. ** indicates those periods that were significantly different from pre-CPB values (P<0.05). Individual outlier values are represented by single black dots.
The patients with clinical significant neurological findings underwent evaluation by a neurologist while in the intensive care unit. The majority (67%) were males. Presenting features were weakness (n=2), depressed mental status (n=3), and fluctuating neurological status (n=2). All stroke patients in this cohort also underwent neurological imaging with either computerized tomographic scanning (n=4) or magnetic resonance imaging (n=2) at the discretion of the clinical care team. All (100%) structural lesions were considered to be thromboembolic neurological injuries and were located primarily in the right brain hemisphere by radiologist’s clinical report. Median time to stroke diagnosis was 3 days (range 1–5 days). All patients had resolution of their neurological symptoms and were discharged to home without reported neurological deficit or planned neurological follow-up. The median Mx in stroke patients was 0.3 (range: 0.1–0.6). The median HVx in stroke patients was 0.2 (range: −0.3–0.8). Both Mx and HVx identified an LLA in five of six stroke patients. The median LLA by Mx was 65 mmHg (range: 55–70 mmHg) and by HVx was 65 mmHg (range: 55–85 mmHg). Both methods identified an ABPopt 100% of patients. The median ABPopt by Mx was 68 mmHg (range: 55–80 mmHg) and by HVx was 75 mmHg (range: 55–90 mmHg). Comparison of autoregulation parameters from the stroke patients to those patients presumed to be without a neurological injury demonstrated no significant differences.
Discussion
Summary of main findings
The main findings of this study are that despite fundamental differences in autoregulatory assessment (CBF versus vascular reactivity), HVx and Mx demonstrate agreement when used to delineate the LLA and ABPopt during CPB. The internal consistency demonstrated by correlating these two metrics is validating of the concept that autoregulation monitoring during CPB can be used to delineate the LLA and optimal blood pressure to support autoregulation and vascular reactivity in clinical practice.
Since both methods of autoregulatory assessment are evaluating aspects of the same autoregulatory phenonmenon, our finding of agreement between the Mx and HVx to identify the LLA and the point of optimal autoregulation (ABPopt) suggests that vascular reactivity and CBF remain closely coupled in this patient cohort undergoing CPB. Our findings emphasize the blood pressure dependence of the autoregulatory mechanism within the brain.
Strengths and limitations of the study
When evaluating autoregulation monitoring modalities, it is always appealing to correlate these changes with outcome. However, the design of this study was to assess the performance of the techniques and not the detection of neurological injury. Thus, this study is not powered to correlate prolonged periods of impaired autoregulation with the incidence of stroke. Further, the retrospective nature of this study fails to identify and delineate those patients with subtle or more transient neurological deficits so become incorrectly included in the group of ‘non-stroke’ patients. The ability to detect stroke events will be the focus of future studies in the application of this monitoring technology to optimize blood pressure management during CPB and will require pre- and post-operative neurological evaluation and neurological imaging of all enrolled patients.
Another limitation is the challenge in obtaining continuous TCD data to construct the autoregulatory index for all phases of the CPB. The frequent use of electrocautery before and after CPB and movement of the patient with resultant need to reposition the Doppler probe creates artificial alterations in the blood flow signal. Even with a bedside Doppler technician, these issues result in noise and variability in the TCD signal that most likely undermines the correlation between the blood pressure and flow velocity. Ultimately, this results in higher Mx values across blood pressure and reduces the ability of the TCD-based method to identify both the LLA (Mx versus HVx: 67.9% versus 82.5%) and ABPopt (Mx versus HVx: 87.2% versus 100%) when compared to the NIRS-based method. There are only limited data comparing CBF autoregulation and vascular reactivity autoregulation monitoring techniques. In animal models with altered intracranial pressure, the LLA by vascular reactivity assessment appears to occur at a higher CPP or ABP than the LLA determined by CBF assessment. However, this difference appears to occur within ±5 mmHg of CPP. In addition, assessments of animal data comparing autoregulatory indices demonstrated by CPP or by ABP have shown comparable results in subjects with naive intracranial pressure.22
Agreement and disagreement with the existing literature
This is the first report of the use of HVx for monitoring cerebral vascular reactivity in patients undergoing CPB. In a laboratory investigation, we found that slow waves of rTHb measured with NIRS were coherent with slow waves of ICP.16 We further found in this piglet model that HVx was significantly related to the pressure reactivity index. In that study, the area under the ROC curve for HVx to detect the LLA was 0.85. In a study of 40 patients with closed head injury, there was significant correlation between the ICP-derived pressure reactivity index and the HVx within individual patient recordings (r=0.49; P<0.0001) and across patients (r=0.56; P=0.0002).17 Our findings extend these results and suggests that HVx monitoring might have potential value for patients undergoing cardiac surgery such as when cerebral oximetry is low (e.g. due to cyanotic heart defects) or for patients with increased ICP (e.g. ischemic stroke) where direct ICP monitoring is not feasible due to anticoagulation.
Some brain injury researchers favor finding an ‘optimal’ ABP for targeting blood pressure management compared with defining an LLA threshold.1 Because LLA identification requires some blood pressure be spent below a subject individual LLA, this potentially exposes the patient to periods of impaired autoregulation and potential cerebral hypoperfusion. Ideally, a patient’s blood pressure is maintained where the autoregulation mechanism is intact and robust. For instance, outcomes of patients with traumatic brain injury have been associated with ABP management that ‘optimizes’ autoregulation.1,5 However, increasing ABP to optimize autoregulation could potentially increase left ventricular afterload and impair cardiac function and visceral perfusion in patients with cardiac disease.
In this study, ABPopt was easily identified in all patients with HVx and was always in a bin of blood pressure greater than the LLA. Similar to patients with traumatic brain injury where a CPPopt is identified and targeted to keep patients above their LLA, our findings suggest that the HVx can be used to find the ABPopt in patients on CPB. Notably, more patients’ autoregulation curves demonstrated an ABPopt than an LLA by both methods. Based on our analysis, this was attributable to patients having a range of blood pressure continually greater than their LLA, thus precluding its identification (see Fig. 2, panel B). Since ABPopt was detectable in 100% of patients on CPB with HVx monitoring, this new method may provide an option for guiding blood pressure management and individualizing blood pressure goals to improve brain perfusion.
In a prior study of CBF autoregulation monitoring on CPB, Joshi et al.23 utilized the Mx to identify the LLA in 232 adult patients, and then performed a multi-variate analysis to indentify potential clinical predictors of the LLA. They found that elevated systolic blood pressure was associated with a higher LLA in patients. However, there was no significant influence on LLA by age, gender, or co-morbid conditions (i.e. diabetes, smoking, hypertension, and prior stroke). While the LLA in those patients with stroke was slightly higher (74±15 mmHg by Mx), there was no difference in their mean LLA compared to the current study (66±12 mmHg versus 66±13 mmHg, respectively). Given the broad range of LLA found in their cohort, they concluded that only direct monitoring by a method of autoregulation could determine an individual’s LLA. Overall, the clinical findings of this study are consistent with our prior studies evaluating clinical predictors of autoregulatory impairment and LLA.24
Other investigators have evaluated the relationship of CBF autoregulation and vascular reactivity monitoring in patients with traumatic brain injury. Budohoski et al.25 in a retrospective study compared Mx and the pressure reactivity index (an index of vascular reactivity) in 345 patients with traumatic brain injuries. They found a correlation between the values (r=0.58; P<0.001). However, in those patients with poor neurological outcome scores, elevated ICP (>30 mmHg), and fatal injuries, there was poor correlation between indices. Similar to our study, they noted limitations in the ability to collect Mx data (approximately 1 hour/day) compared to near continuous (>20 hours/day) of the vascular reactivity index. However, issues of agreement secondary to elevations in ICP and their potential influence on the reactivity index are not necessarily present during CPB nor are they likely to complicate our measurements in the setting of relatively mild neurological injuries compared to a population with traumatic brain injury. Their findings highlight the useful approach to assessing cerebrovascular autoregulation by quantifying different aspects of the phenonemenon (either CBF or vascular reactivity) and the inherent limitations of each. Future studies are warranted to understand the clinical situations where these indices agree and disagree, as they may provide additional insights into the mechanisms of autoregulation.
Implications for future research and clinical practice
In summary, we have presented a novel application and method to continuously monitor NIRS-based autoregulation. Our data suggest that it is possible to estimate the LLA and ABPopt for individual patients during cardiac surgery using this method. The range of blood pressure experienced by the majority of patients during the course of routine CPB resulted in periods of low blood pressure and impaired cerebrovascular autoregulation detectable by the new HVx method. Future studies focused on perioperative blood pressure management strategies in CPB patients, could utilize continuous vascular reactivity monitoring into an algorithm of blood pressure management with a goal of improving neurological outcomes by avoiding or minimizing individual patient exposure to blood pressures that result in impaired vascular reactivity by HVx.
Acknowledgments
This work was funded in part by a grant from the National Institute of Health (R01HL092259) and Grant-in-Aid number 103363 from the Mid-Atlantic Affiliate of the American Heart Association.
References
- 1.Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30(4):733–8. doi: 10.1097/00003246-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, et al. Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. J Neurotrauma. 2007;24(Suppl 1):S59–64. doi: 10.1089/neu.2007.9987. [DOI] [PubMed] [Google Scholar]
- 3.Czosnyka M, Brady K, Reinhard M, Smielewski P, Steiner LA. Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care. 2009;10(3):373–86. doi: 10.1007/s12028-008-9175-7. [DOI] [PubMed] [Google Scholar]
- 4.Joshi B, Brady K, Lee J, Easley B, Panigrahi R, Smielewski P, et al. Impaired autoregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg. 2010;110(2):321–8. doi: 10.1213/ANE.0b013e3181c6fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steiner LA, Coles JP, Johnston AJ, Chatfield DA, Smielewski P, Fryer TD, et al. Assessment of cerebrovascular autoregulation in head-injured patients: a validation study. Stroke. 2003;34(10):2404–9. doi: 10.1161/01.STR.0000089014.59668.04. [DOI] [PubMed] [Google Scholar]
- 6.Lang EW, Mehdorn HM, Dorsch NW, Czosnyka M. Continuous monitoring of cerebrovascular autoregulation: a validation study. J Neurol Neurosurg Psychiatry. 2002;72(5):583–6. doi: 10.1136/jnnp.72.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minhas PS, Smielewski P, Kirkpatrick PJ, Pickard JD, Czosnyka M. Pressure autoregulation and positron emission tomography-derived cerebral blood flow acetazolamide reactivity in patients with carotid artery stenosis. Neurosurgery. 2004;55(1):63–7. doi: 10.1227/01.neu.0000126876.10254.05. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 8.Reinhard M, Roth M, Muller T, Guschlbauer B, Timmer J, Czosnyka M, et al. Effect of carotid endarterectomy or stenting on impairment of dynamic cerebral autoregulation. Stroke. 2004;35(6):1381–7. doi: 10.1161/01.STR.0000127533.46914.31. [DOI] [PubMed] [Google Scholar]
- 9.Reinhard M, Roth M, Guschlbauer B, Harloff A, Timmer J, Czosnyka M, et al. Dynamic cerebral autoregulation in acute ischemic stroke assessed from spontaneous blood pressure fluctuations. Stroke. 2005;36(8):1684–9. doi: 10.1161/01.STR.0000173183.36331.ee. [DOI] [PubMed] [Google Scholar]
- 10.Kasprowicz M, Schmidt E, Kim DJ, Haubrich C, Czosnyka Z, Smielewski P, et al. Evaluation of the cerebrovascular pressure reactivity index using non-invasive finapres arterial blood pressure. Physiol Meas. 2010;31(9):1217–28. doi: 10.1088/0967-3334/31/9/011. [DOI] [PubMed] [Google Scholar]
- 11.Lewis PM, Smielewski P, Pickard JD, Czosnyka M. Dynamic cerebral autoregulation: should intracranial pressure be taken into account? Acta neurochirurgica. 2007;149(6):549–55. doi: 10.1007/s00701-007-1160-y. [DOI] [PubMed] [Google Scholar]
- 12.Moraca R, Lin E, Holmes JHt, Fordyce D, Campbell W, Ditkoff M, et al. Impaired baseline regional cerebral perfusion in patients referred for coronary artery bypass. J Thorac Cardiovasc Surg. 2006;131(3):540–6. doi: 10.1016/j.jtcvs.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 13.Gottesman RF, Sherman PM, Grega MA, Yousem DM, Borowicz LM, Jr, Selnes OA, et al. Watershed strokes after cardiac surgery: diagnosis, etiology, and outcome. Stroke. 2006;37(9):2306–11. doi: 10.1161/01.STR.0000236024.68020.3a. [DOI] [PubMed] [Google Scholar]
- 14.Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41(1):11–7. doi: 10.1097/00006123-199707000-00005. discussion 7–9. [DOI] [PubMed] [Google Scholar]
- 15.Aries MJ, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40(8):2456–63. doi: 10.1097/CCM.0b013e3182514eb6. [DOI] [PubMed] [Google Scholar]
- 16.Lee JK, Kibler KK, Benni PB, Easley RB, Czosnyka M, Smielewski P, et al. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke. 2009;40(5):1820–6. doi: 10.1161/STROKEAHA.108.536094. [DOI] [PubMed] [Google Scholar]
- 17.Zweifel C, Castellani G, Czosnyka M, Helmy A, Manktelow A, Carrera E, et al. Noninvasive monitoring of cerebrovascular reactivity with near infrared spectroscopy in head-injured patients. J Neurotrauma. 2010;27(11):1951–8. doi: 10.1089/neu.2010.1388. [DOI] [PubMed] [Google Scholar]
- 18.Brady K, Joshi B, Zweifel C, Smielewski P, Czosnyka M, Easley RB, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010;41(9):1951–6. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czosnyka M, Smielewski P, Kirkpatrick P, Menon DK, Pickard JD. Monitoring of cerebral autoregulation in head-injured patients. Stroke. 1996;27(10):1829–34. doi: 10.1161/01.str.27.10.1829. [DOI] [PubMed] [Google Scholar]
- 20.Brady KM, Mytar JO, Lee JK, Cameron DE, Vricella LA, Thompson WR, et al. Monitoring cerebral blood flow pressure autoregulation in pediatric patients during cardiac surgery. Stroke. 2010;41(9):1957–62. doi: 10.1161/STROKEAHA.109.575167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30. [PubMed] [Google Scholar]
- 22.Brady KM, Mytar JO, Kibler KK, Hogue CW, Jr, Lee JK, Czosnyka M, et al. Noninvasive autoregulation monitoring with and without intracranial pressure in the naive piglet brain. Anesth Analg. 2010;111(1):191–5. doi: 10.1213/ANE.0b013e3181e054ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi B, Ono M, Brown C, Brady K, Easley RB, Yenokyan G, et al. Predicting the limits of cerebral autoregulation during cardiopulmonary bypass. Anesth Analg. 2012;114(3):503–10. doi: 10.1213/ANE.0b013e31823d292a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ono M, Joshi B, Brady K, Easley RB, Zheng Y, Brown C, et al. Risks for impaired cerebral autoregulation during cardiopulmonary bypass and postoperative stroke. Br J Anaesth. 2012;109(3):391–8. doi: 10.1093/bja/aes148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budohoski KP, Zweifel C, Kasprowicz M, Sorrentino E, Diedler J, Brady KM, et al. What comes first? The dynamics of cerebral oxygenation and blood flow in response to changes in arterial pressure and intracranial pressure after head injury. Br J Anaesth. 2012;108(1):89–99. doi: 10.1093/bja/aer324. [DOI] [PMC free article] [PubMed] [Google Scholar]