Review on lymphatic endothelial progenitors, primarily of myeloid origin, which induce new lymphatic vessels in inflamed tissues and tumors.
Keywords: inflammatory/tumor lymphangiogenesis, endothelial progenitors, lineage development
Abstract
Inflammation triggers an immune cell-driven program committed to restoring homeostasis to injured tissue. Central to this process is vasculature restoration, which includes both blood and lymphatic networks. Generation of new vessels or remodeling of existing vessels are also important steps in metastasis—the major cause of death for cancer patients. Although roles of the lymphatic system in regulation of inflammation and cancer metastasis are firmly established, the mechanisms underlying the formation of new lymphatic vessels remain a subject of debate. Until recently, generation of new lymphatics in adults was thought to occur exclusively through sprouting of existing vessels without help from recruited progenitors. However, emerging findings from clinical and experimental studies show that lymphoendothelial progenitors, particularly those derived from immature myeloid cells, play an important role in this process. This review summarizes current evidence for the existence and significant roles of myeloid-derived lymphatic endothelial cell progenitors (M-LECPs) in generation of new lymphatics. We describe specific markers of M-LECPs and discuss their biologic behavior in culture and in vivo, as well as currently known molecular mechanisms of myeloid-lymphatic transition (MLT). We also discuss the implications of M-LECPs for promoting adaptive immunity, as well as cancer metastasis. We conclude that improved mechanistic understanding of M-LECP differentiation and its role in adult lymphangiogenesis may lead to new therapeutic approaches for correcting lymphatic insufficiency or excessive formation of lymphatic vessels in human disorders.
Introduction
Analogous to blood vessels, the formation of new lymphatic vessels (i.e., lymphangiogenesis) occurs vigorously during embryogenesis but is highly restricted in adults. During adulthood, lymphangiogenesis is limited to sites of chronic inflammation [1], tissue injury or remodeling [2], and cancer [3, 4]. Whereas generation of lymphatic vessels in embryonic tissues involves both de novo vascular formation (i.e., vasculogenesis) and sprouting, postnatal lymphangiogenesis is thought to occur mainly by branching out from pre-existing vessels. This process is initiated by activation of VEGFR-3, expressed on LECs, by its ligands VEGF-C [5] or VEGF-D [6]. VEGFR-3-dependent activation of LECs induces mitotic division, followed by their migration into a matrix-guided shaft and formation of a new sprout from the original “mother” vessel. This canonical concept of lymphatic vessel formation has been updated recently by evidence emerging from both mouse models and human patients for the essential role of LECPs. These cells are currently thought to be mainly derived from myeloid precursors and therefore, designated as M-LECPs. BM-residing immature myeloid cells are known to mobilize to inflamed tissues [7] and tumors [8, 9], where they contribute significantly to vascular formation [10] and in the context of tumors, to metastasis [11]. Current evidence suggests that under inflammatory conditions, some of these cells undergo MLT, reflected by de novo expression of lymphatic-specific markers, acquisition of endothelial-specific functions, and most importantly, the capability to expand the existing lymphatic network (Fig. 1). Although relatively unexplored, differentiation of lymphatic-promoting cells from myeloid precursors is reminiscent of the well-known generation of myeloid-derived blood vascular endothelial progenitors that promote angiogenesis [12, 13]. This review summarizes current evidence for LECP differentiation from myeloid and other progenitor cells, their unique structural and biologic properties, and their impact on inflammatory and tumor lymphangiogenesis.
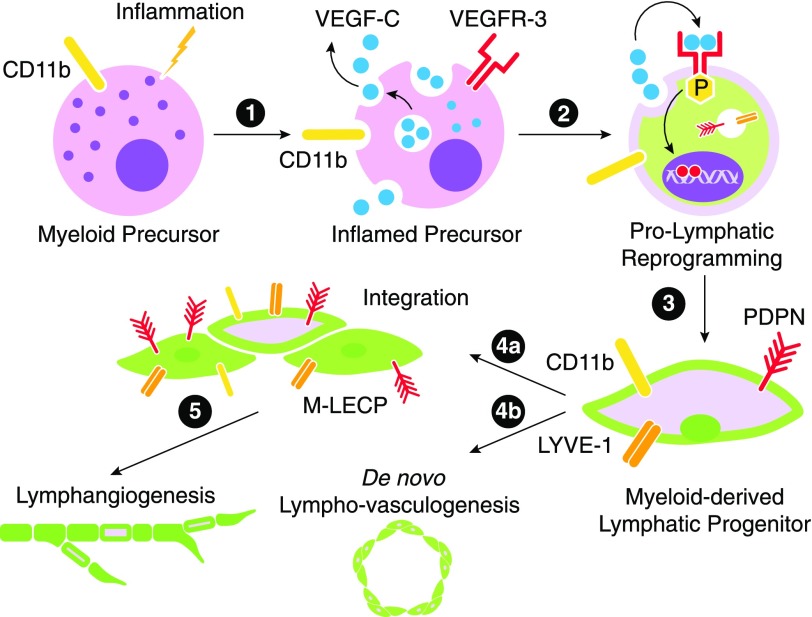
Figure 1. A model for inflammation-induced reprogramming of immature myeloid cells into M-LECP.
Inflammatory stimuli reach the BM, where they activate the NF-κB pathway in HSCs/hematopoietic progenitors and immature myeloid cells. NF-κB activation could be mediated by TLR4 or endothelial receptors if they are already expressed in myeloid cells. (1) Activation of the NF-κB pathway leads to simultaneous up-regulation of VEGFR-3 and its ligand VEGF-C. (2) Coexpression of VEGF-C and VEGFR-3 creates an autocrine loop leading to up-regulation of lymphatic-specific genes. Prolymphatic reprogramming generates M-LECPs, which coexpress monocytic (e.g., CD11b) and lymphatic-specific (e.g., Lyve-1 and PDPN) markers that are normally segregated to distinct cell lineages. (3) Under chronic inflammatory conditions or cancer, BM-generated M-LECPs are mobilized to peripheral blood. (4) Circulating M-LECPs are recruited to inflamed sites and tumors and either integrate into pre-existing lymphatic vessels (4a) or initiate de novo formation of new lymphatic vasculature (i.e., lympho-vasculogenesis; 4b). (5) M-LECP integration might be necessary to induce sprouting of existing vessels that otherwise might be resistant to undergo remodeling.
THE ROLE OF INFLAMMATION-INDUCED LYMPHATICS IN IMMUNITY AND RESTORATION OF HOMEOSTASIS
Inflammation-induced lymphangiogenesis is a hardwired program designed to address drastically altered tissue needs during pathogen invasion, tissue expansion and injury, or other disturbances of homeostasis as a result of molecular imbalances and physical causes. The lymphatic system is primarily responsible for balancing high levels of interstitial fluid and removing massive amounts of immune cells mobilized to inflamed tissues in response to the aforementioned effects. Increasing LVD is critical for alleviating interstitial pressure, reducing inflammation by exporting inflammatory leukocytes, and promoting adaptive immunity by transporting antigen-pulsed immune cells to regional LNs [14, 15].
Not surprisingly, inflamed tissues in human patients and experimental animal models display significantly higher LVD than healthy counterparts [16]. For instance, LVD is increased by at least 2- to 6-fold in humans with psoriasis [17], arthritis [18], and inflammatory bowel disease [19]. Experimentally induced inflammation in mice also leads to a 2- to 10-fold increase in LVD in models of corneal injury [7], wound healing [2, 20, 21], peritonitis [22, 23], and dermatitis [24]. Expansion of the lymphatic network under these circumstances is largely beneficial, as it lessens inflammation, improves immune defense, and restores homeostasis; however, a similar increase in LVD, driven by tumors, has more complicated and less-favorable outcomes.
CONFLICTING ROLES OF LYMPHATICS IN ANTI-TUMOR IMMUNITY AND CANCER PROGRESSION
Anti-tumor role of lymphatics
Expansion of the lymphatic network is frequently observed in inflammatory diseases and cancer leading to increased fluid drainage by at least 10-fold [25]. In humans and mice with intact immune systems, this expansion results in increased antigen delivery to the draining LNs and trafficking of DCs and macrophages that stimulate production of antibodies and cytotoxic T cells against tumor antigens. Besides transporting fluid and immune cells to regional nodes, the lymphatics play a variety of immunomodulatory functions, including antigen presentation, maintenance of peripheral tolerance to self-antigens, production of cytokines, and regulation of DC maturation [26]. All activated LECs produce high levels of the chemokine CCL21 that binds to the CCR7 receptor expressed on antigen-activated DCs and some T cells [27–29]. Mice deficient in CCR7 ligands or receptors demonstrated poor migration of DCs and T cells to LNs, effectively preventing an adaptive immune response [30]. Thus, lymphatic vasculature plays an active role in immunologic surveillance, including defense against tumor cells.
These functions of lymphatics are essential for mounting the anti-tumor immune response upon initial detection of tumor antigens. This is illustrated by several elegant studies performed with transgenic mice and syngeneic tumor models. Mice with defective dermal lymphatics in a K14-VEGFR3-Ig model grew B16 melanoma with substantially reduced cytokines and leukocyte infiltrates compared with wild-type controls [31]. This led to decreased local immunosuppression, resulting in a more efficacious anti-tumor effect of transferred cytotoxic T cells than in controls [31]. Likewise, B16 melanoma and EL-4 lymphoma, grown in mice with severe lymphatic dysfunction (k-cyclin model), had reduced levels of inflammatory cytokines in tumor-draining LNs, resulting in inferior antigen presentation and decreased activity of cytotoxic T cells toward tumor cells in vitro [32]. A syngeneic breast carcinoma grew faster in Chy mice with impaired lymphangiogenesis, as a result of the inactivating VEGFR-3 mutation, than in corresponding controls [33]. Collectively, these studies highlight the importance of functional lymphatics in tumor suppression, particularly at early stages of cancer development.
Prometastatic role of tumor-induced lymphatics
Whereas tumor and inflammatory lymphangiogenesis shares a similar function of restoring homeostasis, tumors add complexity to this task by using newly formed vessels for metastatic spread [34]. Metastasis is a well-recognized cause of mortality from cancer [35], and tumor lymphatics play a major role in initiation of this process [3, 4]. Whereas blood and lymphatic vessels can both support dissemination of cells from the primary tumor, spread to regional LNs typically precedes hematogenous metastasis [36]. The well-documented preference of tumor cells for LN metastasis [3, 4, 37] is thought to be a result of unique properties of lymphatic vessels naturally equipped to preserve viability of immune cells during routine trafficking from tissues to local LNs. The main properties of mouse and human lymphatic vessels that support tumor spread are the following: 1) easy access to the lumen of lymphatic capillaries, which are lined by a single layer of endothelial cells and have a naturally discontinuous basement membrane; 2) low-pressure lymph flow that minimizes shear stress; 3) unidirectional flow from tissues toward LNs, which controls cell destination; and 4) cytoprotective molecular composition of the lymph that promotes cell survival [3]. Consequently, most epithelial cancers spread first to proximate LNs, followed by invasion of nodal blood vessels and entry into systemic circulation. This leads to establishment of life-threatening distant metastases in bones, brain, and visceral organs. As many tumors disseminate sequentially (i.e., first lymphatic followed by hematogenous spread), the entire metastatic cascade might be prevented by intercepting transport of tumor cells via lymphatic vessels or even earlier, by suppressing generation of such vessels. This concept is the major motivation for gaining a greater understanding of the cell types and mechanisms required for formation of tumor lymphatic vessels, as this process is a quintessential prerequisite for tumor spread to distant sites [3, 38, 39] that ultimately leads to death from cancer [4, 40].
M-LECPs IN INFLAMMATORY AND TUMOR LYMPHANGIOGENESIS
Origin of LECP in adults
Although the main origin of mouse embryonic LECs is the cardinal vein [41], the formation of the first lymphatics is also supported by mesenchymal lymphangioblasts [42, 43]. The latter cells have two unusual characteristics: 1) coexpression of myeloid (e.g., CD11b, F4/80) and lymphoendothelial (e.g., Lyve-1) proteins and 2) capacity to integrate into pre-existing lymphatic vessels, which might be necessary for induction of sprouting [42]. Early studies questioned whether similar progenitor cells are necessary for adult lymphangiogenesis [44]; however, emerging evidence supports not only the existence of such cells in adult humans and mice but also their essential role in the formation of new lymphatics.
Adult LECPs of mice and humans have been reported to derive from various precursors, including HSC [45], MSC [46], ADSC [47], and myeloid [21] stem or progenitor cells (Table 1), with the myeloid lineage drawing increasing support as the primary source [7, 48–51]. HSCs were initially suggested as the main source of LECP, based on studies in mice transplanted with a c-Kit+ and Sca-1+ HSC population that also constitutively expressed GFP [45]. Such GFP+ cells were detected in 1–3% of normal lymphatic vessels, up to 20 mo after injection [45], indicating long-term residence of the integrated progenitors within the recipient vessels. When isolated months after injection, GFP+ cells were negative for CD45 and CD11b, leading to the conclusion that myeloid cells are not involved in generation of LECP [45]. However, this was not supported by follow-up studies monitoring native or in vitro-differentiated M-LECP within a few weeks of adoptive transfer [21, 48]. The discrepancy in these findings might be a result of progressive loss of myeloid and hematopoietic markers, as shown during prolymphatic differentiation in vitro [21, 52, 53]. Thus, lack of detectable levels of CD11b in fully differentiated LECPs does not exclude myeloid cells as their precursors.
TABLE 1.
Phenotypes of stem/progenitor populations that may contribute to development of adult LEC lineage
| Population or cell type |
Species | Typical phenotype-defining markers | Reference (example) | |
|---|---|---|---|---|
| Full name | Short name | |||
| Adult lymphatic endothelial cell | LEC | Mouse or human | VEGFR-3, LYVE-1, PDPN, PROX-1 | [95] |
| Hematopoietic stem cell | HSC | Mouse | CD34+, c-Kit+, Sca-1+ | [45] |
| Hematopoietic stem cell | HSC | Human | CD34+, CD133+ | [52] |
| Common myeloid progenitor | CMP | Mouse | Lin−, Sca-1+, IL-7Rα−, c-Kit+ | [48] |
| Mesenchymal stem cell | MSC | Mouse | Sca-1+, CD34−, CD45−, CD11b−, | [96] |
| Mesenchymal stem cell | MSC | Human | CD73+, CD105+, CD90+, CD14−, CD31−, CD34−, CD45− | [97] |
| Adult adipose-derived stem cell | ADSC | Human | CD13+, CD29+, CD44+, CD105+ | [47] |
| CD31−, CD34−, CD45−, HLA-DR− | ||||
| Endothelial progenitor cell | EPC | Human | CD34+, CD133+, VEGFR-2+ | [98, 99] |
| Lymphatic endothelial cell progenitor or lymphatic endothelial precursor cell | LECP or LEPC | Human | CD34+, CD133+, VEGFR-3+ | [57, 76] |
| Myeloid-derived lymphatic endothelial cell progenitor | M-LECP | Human | Combined markers for early progenitors (CD133, CD34), myeloid cells (CD14, CD11b), and adult LECs (VEGFR-3, PDPN, LYVE-1) | [57] |
| Myeloid-derived lymphatic endothelial cell progenitor | M-LECP | Mouse | Combined markers for myeloid cells (CD11b, F4/80) and adult LECs (Vegfr-3, Pdpn, Lyve-1) | [23] |
PROX-1, Prospero homeobox 1.
One of the most supportive pieces of evidence for the myeloid origin of LECPs was obtained in mice reconstituted with BM cells expressing GFP under transcriptional control of the CX3CR1 gene promoter, a specific marker of the myeloid lineage. Under inflammatory conditions, these transplanted cells up-regulated LEC markers and integrated into inflamed lymphatic vessels. Identical properties were detected in a subset of GFP-tagged CMPs, identified as Lin−/Sca-1−/IL-7Rα−/c-Kit+ cells [48]. In contrast, representatives of the lymphoid lineage CD3+ T cells and CD19+ B cells did not integrate or increase LVD [48]. Derivation of M-LECPs was independently confirmed in a study that demonstrated the lymphangiogenic potential of murine CD11b+ cells coexpressing c-Kit and the lymphatic marker Pdpn [21]. FACS sorting of these myeloid cells into Pdpn-positive and -negative populations showed that LEC markers were exclusively associated with the Pdpn+ fraction. This is consistent with the idea that only Pdpn+ myeloid cells are precursors for M-LECPs, whereas other CD11b+ cells perform unrelated functions.
Although the origin of adult LECP is still debatable, it is possible that seemingly heterogeneous sources of these cells in humans and mice (Table 1) are, in fact, interconnected and simply represent different milestones in differentiation of the lymphoendothelial lineage. For instance, it is not surprising that mouse ESCs, HSCs, and BM myeloid cells all give rise to LECPs, because the first two populations harbor precursors of the myeloid lineage. Likewise, MSCs give rise to adipose progenitors that can subsequently contribute to local production of LECPs in fat tissue [47]. Regardless of origin, all described types of lymphatic progenitors share several properties that structurally distinguish them from other progenitors and differentiated cells and are indicative of their main function, i.e., generation of new lymphatics.
Properties that distinguish LECP from precursors of other lineages
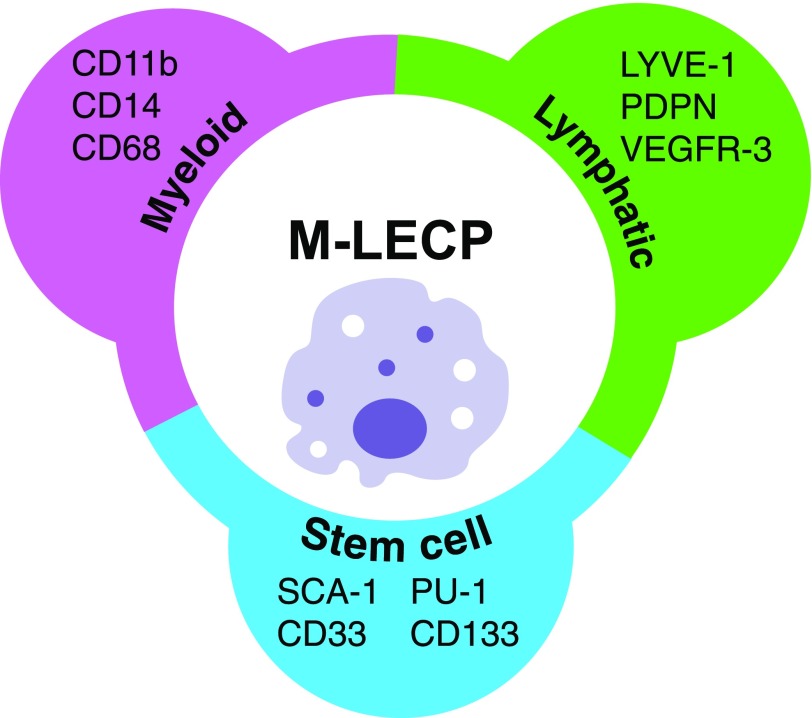
In humans and mice, LECP can be identified by coexpression of three types of markers: 1) indicators of the parental lineage (e.g., myeloid or mesenchymal); 2) markers of stem/progenitor cells indicative of their immature status; and 3) specific markers of the terminal/destination lineage, that is, differentiated LECs. M-LECPs, for instance, can be identified by immunostaining for coexpression of myeloid-specific markers (e.g., CD14, CD11b, and CD68); lymphatic-specific markers (e.g., LYVE-1 or PDPN); and stem/progenitor markers, such as CD133 (Table 1 and Fig. 2). Analogously, MSC-derived LECP express markers of mesenchymal cells and LECs [46] typically segregated to distinct lineages.
Figure 2. Typical markers of M-LECPs.
This subset can be specifically identified by coexpression of myeloid and lymphatic-specific proteins in conjunction with expression of stem/progenitor cell markers indicating lack of maturity.
Coexpression of myeloid and lymphatic markers is particularly well documented in M-LECPs induced by inflammatory conditions in vivo [54–56] or generated from myeloid cells in vitro [23, 49, 57]. Although widely used in humans and mice, VEGFR-3 is a less-precise marker of M-LECP, as in contrast to sustained expression by mature LECs, its expression in lymphatic progenitors is transient [23]. In contrast, LYVE-1 and PDPN are more consistent markers for identifying lymphatic progenitors, as their expression is sustained from the onset of MLT [23] to terminal differentiation [21, 48]. M-LECPs also express general vascular markers, such as neuropilin-2 and stabilin-1 [56, 57]. Mouse-committed lymphatic progenitors express low levels of blood vascular-specific markers, such as CD31 [21] and Tie-2 [7], suggesting a bifurcation of the LEC lineage from the bipotent common endothelial progenitors at an early stage of differentiation. The pan-leukocyte marker CD45 is also expressed by M-LECPs [21], suggesting the existence of a common multipotent precursor to hematopoietic, myeloid, and endothelial lineages during adulthood. This conclusion is based mainly on studies in mice with potential to extrapolate to humans.
Along with myeloid and lymphatic proteins, LECPs express stem/progenitor markers (Table 1 and Fig. 2), indicating their early developmental stage. The most commonly reported HSC markers, CD133 and CD34, were shown to be expressed by M-LECPs, derived from myeloid cells and isolated from human umbilical cord blood [57], human circulating monocytes [49], and mouse BM mononuclear cells [21, 48]. The immature status of mouse LECPs was also indicated by expression of stem cell markers c-Kit and Sca-1 [21, 45], as well as by the absence of costimulatory CD80, CD86, and MHC class II proteins [58, 59].
In summary, definitive structural identification of LECP requires evidence for coexpression of specific markers of myeloid or mesenchymal lineage, markers of adult LECs, and early stem/progenitor proteins, along with the absence of CD80/CD86 or other proteins indicating maturity (Fig. 2).
Functional properties of lymphatic progenitors in endothelial cell assays in vitro
One of the most compelling lines of evidence for existence of M-LECPs is the ability to differentiate such cells from primary human monocytes [52, 57] or mouse myeloid cells [21, 48] under controlled in vitro conditions (Tables 2 and 3). Although differentiation protocols vary among different research groups, the resultant cells demonstrated conserved properties, such as acquisition of the lymphatic phenotype, endothelial-specific morphology, and functional traits restricted to vascular cells [46, 60]. In vitro-produced M-LECPs were able to form tubes in Matrigel [48, 60–62], perform uptake of acetylated low-density lipoproteins [60, 62], and respond to a key lymphangiogenic factor VEGF-C by increasing proliferation and migration [52, 60]. This functional maturation was concomitant with loss or reduced expression of stem, myeloid, or hematopoietic markers [21, 52, 53], providing evidence for MLT, that is, transition from myeloid to lymphatic endothelial lineage.
TABLE 2.
Evidence for differentiation of mouse or rat LECP
| Source of precursors | Comments | Year | Reference |
|---|---|---|---|
| BM | Mouse B16 melanoma conditioned medium induced prolymphatic differentiation in up to 40% of BM myeloid CD11b+ cells. | 2006 | [56] |
| BM | LPS-activated mouse CD11b+ BM myeloid cells acquired the expression of lymphatic markers and functional capacities of endothelial cells. CD11b+ BM cells integrated into tumor lymphatic but not blood vessels. CD3+ T cells and CD19+ B cells did not integrate into either type of vessels. | 2009 | [48] |
| BM | Mouse CD11b+/Pdpn+ BM-derived cells express lymphatic markers and increased lymphatic formation in tissue repair and melanoma models. | 2010 | [21] |
| BM | Colorectal LS174T and breast SK-BR-3 cancers implanted in mice recruited BM monocytes expressing LEC markers. VEGF-A was shown to control differentiation and tumor recruitment of M-LECP. | 2014 | [66] |
| ESC lines | Forced expression of intact VEGFR-3 in mouse ESCs enhanced lymphatic differentiation, whereas mutated VEGFR-3 inhibited it. | 2005 | [80] |
| ESC lines | Soluble decoy VEGFR-3 receptor prevented prolymphatic reprogramming in mouse ESC. | 2006 | [81] |
| Immortalized macrophage cell line | Mouse BM-derived M-LECP recruited to LPS-inflamed tissues integrated into lymphatic vessels before sprouting. A mouse macrophage cell line RAW264.7 treated with LPS recapitulated myeloid-lymphatic reprogramming observed in vivo. | 2012 | [23] |
| MSC | A subset of mouse embryonic mesenchymal cells expressing lymphoendothelial and hematopoietic markers was shown to integrate into growing lymphatic vessels before sprouting. | 2006 | [100] |
| MSC | Rat mesenchymal BM-derived cells, positive for CD34 and CD14, gave rise to LECP in the presence of VEGF-A and VEGF-C factors. | 2012 | [101] |
| MSC | VEGF-C promoted prolymphatic differentiation of adult mouse MSCs and suppressed development of the osteogenic lineage. | 2016 | [102] |
| Adult lungs | Mouse lung-derived bipotent endothelial progenitors gave rise to blood vascular cells and LECs, suggesting a common origin of these lineages. | 2010 | [94] |
TABLE 3.
Evidence for differentiation of human LECP
| Source of precursors | Comments | Year | Reference |
|---|---|---|---|
| Cord blood | Hematopoietic CD34+ cells treated with VEGF-A differentiated into cells expressing LYVE-1 but not PROX-1 or PDPN. | 2009 | [53] |
| Cord blood | CD34+/VEGFR-3+ progenitors stimulated with VEGF-C differentiated into LYVE-1+/PROX-1+ cells that displayed endothelial morphology and performed endothelial functions. | 2014 | [60] |
| Cord blood | CD34+/VEGFR-3+ progenitors stimulated with VEGF-C differentiated into LYVE-1+ cells. The silencing of VEGFR-3 before differentiation prevented acquisition of the lymphatic phenotype. | 2014 | [50] |
| Cord blood | LECPs induced by VEGF-A and VEGF-C express lymphatic markers VEGFR-3, LYVE-1, and PDPN. Injection of these cells into the eyes of the mice induced lymphovasculogenesis. | 2014 | [57] |
| Peripheral blood | CD34+/CD133+/VEGFR-3+ progenitors stimulated with VEGF-A and VEGF-C differentiated into LYVE-1+/VE-cadherin+/PDPN+ cells that displayed endothelial morphology and performed endothelial-specific functions. | 2003 | [52] |
| Peripheral blood | CD14+ monocytes, stimulated with LPS, VEGF-C, IL-3, and TNF-α, differentiated into LYVE-1+/PROX-1+/PDPN+ cells. | 2011 | [49] |
| Peripheral blood | Monocyte-derived, lymphatic-like cells complexed with platelets significantly accelerated wound healing via increase of new lymphatic vessels. | 2014 | [20] |
| Peripheral blood | Aberrant LECPs in patients in lymphatic malformations coexpressed markers for stem cells, endothelial progenitors, and differentiated LECs. | 2016 | [62] |
| Pluripotent stem cell line | Pluripotent immortalized stem cell lines stimulated with VEGF-A and VEGF-C differentiated into LECP. When injected into wound healing and melanoma models, differentiated cells significantly increased lymphatic vessel formation. | 2015 | [2] |
| MSC | Human mesenchymal cells and multipotent mesenchymal cell lines differentiated into LECP-enhanced lymphatic vascularization in vivo. | 2009 | [46] |
| Adipose tissue progenitors | ADSCs were differentiated into LEC-like cells using VEGF-C. Differentiated cells implanted into Matrigel plugs increased lymphangiogenesis in vivo. | 2011 | [103] |
| Adipose tissue progenitors | Differentiation of ADSCs with a VEGFR-3-specific ligand VEGF-C156S up-regulated LEC-specific but not blood vascular-specific markers, such as CD31. | 2015 | [47] |
Functional properties of lymphatic progenitors in inflammatory models in vivo
The main evidence for the function and impact of all described LECPs is derived from inflammatory murine models typically involving tissue injury [7] or administration of an inflammatory agent, such as LPS [23]. For many of these studies, lethally irradiated mice were reconstituted with BM of transgenic mice with constitutive GFP expression to facilitate tracking of cell recruitment and behavior at the inflamed site. Mouse myeloid-derived LECPs were typically identified by coexpression of GFP, indicating BM origin; myeloid markers CD11b or F4/80 [51, 56]; and specific LEC markers, such as Lyve-1 [7, 48, 51, 56], Vegfr-3 [51, 59], or Pdpn [21]. These studies yielded a number of similar conclusions. First, inflamed tissues recruit substantial numbers of BM-derived myeloid cells expressing specific LEC markers, reflected by accumulation of GFP+/CD11b+/VEGFR-3+ or Lyve-1+ cells in inflamed tissues [21, 48, 63], whereas few or no such cells were detected in controls [7].
Second, studies in mice showed that inflammation-mobilized cells with dual myeloid-lymphatic identity were often detected near or integrated within nascent lymphatic vessels, a behavior that was previously described for embryonic lymphangioblasts expressing myeloid markers [42]. Depending on the model and experimental design, between 2 and 50% of Lyve-1+ inflamed vessels contained integrated M-LECPs. Lymphovascular insertion of progenitors was detected in inflammatory models of LPS-induced peritonitis [23], radiation-induced lymphangiogenesis [45], as well as various models of wound healing [7, 21, 51, 64]. Interestingly, M-LECPs tended to integrate preferentially into Lyve-1+ structures despite physical proximity to both blood and lymphatic vessels [48]. Although poorly understood, reproducible reports of progenitor integration into pre-existing vessels suggest high significance of this event for the formation of new lymphatics.
The third intriguing feature of mouse and human lymphatic progenitors is their capacity to create de novo lymphatic vasculature in the absence of pre-existing vessels through the process of lymphovasculogenesis, reminiscent of embryonic vessel formation. Although observed in a minority of studies, de novo lymphatic vasculature formation is unmistakable at sites naturally lacking pre-existing vessels, such as the cornea of the eye [7, 51] or Matrigel plugs that have no cellular structures before implantation into mice [65]. It is yet to be determined, however, whether new vessels constructed through vasculogenesis are functionally equal to those induced by integration of LECPs.
Finally, recent studies showed the biologic significance of lymphatic progenitors by demonstrating an increase in LVD and the function of the remodeled lymphatic network after injection of in vitro-differentiated or native M-LECPs. For instance, injection of syngeneic CD11b+/Pdpn+ cells into mice with ear or skin injuries significantly increased LVD and accelerated wound healing [2]. Injection of in vitro-differentiated mouse M-LECPs promoted relief from lymphedema [20]. Similarly produced human M-LECPs induced formation of lymphatic vessels in the normally avascular cornea of immunodeficient mice [57], consistent with the previously noted ability of mouse M-LECPs to create de novo corneal vessels after eye injury [7, 51].
Functional properties of myeloid-derived lymphatic progenitors in tumor models in vivo
Tumor-induced lymphatic formation is governed by principles similar to inflammatory lymphangiogenesis with the distinction that new tumor vessels are used for trafficking of both immune and malignant cells. Tumor lymphangiogenesis is also assisted by LECPs that have similar properties to those promoting inflammatory lymphatic formation. Mouse tumor models showed that mobilized M-LECPs express LEC-specific proteins [48], integrate into pre-existing lymphatic vessels [54, 66], have potential to induce lymphovasculogenesis [67], and increase tumor LVD [21]. The tracking of GFP+ mouse BM-derived myeloid cells identified integration of M-LECPs in 2% and up to 50% of tumor-associated lymphatics [66] in various syngeneic and human xenograft tumors modeling melanoma [21, 56], insulinoma [48], fibrosarcoma [51], and gastric [54], prostate [48], pancreatic [48], breast [66], and colorectal [66] cancers. In addition to GFP and CD11b markers indicating, respectively, the BM origin and the myelomonocytic lineage, tumor M-LECPs also express a vascular protein VE-cadherin, an LEC-specific marker Lyve-1, and a macrophage marker F4/80 [48]. These similarities between tumor- and inflammation-induced M-LECPs suggest that they are recruited from the same subset of BM cells and undergo similar differentiation programs. This idea has been supported by analysis of a double-positive CD11b+/Pdpn+ population in the BM of healthy and tumor-bearing mice. Whereas only 2% of these BM cells are present in cancer-free mice, implantation of syngeneic B16 melanoma expanded this population by 15-fold [21]. This is in line with the emerging concept that inflammatory substances up-regulated by pathogens, injury, or tumors expand and cause differentiation of BM-derived lymphatic progenitors that upon recruitment to inflamed tissues promote new vasculature.
Tumor M-LECPs are an integral part of M2-type TAMs
TAMs, in both mice and human patients, have been long associated with tumor lymphangiogenesis [68, 69] and lymphatic metastasis [68, 70]. Similar to myeloid-derived blood vascular progenitors, TAMs are mainly mobilized from BM [71, 72] and exhibit the “wound healing” M2-type phenotype [73]. This TAM phenotype is dictated by continuous remodeling of the tumor microenvironment that induces reparative rather than cytotoxic functions in recruited immune cells. M2-type macrophages, derived from BM myeloid cells, also dominate the late stages of wound healing in which these cells are programmed to restore postinjury homeostasis—a task that begins with rebuilding functional vasculature [74]. Reparative rather than cytotoxic behavior of TAMs might be a result of difficulty in assembling complex vascular structures in the presence of tissue-disruptive cytotoxic immune cells. Thus, the well-known immunosuppressive behavior of TAMs is likely an essential prerequisite for generating new vessels. Favoring this concept is evidence derived from multiple studies demonstrating expression of specific lymphatic proteins (Lyve-1 and Pdpn) in a subset of mouse M2-type macrophages identified by CD204 and CD163 [48, 56, 75]. This dual myeloid-lymphatic phenotype firmly places this subset in the category of M-LECP, suggesting that these cells are part of the global host program responding to signals of tumor remodeling. This is supported further by the presence of myeloid-lymphatic cells in other tumor models and their contribution to increasing density of tumor lymphatics [2].
M-LECPs in clinical cancers
At present, the evidence for M-LECPs in clinical cancers is limited to a small number of studies. The levels of blood-circulating LECPs, defined as CD34+/VEGFR-3+ cells, were found to be 3- to 4-fold higher in patients with small cell lung carcinoma (n = 88) and ovarian cancer (n = 54) compared with healthy subjects (n = 31 and 32, respectively) [76, 77]. Both studies showed that LN status correlated highly with the level of circulating LECPs (P < 0.01) but not with plasma concentration of the principal lymphangiogenic factor VEGF-C. These data suggest that without significant contribution of M-LECPs, VEGF-C alone might be insufficient to impact the metastatic efficiency of tumor-associated lymphatics, because of limited sprouting or poor functionality of new vessels. Higher levels of circulating CD14+ M-LECPs were also detected in breast cancer patients [57]. These cells were positive for neuropilin-1/2, receptors that facilitate binding of VEGF-A and VEGF-C to their high-affinity receptors VEGFR-2/3 [58, 78]. Interestingly, plasma from cancer patients, but not from healthy volunteers, significantly shortened the time required for primitive CD34+/CD133+ precursors to differentiate into M-LECPs [57]. These in vitro-produced human M-LECPs were able to induce new lymphatic vessels in vivo in an assay of cornea vascularization [57]. Collectively, these studies show many structural and functional similarities between inflammatory and tumor LECPs, further solidifying the concept of their shared origin and mechanisms underlying lymphangiogenesis in adults.
In summary, chronic inflammatory conditions, including cancers, induce differentiation of lymphatic progenitors, primarily from BM immature myeloid cells (Fig. 1, Step 1). Consequently, this subset coexpresses newly acquired lymphatic markers in conjunction with myeloid and stem/progenitor proteins (Fig. 2). Coexpression of a key lymphangiogenic receptor VEGFR-3 and its ligand VEGF-C in M-LECP promotes MLT (Fig. 1, Steps 2 and 3). This subset is present at low levels under steady-state conditions but rapidly expands and mobilizes to the blood during inflammation. Upon arrival to sites requiring expansion of the lymphatic network as a result of inflammation-imposed demands, M-LECPs preferentially integrate into pre-existing lymphatic vessels (Fig. 1, Step 4a), an event that precedes and presumably prompts sprouting (Fig. 1, Step 5). M-LECP can also promote creation of new vessels through a process of lymphovasculogenesis, an embryonic mechanism of vascular formation that does not require pre-existing vessels (Fig. 1, Step 4b).
MECHANISMS OF MLT THAT GENERATE LYMPHATIC PROGENITORS
Factors that induce M-LECP differentiation from myeloid stem cells or ESCs in vitro
Differentiation of M-LECP has been achieved in vitro using myeloid cells or their hematopoietic precursors isolated from mice or humans (Tables 2 and 3). Generation of mouse M-LECP was shown using primary BM-derived CD11b+ cells [7, 21, 48, 56, 66] or an immortalized semidifferentiated macrophage cell line RAW264.7 [23]. Mouse ESCs harboring myeloid precursors were also used successfully to generate lymphatic-like cells [79–82]. Similar differentiation protocols using VEGF-A [2, 57, 81] and VEGF-C [52] resulted in lymphatic reprogramming of human peripheral blood monocytes [20, 49, 62, 83], stem cells from umbilical cord blood [50, 52, 53, 57, 60], and human pluripotent stem cell lines [84, 85]. In all instances, differentiated cells displayed de novo-expressed, LEC-specific markers and exhibited traits reserved for vascular and specifically, LECs.
M-LECPs have also been generated from murine myeloid cells by stimulating the TLR4 pathway, which leads to NF-κB activation and subsequent up-regulation of VEGFR-3 [23], as well as VEGFR-2 [86], VEGF-A [87], and VEGF-C [88]. A TLR4-dependent increase in the key endothelial receptors and corresponding ligands in myeloid cells is likely a transformative event for MLT (Fig. 1). This contribution of TLR4 to an MLT might be the underlying cause for increased LVD in mice treated with a TLR4 ligand (LPS) [22, 23] and decreased LVD in mice lacking functional TLR4 [89]. This hypothesis is consistent with the study showing that treatment of tumor-bearing mice with a potent LPS mimetic—a chemo drug, paclitaxel—increases the density of tumor-associated lymphatics [67]. In mouse models, both LPS- and paclitaxel-induced lymphangiogenesis was accompanied by massive recruitment of BM-derived CD11b+ monocytes positive for Lyve-1 and other LEC markers [22, 23, 67], suggesting that tumor-mobilized M-LECPs play a critical role in formation of new lymphatics. Thus, in contrast to TLR4 activation of DCs that improve anti-tumor immunity, similar activation of immature myeloid cells leads to generation of M-LECP and ultimately, increased tumor lymphangiogenesis and metastasis [67, 90].
A transient but essential role of VEGFR-3 in myeloid cells undergoing prolymphatic reprogramming
In humans and mice, VEGFR-3 is best known as a specific marker of lymphatic endothelium, in which it plays a central role in LEC proliferation and migration—2 critical processes for vascular sprouting [91]. Although VEGFR-3+ TAMs have been long known to correlate with tumor lymphangiogenesis and lymphatic metastasis [68], the underlying causes were initially unclear. We were the first group to show that myeloid-expressed VEGFR-3 plays a nonredundant role in MLT [23]. This was indicated by the following observations: 1) VEGFR-3 is not expressed by resting myeloid cells; however, it is transcribed by myeloid cells after induction of prolymphatic differentiation. 2) Early expression of VEGFR-3 during differentiation coincides with up-regulation of its ligand VEGF-C, and this coordinated expression creates an autocrine reprogramming-promoting loop. 3) The autocrine activation of the VEGFR-3/VEGF-C loop precedes transcription of other lymphatic genes. 4) Blockade of this loop by a soluble decoy receptor completely abolishes the MLT, as indicated by suppressed up-regulation of lymphatic target genes [23].
These observations have been reproduced in several independent studies. The transient nature of VEGFR-3 expression has been shown during differentiation of mouse CD11b+ BM-derived cells [2]. The critical role of VEGFR-3 was shown in mouse ESCs overexpressing a nonfunctional VEGFR-3, an event that prevented expression of lymphatic markers during MLT [80]. A separate study showed that treatment of mouse ESCs with a decoy VEGFR-3 receptor also inhibited MLT [81]. The silencing of VEGFR-3 by small interfering RNA in human monocytes resulted in an identical outcome [50]. This is in line with the study showing that only VEGFR-3+ human monocytes, but not cells lacking this receptor, can undergo MLT [62]. Collectively, this evidence suggests an essential role for VEGFR-3 expressed in myeloid progenitors undergoing the MLT program. The fact that high but transient VEGFR-3 expression in myeloid precursors is restricted to the early phase of differentiation suggests that autocrine signaling induced by its ligand VEGF-C is required only for initiation of MLT and not for propagation of reprogramming. Thus, despite its critical role in MLT, VEGFR-3 might be a suboptimal marker for identification of circulating and tissue-mobilized M-LECPs as it is down-regulated to basal levels before full acquisition of the lymphatic phenotype.
Paracrine versus cell-autonomous prolymphangiogenic roles of myeloid-produced VEGF-C
Multiple studies found human and mouse M-LECPs to be ardent producers of VEGF-A and VEGF-C [11, 68, 92]. This led to a logical conclusion that the ability of M-LECPs to promote lymphatic formation directly relates to production of these lymphangiogenic factors [2, 57, 77, 93]. Although straightforward, this explanation does not account for unique properties of lymphatic progenitors, such as de novo expression of LEC-specific proteins [52, 57] and even a more peculiar ability to integrate into pre-existing lymphatic vessels [51]. These properties, shared by all reported lymphatic progenitors, indicate the importance of cell-autonomous mechanisms, in addition to production of paracrine factors that are already amply supplied by tumor and stromal cells. In fact, it has been empirically determined that for every 100 transcripts of VEGF-C produced by tumor cells, macrophages from the same tumor contribute only 1–2 molecules [48]. This evidence strongly argues that production of lymphangiogenic mediators by M-LECPs serves broader functions. We propose that VEGF-C up-regulation is required, first and foremost, for induction of MLT, a cell-autonomous reprogramming of myeloid precursors into lymphatic-like cells (Fig. 1, Steps 1–3). Acquisition of a new lymphatic phenotype might be necessary for M-LECP integration into lymphatic vessels or other functions promoting sprouting. As suggested by transient VEGFR-3 expression as opposed to sustained up-regulation of VEGF-C [23], after completing its autocrine function, VEGF-C can play a paracrine role by stimulating VEGFR-3 expressed on lymphatic vessels. Our model presented in Fig. 1 suggests that VEGF-C promotes lymphangiogenesis by two distinct mechanisms: autocrine induction of M-LECP reprogramming through activation of myeloid VEGFR-3 and paracrine activation of lymphatic vessels through stimulation of LEC-expressed VEGFR-3. The other tentative conclusion is that inhibition of lymphangiogenesis by antibodies targeting VEGF-C or VEGFR-3 also might have two separate causes: preventing myeloid cell differentiation into M-LECPs and blocking effects of VEGF-C on preformed LECs. The multiple ramifications of disrupting VEGF-C/VEGFR-3 signaling might be important for development and application of future therapies.
UNRESOLVED QUESTIONS REGARDING MLT AND LECPs INDUCED BY INFLAMMATION OR TUMOR
Development of adult lymphatic progenitors is an emerging field of study that features many intriguing questions. The multiplicity of origins for LECPs poses a fundamental question about the relationships among HSCs [45], MSCs [46], immature myeloid cells [7, 51, 55], and tissue-derived endothelial progenitors [94] as the primary source of lymphatic-inducing cells. VEGF-A/C and TLR4 ligands can induce experimental MLT, but natural stimuli regulating this process in vivo have not been clearly defined. Only the bare essentials of the MLT program are currently known, whereas the majority of its transcriptional, metabolic, and cellular regulators are yet to be discovered. The hallmarks of LECPs, such as de novo expression of lymphatic proteins and structural contribution to existing lymphatic vessels, have been well documented, but the significance and underlying mechanisms of both phenomena remain unclear. The pathologic circumstances surrounding infrequent but well-documented lymphovasculogenesis [7, 57, 67]—a process normally restricted to embryonic development—are equally poorly understood. The answers to these questions will lay the foundation for future studies aimed at better understanding of MLT and development of rationally designed therapies.
CONCLUDING REMARKS
In this review, we summarized and discussed current knowledge of BM-derived endothelial progenitors exhibiting a myeloid-lymphatic phenotype that increases the density of inflamed and tumor lymphatic vessels. Such cells are detected in mouse inflammatory and tumor models, as well as in the blood of cancer patients. Recruitment of these cells to inflamed sites correlates with increased number and function of lymphatics, whereas their mobilization to tumors correlates with lymphatic metastasis and poor outcome. MLT can be reproduced in vitro using defined lymphangiogenic factors, plasma from cancer patients, and activators of TLR4. We envision that unraveling the mechanisms of M-LECP differentiation will advance our understanding of adult lymphangiogenesis, leading to development of new therapies to correct deficient or excessive formation of lymphatic vessels.
AUTHORSHIP
S.R. and A.W. wrote the review.
ACKNOWLEDGMENTS
This work was supported by grants from the U.S. National Institutes of Health National Cancer Institute (5R01CA140732 and 1R01CA199649-01A1 to S.R. and R15CA173657 to A.W.) and Illinois William E. McElroy Charitable Foundation (to S.R.) and Team Science Grants from the Simmons Cancer Institute (to S.R. and A.W.). The authors are grateful to Ms. Kelly Hall for help with preparation of figures for this manuscript and to Ms. Lisa Volk-Draper for careful proofreading.
Glossary
- ADSC
adipose-derived stem cell
- BM
bone marrow
- c-Kit
receptor tyrosine protein kinase Kit
- CMP
common myeloid progenitor
- DC
dendritic cell
- ESC
embryonic stem cell
- HSC
hematopoietic stem cell
- LEC
lymphatic endothelial cell
- LECP
lymphatic endothelial cell progenitor
- Lin−
lineage marker negative
- LN
lymph node
- LVD
lymphatic vessel density
- LYVE-1
lymphatic vessel endothelial hyaluronan receptor 1
- M-LECP
myeloid-derived lymphatic endothelial cell progenitor
- M2
alternatively activated macrophage
- MLT
myeloid-lymphatic transition
- MSC
mesenchymal stem cell
- PDPN
(human) podoplanin
- Pdpn
(mouse) podoplanin
- Sca-1
stem cell antigen-1
- TAM
tumor-associated macrophage
- VE-cadherin
vascular endothelial-cadherin
- VEGF-A, -C, or -D
vascular endothelial growth factor A, C, or D
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Kim H., Kataru R. P., Koh G. Y. (2014) Inflammation-associated lymphangiogenesis: a double-edged sword? J. Clin. Invest. 124, 936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee S. J., Park C., Lee J. Y., Kim S., Kwon P. J., Kim W., Jeon Y. H., Lee E., Yoon Y. S. (2015) Generation of pure lymphatic endothelial cells from human pluripotent stem cells and their therapeutic effects on wound repair. Sci. Rep. 5, 11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht I., Christofori G. (2011) Molecular mechanisms of lymphangiogenesis in development and cancer. Int. J. Dev. Biol. 55, 483–494. [DOI] [PubMed] [Google Scholar]
- 4.Ran S., Volk L., Hall K., Flister M. J. (2010) Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology 17, 229–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karpanen T., Egeblad M., Karkkainen M. J., Kubo H., Ylä-Herttuala S., Jäättelä M., Alitalo K. (2001) Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 61, 1786–1790. [PubMed] [Google Scholar]
- 6.Achen M. G., Williams R. A., Baldwin M. E., Lai P., Roufail S., Alitalo K., Stacker S. A. (2002) The angiogenic and lymphangiogenic factor vascular endothelial growth factor-D exhibits a paracrine mode of action in cancer. Growth Factors 20, 99–107. [DOI] [PubMed] [Google Scholar]
- 7.Maruyama K., Ii M., Cursiefen C., Jackson D. G., Keino H., Tomita M., Van Rooijen N., Takenaka H., D’Amore P. A., Stein-Streilein J., Losordo D. W., Streilein J. W. (2005) Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 115, 2363–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allavena P., Sica A., Solinas G., Porta C., Mantovani A. (2008) The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit. Rev. Oncol. Hematol. 66, 1–9. [DOI] [PubMed] [Google Scholar]
- 9.Pollard J. W. (2008) Macrophages define the invasive microenvironment in breast cancer. J. Leukoc. Biol. 84, 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding M., Fu X., Tan H., Wang R., Chen Z., Ding S. (2012) The effect of vascular endothelial growth factor C expression in tumor-associated macrophages on lymphangiogenesis and lymphatic metastasis in breast cancer. Mol. Med. Rep. 6, 1023–1029. [DOI] [PubMed] [Google Scholar]
- 11.Schoppmann S. F., Fenzl A., Nagy K., Unger S., Bayer G., Geleff S., Gnant M., Horvat R., Jakesz R., Birner P. (2006) VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery 139, 839–846. [DOI] [PubMed] [Google Scholar]
- 12.Nolan D. J., Ciarrocchi A., Mellick A. S., Jaggi J. S., Bambino K., Gupta S., Heikamp E., McDevitt M. R., Scheinberg D. A., Benezra R., Mittal V. (2007) Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 21, 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Artus J., Hadjantonakis A. K. (2012) Troika of the mouse blastocyst: lineage segregation and stem cells. Curr. Stem Cell Res. Ther. 7, 78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Card C. M., Yu S. S., Swartz M. A. (2014) Emerging roles of lymphatic endothelium in regulating adaptive immunity. J. Clin. Invest. 124, 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H., Kataru R. P., Koh G. Y. (2012) Regulation and implications of inflammatory lymphangiogenesis. Trends Immunol. 33, 350–356. [DOI] [PubMed] [Google Scholar]
- 16.Ran S., Montgomery K. E. (2012) Macrophage-mediated lymphangiogenesis: the emerging role of macrophages as lymphatic endothelial progenitors. Cancers (Basel) 4, 618–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henno A., Blacher S., Lambert C., Colige A., Seidel L., Noël A., Lapière C., de la Brassinne M., Nusgens B. V. (2009) Altered expression of angiogenesis and lymphangiogenesis markers in the uninvolved skin of plaque-type psoriasis. Br. J. Dermatol. 160, 581–590. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q., Lu Y., Proulx S. T., Guo R., Yao Z., Schwarz E. M., Boyce B. F., Xing L. (2007) Increased lymphangiogenesis in joints of mice with inflammatory arthritis. Arthritis Res. Ther. 9, R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geleff S., Schoppmann S. F., Oberhuber G. (2003) Increase in podoplanin-expressing intestinal lymphatic vessels in inflammatory bowel disease. Virchows Arch. 442, 231–237. [DOI] [PubMed] [Google Scholar]
- 20.Hur J., Jang J. H., Oh I. Y., Choi J. I., Yun J. Y., Kim J., Choi Y. E., Ko S. B., Kang J. A., Kang J., Lee S. E., Lee H., Park Y. B., Kim H. S. (2014) Human podoplanin-positive monocytes and platelets enhance lymphangiogenesis through the activation of the podoplanin/CLEC-2 axis. Mol. Ther. 22, 1518–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J. Y., Park C., Cho Y. P., Lee E., Kim H., Kim P., Yun S. H., Yoon Y. S. (2010) Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation 122, 1413–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang S., Lee S. P., Kim K. E., Kim H. Z., Mémet S., Koh G. Y. (2009) Toll-like receptor 4 in lymphatic endothelial cells contributes to LPS-induced lymphangiogenesis by chemotactic recruitment of macrophages. Blood 113, 2605–2613. [DOI] [PubMed] [Google Scholar]
- 23.Hall K. L., Volk-Draper L. D., Flister M. J., Ran S. (2012) New model of macrophage acquisition of the lymphatic endothelial phenotype. PLoS One 7, e31794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi V. Y., Bao L., Chan L. S. (2012) Inflammation-driven dermal lymphangiogenesis in atopic dermatitis is associated with CD11b+ macrophage recruitment and VEGF-C up-regulation in the IL-4-transgenic mouse model. Microcirculation 19, 567–579. [DOI] [PubMed] [Google Scholar]
- 25.Brand C. U., Hunziker T., Braathen L. R. (1992) Isolation of human skin-derived lymph: flow and output of cells following sodium lauryl sulphate-induced contact dermatitis. Arch. Dermatol. Res. 284, 123–126. [DOI] [PubMed] [Google Scholar]
- 26.Swartz M. A. (2014) Immunomodulatory roles of lymphatic vessels in cancer progression. Cancer Immunol. Res. 2, 701–707. [DOI] [PubMed] [Google Scholar]
- 27.Saeki H., Moore A. M., Brown M. J., Hwang S. T. (1999) Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 162, 2472–2475. [PubMed] [Google Scholar]
- 28.Gunn M. D., Kyuwa S., Tam C., Kakiuchi T., Matsuzawa A., Williams L. T., Nakano H. (1999) Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189, 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willimann K., Legler D. F., Loetscher M., Roos R. S., Delgado M. B., Clark-Lewis I., Baggiolini M., Moser B. (1998) The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur. J. Immunol. 28, 2025–2034. [DOI] [PubMed] [Google Scholar]
- 30.Ohl L., Mohaupt M., Czeloth N., Hintzen G., Kiafard Z., Zwirner J., Blankenstein T., Henning G., Förster R. (2004) CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21, 279–288. [DOI] [PubMed] [Google Scholar]
- 31.Lund A. W., Wagner M., Fankhauser M., Steinskog E. S., Broggi M. A., Spranger S., Gajewski T. F., Alitalo K., Eikesdal H. P., Wiig H., Swartz M. A. (2016) Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J. Clin. Invest. 126, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura T., Sugaya M., Oka T., Blauvelt A., Okochi H., Sato S. (2015) Lymphatic dysfunction attenuates tumor immunity through impaired antigen presentation. Oncotarget 6, 18081–18093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinskog E. S., Sagstad S. J., Wagner M., Karlsen T. V., Yang N., Markhus C. E., Yndestad S., Wiig H., Eikesdal H. P. (2016) Impaired lymphatic function accelerates cancer growth. Oncotarget 7, 45789–45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stachura J., Wachowska M., Kilarski W. W., Güç E., Golab J., Muchowicz A. (2016) The dual role of tumor lymphatic vessels in dissemination of metastases and immune response development. OncoImmunology 5, e1182278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenberg P. A., Hortobagyi G. N., Smith T. L., Ziegler L. D., Frye D. K., Buzdar A. U. (1996) Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J. Clin. Oncol. 14, 2197–2205. [DOI] [PubMed] [Google Scholar]
- 36.Volk L. D., Flister M. J., Chihade D., Desai N., Trieu V., Ran S. (2011) Synergy of nab-paclitaxel and bevacizumab in eradicating large orthotopic breast tumors and preexisting metastases. Neoplasia 13, 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Podgrabinska S., Skobe M. (2014) Role of lymphatic vasculature in regional and distant metastases. Microvasc. Res. 95, 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sleeman J. P., Thiele W. (2009) Tumor metastasis and the lymphatic vasculature. Int. J. Cancer 125, 2747–2756. [DOI] [PubMed] [Google Scholar]
- 39.Hirakawa S., Brown L. F., Kodama S., Paavonen K., Alitalo K., Detmar M. (2007) VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 109, 1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Algars A., Irjala H., Vaittinen S., Huhtinen H., Sundström J., Salmi M., Ristamäki R., Jalkanen S. (2012) Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int. J. Cancer 131, 864–873. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y., García-Verdugo J. M., Soriano-Navarro M., Srinivasan R. S., Scallan J. P., Singh M. K., Epstein J. A., Oliver G. (2012) Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood 120, 2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buttler K., Ezaki T., Wilting J. (2008) Proliferating mesodermal cells in murine embryos exhibiting macrophage and lymphendothelial characteristics. BMC Dev. Biol. 8, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zumsteg A., Christofori G. (2012) Myeloid cells and lymphangiogenesis. Cold Spring Harb. Perspect. Med. 2, a006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He Y., Rajantie I., Ilmonen M., Makinen T., Karkkainen M. J., Haiko P., Salven P., Alitalo K. (2004) Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 64, 3737–3740. [DOI] [PubMed] [Google Scholar]
- 45.Jiang S., Bailey A. S., Goldman D. C., Swain J. R., Wong M. H., Streeter P. R., Fleming W. H. (2008) Hematopoietic stem cells contribute to lymphatic endothelium. PLoS One 3, e3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conrad C., Niess H., Huss R., Huber S., von Luettichau I., Nelson P. J., Ott H. C., Jauch K. W., Bruns C. J. (2009) Multipotent mesenchymal stem cells acquire a lymphendothelial phenotype and enhance lymphatic regeneration in vivo. Circulation 119, 281–289. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y., Chen X. H., Li F. G., Chen Y. X., Gu L. Q., Zhu J. K., Li P. (2015) In vitro induction of human adipose-derived stem cells into lymphatic endothelial-like cells. Cell. Reprogram. 17, 69–76. [DOI] [PubMed] [Google Scholar]
- 48.Zumsteg A., Baeriswyl V., Imaizumi N., Schwendener R., Rüegg C., Christofori G. (2009) Myeloid cells contribute to tumor lymphangiogenesis. PLoS One 4, e7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Changming W., Xin L., Hua T., Shikun W., Qiong X., Zhigeng Z., Xueying W. (2011) Monocytes can be induced to express lymphatic phenotypes. Lymphology 44, 48–53. [PubMed] [Google Scholar]
- 50.Li T., Wang G. D., Tan Y. Z., Wang H. J. (2014) Inhibition of lymphangiogenesis of endothelial progenitor cells with VEGFR-3 siRNA delivered with PEI-alginate nanoparticles. Int. J. Biol. Sci. 10, 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Religa P., Cao R., Bjorndahl M., Zhou Z., Zhu Z., Cao Y. (2005) Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood 106, 4184–4190. [DOI] [PubMed] [Google Scholar]
- 52.Salven P., Mustjoki S., Alitalo R., Alitalo K., Rafii S. (2003) VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood 101, 168–172. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen V. A., Fürhapter C., Obexer P., Stössel H., Romani N., Sepp N. (2009) Endothelial cells from cord blood CD133+CD34+ progenitors share phenotypic, functional and gene expression profile similarities with lymphatics. J. Cell. Mol. Med. 13, 522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tawada M., Hayashi S., Osada S., Nakashima S., Yoshida K. (2012) Human gastric cancer organizes neighboring lymphatic vessels via recruitment of bone marrow-derived lymphatic endothelial progenitor cells. J. Gastroenterol. 47, 1057–1060. [DOI] [PubMed] [Google Scholar]
- 55.Kerjaschki D., Huttary N., Raab I., Regele H., Bojarski-Nagy K., Bartel G., Kröber S. M., Greinix H., Rosenmaier A., Karlhofer F., Wick N., Mazal P. R. (2006) Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat. Med. 12, 230–234. [DOI] [PubMed] [Google Scholar]
- 56.Schledzewski K., Falkowski M., Moldenhauer G., Metharom P., Kzhyshkowska J., Ganss R., Demory A., Falkowska-Hansen B., Kurzen H., Ugurel S., Geginat G., Arnold B., Goerdt S. (2006) Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J. Pathol. 209, 67–77. [DOI] [PubMed] [Google Scholar]
- 57.Van’t Hull E. F., Bron S., Henry L., Ifticene-Treboux A., Turrini R., Coukos G., Delaloye J. F., Doucey M. A. (2014) Bone marrow-derived cells are implicated as a source of lymphatic endothelial progenitors in human breast cancer. OncoImmunology 3, e29080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamrah P., Chen L., Zhang Q., Dana M. R. (2003) Novel expression of vascular endothelial growth factor receptor (VEGFR)-3 and VEGF-C on corneal dendritic cells. Am. J. Pathol. 163, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamrah P., Chen L., Cursiefen C., Zhang Q., Joyce N. C., Dana M. R. (2004) Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) on monocytic bone marrow-derived cells in the conjunctiva. Exp. Eye Res. 79, 553–561. [DOI] [PubMed] [Google Scholar]
- 60.Tan Y. Z., Wang H. J., Zhang M. H., Quan Z., Li T., He Q. Z. (2014) CD34+ VEGFR-3+ progenitor cells have a potential to differentiate towards lymphatic endothelial cells. J. Cell. Mol. Med. 18, 422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cooley L. S., Handsley M. M., Zhou Z., Lafleur M. A., Pennington C. J., Thompson E. W., Pöschl E., Edwards D. R. (2010) Reversible transdifferentiation of blood vascular endothelial cells to a lymphatic-like phenotype in vitro. J. Cell Sci. 123, 3808–3816. [DOI] [PubMed] [Google Scholar]
- 62.DiMaio T. A., Wentz B. L., Lagunoff M. (2016) Isolation and characterization of circulating lymphatic endothelial colony forming cells. Exp. Cell Res. 340, 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cursiefen C., Chen L., Borges L. P., Jackson D., Cao J., Radziejewski C., D’Amore P. A., Dana M. R., Wiegand S. J., Streilein J. W. (2004) VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest. 113, 1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maruyama K., Asai J., Ii M., Thorne T., Losordo D. W., D’Amore P. A. (2007) Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am. J. Pathol. 170, 1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buttler K., Lohrberg M., Gross G., Weich H. A., Wilting J. (2016) Integration of CD45-positive leukocytes into newly forming lymphatics of adult mice. Histochem. Cell Biol. 145, 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tawada M., Hayashi S., Ikegame Y., Nakashima S., Yoshida K. (2014) Possible involvement of tumor-producing VEGF-A in the recruitment of lymphatic endothelial progenitor cells from bone marrow. Oncol. Rep. 32, 2359–2364. [DOI] [PubMed] [Google Scholar]
- 67.Volk-Draper L., Hall K., Griggs C., Rajput S., Kohio P., DeNardo D., Ran S. (2014) Paclitaxel therapy promotes breast cancer metastasis in a TLR4-dependent manner. Cancer Res. 74, 5421–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schoppmann S. F., Birner P., Stöckl J., Kalt R., Ullrich R., Caucig C., Kriehuber E., Nagy K., Alitalo K., Kerjaschki D. (2002) Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 161, 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kurahara H., Shinchi H., Mataki Y., Maemura K., Noma H., Kubo F., Sakoda M., Ueno S., Natsugoe S., Takao S. (2011) Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J. Surg. Res. 167, e211–e219. [DOI] [PubMed] [Google Scholar]
- 70.Yang H., Kim C., Kim M. J., Schwendener R. A., Alitalo K., Heston W., Kim I., Kim W. J., Koh G. Y. (2011) Soluble vascular endothelial growth factor receptor-3 suppresses lymphangiogenesis and lymphatic metastasis in bladder cancer. Mol. Cancer 10, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shand F. H., Ueha S., Otsuji M., Koid S. S., Shichino S., Tsukui T., Kosugi-Kanaya M., Abe J., Tomura M., Ziogas J., Matsushima K. (2014) Tracking of intertissue migration reveals the origins of tumor-infiltrating monocytes. Proc. Natl. Acad. Sci. USA 111, 7771–7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DeNardo D. G., Brennan D. J., Rexhepaj E., Ruffell B., Shiao S. L., Madden S. F., Gallagher W. M., Wadhwani N., Keil S. D., Junaid S. A., Rugo H. S., Hwang E. S., Jirström K., West B. L., Coussens L. M. (2011) Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 1, 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chanmee T., Ontong P., Konno K., Itano N. (2014) Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 6, 1670–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mantovani A., Biswas S. K., Galdiero M. R., Sica A., Locati M. (2013) Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 229, 176–185. [DOI] [PubMed] [Google Scholar]
- 75.Espagnolle N., Barron P., Mandron M., Blanc I., Bonnin J., Agnel M., Kerbelec E., Herault J. P., Savi P., Bono F., Alam A. (2014) Specific inhibition of the VEGFR-3 tyrosine kinase by SAR131675 reduces peripheral and tumor associated immunosuppressive myeloid cells. Cancers (Basel) 6, 472–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bogos K., Renyi-Vamos F., Dobos J., Kenessey I., Tovari J., Timar J., Strausz J., Ostoros G., Klepetko W., Ankersmit H. J., Lang G., Hoda M. A., Nierlich P., Dome B. (2009) High VEGFR-3-positive circulating lymphatic/vascular endothelial progenitor cell level is associated with poor prognosis in human small cell lung cancer. Clin. Cancer Res. 15, 1741–1746. [DOI] [PubMed] [Google Scholar]
- 77.Qiu H., Cao L., Wang D., Xu H., Liang Z. (2013) High levels of circulating CD34+/VEGFR3+ lymphatic/vascular endothelial progenitor cells is correlated with lymph node metastasis in patients with epithelial ovarian cancer. J. Obstet. Gynaecol. Res. 39, 1268–1275. [DOI] [PubMed] [Google Scholar]
- 78.Robinson C. J., Stringer S. E. (2001) The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J. Cell Sci. 114, 853–865. [DOI] [PubMed] [Google Scholar]
- 79.Liersch R., Nay F., Lu L., Detmar M. (2006) Induction of lymphatic endothelial cell differentiation in embryoid bodies. Blood 107, 1214–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suzuki H., Watabe T., Kato M., Miyazawa K., Miyazono K. (2005) Roles of vascular endothelial growth factor receptor 3 signaling in differentiation of mouse embryonic stem cell-derived vascular progenitor cells into endothelial cells. Blood 105, 2372–2379. [DOI] [PubMed] [Google Scholar]
- 81.Kono T., Kubo H., Shimazu C., Ueda Y., Takahashi M., Yanagi K., Fujita N., Tsuruo T., Wada H., Yamashita J. K. (2006) Differentiation of lymphatic endothelial cells from embryonic stem cells on OP9 stromal cells. Arterioscler. Thromb. Vasc. Biol. 26, 2070–2076. [DOI] [PubMed] [Google Scholar]
- 82.Vittet D., Merdzhanova G., Prandini M. H., Feige J. J., Bailly S. (2012) TGFβ1 inhibits lymphatic endothelial cell differentiation from mouse embryonic stem cells. J. Cell. Physiol. 227, 3593–3602. [DOI] [PubMed] [Google Scholar]
- 83.Zhao Y., Glesne D., Huberman E. (2003) A human peripheral blood monocyte-derived subset acts as pluripotent stem cells. Proc. Natl. Acad. Sci. USA 100, 2426–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Laos S., Baeckström D., Hansson G. C. (2006) Inhibition of NF-kappaB activation and chemokine expression by the leukocyte glycoprotein, CD43, in colon cancer cells. Int. J. Oncol. 28, 695–704. [PubMed] [Google Scholar]
- 85.Stanczuk L., Martinez-Corral I., Ulvmar M. H., Zhang Y., Laviña B., Fruttiger M., Adams R. H., Saur D., Betsholtz C., Ortega S., Alitalo K., Graupera M., Mäkinen T. (2015) cKit lineage hemogenic endothelium-derived cells contribute to mesenteric lymphatic vessels. Cell Reports 10, 1708–1721. [DOI] [PubMed] [Google Scholar]
- 86.Lin C. M., Chang H., Li S. Y., Wu I. H., Chiu J. H. (2006) Chrysin inhibits lipopolysaccharide-induced angiogenesis via down-regulation of VEGF/VEGFR-2(KDR) and IL-6/IL-6R pathways. Planta Med. 72, 708–714. [DOI] [PubMed] [Google Scholar]
- 87.Huang S., Pettaway C. A., Uehara H., Bucana C. D., Fidler I. J. (2001) Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene 20, 4188–4197. [DOI] [PubMed] [Google Scholar]
- 88.Li Y., He J., Zhong D., Li J., Liang H. (2015) High-mobility group box 1 protein activating nuclear factor-κB to upregulate vascular endothelial growth factor C is involved in lymphangiogenesis and lymphatic node metastasis in colon cancer. J. Int. Med. Res. 43, 494–505. [DOI] [PubMed] [Google Scholar]
- 89.Zampell J., Elhadad S., Avraham T., Weitman E., Aschen S., Yan A., Mehrara B. J. (2011) Toll-like receptor deficiency worsens inflammation and lymphedema after lymphatic injury. Am. J. Physiol. Cell Physiol. 302, C709–C719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ran S. (2015) The role of TLR4 in chemotherapy-driven metastasis. Cancer Res. 75, 2405–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adams R. H., Alitalo K. (2007) Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 8, 464–478. [DOI] [PubMed] [Google Scholar]
- 92.Ji R. C. (2012) Macrophages are important mediators of either tumor- or inflammation-induced lymphangiogenesis. Cell. Mol. Life Sci. 69, 897–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takeda K., Sowa Y., Nishino K., Itoh K., Fushiki S. (2015) Adipose-derived stem cells promote proliferation, migration, and tube formation of lymphatic endothelial cells in vitro by secreting lymphangiogenic factors. Ann. Plast. Surg. 74, 728–736. [DOI] [PubMed] [Google Scholar]
- 94.Schniedermann J., Rennecke M., Buttler K., Richter G., Städtler A. M., Norgall S., Badar M., Barleon B., May T., Wilting J., Weich H. A. (2010) Mouse lung contains endothelial progenitors with high capacity to form blood and lymphatic vessels. BMC Cell Biol. 11, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirakawa S., Hong Y. K., Harvey N., Schacht V., Matsuda K., Libermann T., Detmar M. (2003) Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am. J. Pathol. 162, 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maertens L., Erpicum C., Detry B., Blacher S., Lenoir B., Carnet O., Péqueux C., Cataldo D., Lecomte J., Paupert J., Noel A. (2014) Bone marrow-derived mesenchymal stem cells drive lymphangiogenesis. PLoS One 9, e106976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brunt K. R., Hall S. R., Ward C. A., Melo L. G. (2007) Endothelial progenitor cell and mesenchymal stem cell isolation, characterization, viral transduction. Methods Mol. Med. 139, 197–210. [DOI] [PubMed] [Google Scholar]
- 98.Hristov M., Schmitz S., Schuhmann C., Leyendecker T., von Hundelshausen P., Krötz F., Sohn H. Y., Nauwelaers F. A., Weber C. (2009) An optimized flow cytometry protocol for analysis of angiogenic monocytes and endothelial progenitor cells in peripheral blood. Cytometry A 75, 848–853. [DOI] [PubMed] [Google Scholar]
- 99.Lanuti P., Rotta G., Almici C., Avvisati G., Budillon A., Doretto P., Malara N., Marini M., Neva A., Simeone P., Di Gennaro E., Leone A., Falda A., Tozzoli R., Gregorj C., Di Cerbo M., Trunzo V., Mollace V., Marchisio M., Miscia S. (2016) Endothelial progenitor cells, defined by the simultaneous surface expression of VEGFR2 and CD133, are not detectable in healthy peripheral and cord blood. Cytometry A 89, 259–270. [DOI] [PubMed] [Google Scholar]
- 100.Buttler K., Kreysing A., von Kaisenberg C. S., Schweigerer L., Gale N., Papoutsi M., Wilting J. (2006) Mesenchymal cells with leukocyte and lymphendothelial characteristics in murine embryos. Dev. Dyn. 235, 1554–1562. [DOI] [PubMed] [Google Scholar]
- 101.Wei L., Liu Y., Chen G., Fang Y., Song X., Dong P., Gao J., Liu R., Ding Z., Bi Y., Liu Z. (2012) Differentiation of lymphatic endothelial cells from bone marrow mesenchymal stem cells with VEGFs. Lymphology 45, 177–187. [PubMed] [Google Scholar]
- 102.Igarashi Y., Chosa N., Sawada S., Kondo H., Yaegashi T., Ishisaki A. (2016) VEGF-C and TGF-β reciprocally regulate mesenchymal stem cell commitment to differentiation into lymphatic endothelial or osteoblastic phenotypes. Int. J. Mol. Med. 37, 1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yan A., Avraham T., Zampell J. C., Haviv Y. S., Weitman E., Mehrara B. J. (2011) Adipose-derived stem cells promote lymphangiogenesis in response to VEGF-C stimulation or TGF-β1 inhibition. Future Oncol. 7, 1457–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]