Elevating circulating ST6Gal-1 sialyltrasferase blunts granulopoiesis at the level of GMP.
Keywords: neutrophils, hematopoiesis, sialylation, glycosylation
Abstract
Responding to systemic demands in producing and replenishing end-effector blood cells is predicated on the appropriate delivery and interpretation of extrinsic signals to the HSPCs. The data presented herein implicate the systemic, extracellular form of the glycosyltransferase ST6Gal-1 in the regulation of late-stage neutrophil development. ST6Gal-1 is typically a membrane-bound enzyme sequestered within the intracellular secretory apparatus, but an extracellular form is released into the blood from the liver. Both human and murine HSPCs, upon exposure to extracellular ST6Gal-1 ex vivo, exhibited decreased proliferation, diminished expression of the neutrophilic primary granule protein MPO, and decreased appearance of CD11b+ cells. HSPC suppression was preceded by decreased STAT-3 phosphorylation and diminished C/EBPα expression, without increased apoptosis, indicating attenuated G-CSF receptor signaling. A murine model to raise systemic ST6Gal-1 level was developed to examine the role of the circulatory enzyme in vivo. Our results show that systemic ST6Gal-1 modified the cell surface of the GMP subset of HSPCs and decreased marrow neutrophil reserves. Acute airway neutrophilic inflammation by LPS challenge was used to drive demand for new neutrophil production. Reduced neutrophil infiltration into the airway was observed in mice with elevated circulatory ST6Gal-1 levels. The blunted transition of GMPs into GPs in vitro is consistent with ST6Gal-1-attenuated granulopoiesis. The data confirm that circulatory ST6Gal-1 is a negative systemic regulator of granulopoiesis and moreover suggest a clinical potential to limit the number of inflammatory cells by manipulating blood ST6Gal-1 levels.
Introduction
The hematopoietic response to immune challenge is essential to the welfare of the organism, whether it is to meet demands to combat invading pathogens or to re-establish blood cell compartments after myelodepleting therapies. Particularly urgent is the maintenance of the granulocytic compartment in a context-appropriate way to effect a balance between immune-compromised and inflammatory states. Neutropenia carries with it the risk of infection, whereas neutrophilia and inappropriate inflammatory cell activation are associated with the pathogenesis of sickle cell disease, rheumatoid arthritis, and chronic obstructive pulmonary disease [1–3]. Neutrophils are among the first cells to access tissues under most inflammatory conditions, and are known to recruit and activate subsequent waves of effector lymphocytes during bacterial and viral infections and in chronic inflammatory diseases via chemokine and proinflammatory cytokine release [4–7]. Managing inflammatory cell production is an attractive treatment modality, whether the goal is treating excessive inflammation or hastening the recovery of blood cells after myelodepletion episodes.
The systemic demand for end-effector blood cells is communicated to marrow HSPCs by the delivery of instructive cues, chiefly in the form of soluble cytokine factors [8, 9]. Glycans, by virtue of their position on cell surfaces, are situated to mediate how these cues are interpreted. The participation of cell surface glycans in multiple aspects of immunity and trafficking is well documented [10]. Sialyl- and fucosyl-glycans participate in the adhesive interactions critical to stem cell homeostasis, including the homing of CD34+ hematopoietic progenitors to the bone marrow [11–13]. However, the involvement of these critical glycan structures in cytokine signaling remains largely unexplored.
ST6Gal-1 is the sialyltransferase responsible for the construction of the α2,6-sialyl moiety on Gal(β1,4)GlcNAc termini. It has been implicated in suppressing differentiation of human pluripotent stem cells, as well as conferring radiation and chemoresistance in cancer cell lines [14–17]. A significant body of literature has accumulated that documents the association of ST6Gal-1 with a diverse array of clinical conditions, including stress, atherosclerosis, alcoholism, and malignancies—particularly colon and breast cancers and multiple myeloma [18–25]. ST6Gal-1 is typically regarded as a resident within the Golgi-ER secretory network, where it glycosylates nascent glycoproteins during intracellular biosynthetic transit. Enzymatically active ST6Gal-1 is also present in an extracellular form in the blood, and changes in the level of circulatory ST6Gal-1 have long been associated with inflammatory states, malignancy, and other disease conditions [26–31]. Recent work in our laboratory has elucidated a basis for this extrinsic mechanism of glycosylation. We have found that platelets can supply the necessary sugar donor to extracellularly glycosylate the cell surface, and insufficiency in the circulatory form of ST6Gal-1 is associated with overly robust inflammation, exaggerated inflammatory cell production, and decreased sialylation in the bone marrow compartment [32–34]. However, the function of extrinsic glycosylation by circulatory ST6Gal-1 in regulating granulopoiesis during inflammatory events has not been explored.
We present data showing that ST6Gal-1 modifies the cell surface glycans of HSPCs and alters the response of committed GMPs to differentiating cytokines. Our data demonstrate that human and murine hematopoietic progenitor cell expansion and differentiation were suppressed by the presence of ST6Gal-1 in the extracellular milieu, to be called extrinsic ST6Gal-1. In a mouse model with an inflated circulatory level of ST6Gal-1, LPS-elicited acute airway inflammation markedly reduced airway neutrophil infiltration, concomitant with diminished neutrophil marrow reserves. Enhanced cell surface sialylation of FcγRII/III+ progenitors was observed, indicating specific extrinsic ST6Gal-1-mediated modification of the granulocyte-monocyte progenitor. This modification resulted in attenuated STAT-3 phosphorylation and impeded transition of GMPs to GPs. The data show that circulatory ST6Gal-1 is a natural negative regulator of neutrophil production and suggest that manipulating blood ST6Gal-1 levels is a novel and viable approach to controlling the availability of neutrophils.
MATERIALS AND METHODS
Animals and inflammation models
The St6gal1-dP1 mouse (dP1), demonstrating a liver-restricted ST6Gal-1 deficiency, was obtained from the Consortium for Functional Glycomics (http://www.functionalglycomics.org/). The dP1 mouse was generated by a specific disruption to the P1 promoter of the St6gal1 gene, including removal of the 1.2-kb region containing Exon H, and backcrossed for >10 generations into the C57BL/6 background [35–37]. Age- and sex-matched C57BL/6 (CD45.2) animals were used as controls and are commonly referenced as WT.
The LPS model of acute pulmonary inflammation was performed as described elsewhere [38]. Mice received an oropharyngeal instillation of 50 μg of LPS. After 18 h, BALF analysis was performed. The trachea was cannulated with a 22-gauge i.v. catheter. PBS (800 μl) was injected and withdrawn from the lung 2 times with a tuberculin syringe. The WBC composition of BALF was assessed with a TC20 automated cell counter (Bio-Rad, Hercules, CA, USA) and flow cytometry. After lavage, the lungs were excised and fixed in 10% formaldehyde in PBS, embedded in paraffin, sectioned, and stained with H&E. A pulmonary pathologist blinded to the identity of the slides evaluated lung pathology.
The Institutional Animal Care and Use Committee of the Roswell Park Cancer Institute approved all animal protocols.
Ex vivo sialylation
Exo-sialylation was performed on human CD34+ (Lonza, Walkersville, MD, USA), and various populations were isolated from the WT mouse. Cells, referred to as “rST6Gal-1,” were treated with 3.5 μU/ml rat ST6Gal-1 and 50 μM CMP-Neu5Ac for 3 h at 37°C before addition of cytokines into the 1 ml normal growth medium. For control, cells were resuspended in the same buffer with CMP-Neu5Ac, but lacking ST6Gal-1.
Sialyltransferase assays
Sialyltransferase assays were performed as described in several publications [35, 36, 39].
HSPC isolation and analysis
Bone marrow cells were flushed from femur and tibia with PBS containing 0.5% FBS and 1 mM EDTA (FACS buffer). Hypotonic lysis buffer (0.8% NH4Cl and 0.1 mM EDTA buffered with KHCO3 to pH 7.4) was added to remove RBCs. Cells were washed and resuspended in FACS buffer after centrifugation at 1200 rpm for 10 min at 4°C. Where indicated, cellularity was assessed with an automated cell counter.
Immunofluorescent staining and flow cytometric analysis were performed. Samples were incubated with combinations of fluorescently labeled anti-mouse antibodies 1A8 (anti-Ly6G), RB6-8C5 (Gr-1; anti-Ly6G, anti-Ly6C), D7 (anti-Sca-1, Ly6A/E), AFS98 (anti-CD115), 2B8 (anti-c-Kit), HM48-1 (anti-CD48), N418 (anti-CD11c), BM8 (anti-F4/80), M1/70 (anti-CD11b), 93 (anti-CD16/32), RA3-6B2 (anti-B220), TC15-12F12.2 (anti-CD150), MJ7/18 (anti-CD105), MWReg30 (anti-CD41), HK1.4 (anti-Ly6C), anti-human antibodies 561 (anti-CD34), ICRF44 (anti-CD11b), and Sambucus nigra lectin (SNA; all from Vector Laboratories, Peterborough, UK). The cell populations examined and surface phenotypes were as follows: (LK; Lin− Sca-1− cKit+), (LSK; Lin− Sca-1+ cKit+), megakaryocyte progenitor (MkP; LK/CD41+ CD150+), CMP (LK/CD41− FcγRII/III−), Pre-GM (LK/CD41− FcγRII/III− CD105− CD150−), FcγRII/III+ progenitor (LK/CD41− CD150− FcγRII/III+), GMP (LK/CD41− CD150− FcγRII/III+ CD115− Ly6C−), GP (LK/CD41− CD150− FcγRII/III+ CD115− Ly6C+), monocyte progenitor (MP; LK/CD41− CD150− FcγRII/III+ CD115+ Ly6C+), erythroid progenitor (LK/CD41− FcγRII/III− CD105+), B lymphocytes (B220+ Gr-1−), monocytes (CD11b+ F4/80− Ly6C+), and neutrophils (CD11b+ Gr-1+, alternatively CD11b+ Ly6G+). Analysis was performed with an LSR-II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and FlowJo software (FlowJo LLC, Ashland, OR, USA).
Progenitor purification for culture was accomplished with a combination of negative selection and cell sorting. Pooled WT marrow was lineage depleted with magnetic microparticles, according to the manufacturer’s protocol (Stemcell Technologies, Vancouver, BC, Canada). LK, FcγRII/III+ progenitors, and GMPs were isolated from lineage-depleted pools by FACS with a FACSAria II (BD Biosciences).
For PCR analysis, RNA was isolated from live cell pellets with TRI Reagent (Sigma-Aldrich, St. Louis, MO, USA), used according to the manufacturer’s instructions. Total RNA (1.5 μg) was used as a template for cDNA synthesis with the iScript reverse transcriptase kit (Bio-Rad) in a volume of 50 μl. Quantitative RT-PCR reactions were performed with iQ SYBR-green Supermix and the CFX96 Touch Real-Time PCR Detection System (both from Bio-Rad). Relative mRNA levels of test genes were normalized to B2-microglobulin mRNA control, and relative fold expression was normalized to G-CSF-stimulated cells without ST6Gal-1 treatment. Primer sequences are as follows: MPO: F- 5′-GCTGGCTATCAATACACGCT-3′, R- 3′-CTCGAACAAAGAGGGTGTGC-5′; C/EBP-α: F- 5′-CGGTGCGCAAGAGCCGAGAT-3′, R- 3′-CCCGCAGCGTGTCCAGTTCA-5′; C/EBP-β: F- 5′-AACTCTCTGCTTCTCCCTCTG-3′, R- 3′-AAGCCCGTAGGAAGATCTTT-5′; SOCS-3: F- 5′-GGAGATTTCGCTTCGGGACTA-3′, R- 3′-GGAAACTTGCTGTGGGTGAC-5′; and B2M: F- 5′-CTGACCGGCCTGTATGCTAT-3′; R- 3′-TTCCCGTTCTTCAGCATTTGGAT-5′.
For Western blot analysis, LK cells were either cultured for 6 d as detailed below, then purified by Gr-1 expression, or were subjected to 100 ng/ml rmG-CSF for various times. For p-STAT-3 analysis, cells were lysed in the presence of 4 mM sodium orthovanadate (Sigma-Aldrich). Lysates were separated by 10% SDS-PAGE and transferred to a PVDF membrane (EMS Millipore, Billerica, MA, USA). Blots were blocked in TBST containing 5% BSA at 4°C overnight. Blots were probed with anti-p-STAT-3 (Tyr705) (cat. no. 9145S, dilution 1:1000), anti-STAT-3 (cat. no. 4904S, dilution 1:1000), and anti-β-actin (cat. no. 3700S, dilution 1:1000; all from Cell Signaling Technology, Danvers, MA, USA) and anti-MPO (cat. no. AF3667, dilution 1:1000; R&D Systems, Minneapolis, MN, USA) for 1 h at room temperature. All were subsequently incubated with IgG-HRP secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a 1:10,000 dilution. Visualization was performed with Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, Waltham, MA, USA) on a ChemiDoc Touch (Bio-Rad). Band intensities were quantified by densitometry analysis with ImageLab software (Bio-Rad).
Ex vivo cultivation of HSPCs
Purified HSPCs were cultured ex vivo, as follows: 105 WT (C57BL/6) LK cells, 0.1 × 105 GMPs, or 0.75 × 105 FcγRII/III+ progenitors were placed in serum-free medium (StemSpan serum-free expansion medium; Stemcell Technologies). Where indicated, recombinant ST6Gal-1 (3.4 μU/ml), CMP-Neu5Ac (50 μM), or both (see below) were included for 3 h before addition of the cytokine mixture (50 ng/ml rmSCF, 5 ng/ml IL-3, 50 ng/ml rmG-CSF, and 50 ng/ml rmM-CSF where indicated; purchased from BioVision, Milpitas, CA, USA). Liquid cultures were maintained at 37°C in 5% CO2 for 72 h.
Differentiation of hHSCs into mature neutrophils after sialylation
Human CD34+ cells from bone marrow were plated on a 24-well tissue culture plate at 0.5 × 106 cells/ml for expansion [40–42]. Cells were plated in complete Stemspan II (Stemcell Technologies) and ex vivo sialylated for 3 h. The cells were then supplemented with 50 ng/ml rhSCF and IL-3 for 2 d. The concentration of SCF and IL-3 was reduced 2-fold every 48 h thereafter until day 7, when an additional 25 ng/ml rhG-CSF was added. On days 2 and 7, cells were resialylated 3 h before the addition of new cytokines. CD11b and CD34 levels were measured every 2 d to monitor differentiation toward neutrophils. Cultured cells obtained on days 12–14 were stained with MPO, Wright-Giemsa, and combined chloroacetate esterase and nonspecific esterase (CAE-NSE) stains.
Statistics
Testing for differences between mean values was determined by ANOVA or Student’s t test, where appropriate, in Prism 6 software (Graph Pad, La Jolla, CA, USA). P < 0.05 is considered significant.
RESULTS
Animals with elevated ST6Gal-1 have depressed neutrophil counts in a lung inflammation model
We have observed that excessive neutrophilic and eosinophilic acute inflammation presents in genetically altered mice with circulating ST6Gal-1 deficiency [33, 34]. We hypothesize that boosting circulating ST6Gal-1 levels can diminish inflammation, putatively by limiting the production of new inflammatory effector cells. To test this hypothesis, a murine system with transiently elevated circulatory ST6Gal-1 was developed and implemented. We used the s.c. implantable B16-F10 melanoma cell line, a well-documented system that delivers soluble factors into systemic circulation in murine models [43, 44]. Native B16-F10 was engineered to suppress the expression of the endogenously encoded ST6Gal-1 by shRNA to generate a control B16 clone (B16-cntl) [45]. B16-cntl was further transduced with a second vector to express high levels of the rat soluble, secreted form of ST6Gal-1 (B16-S6G). For the duration of these studies, s.c. implanted B16 formed primary s.c. tumors and did not disseminate to other sites (Supplemental Materials and Methods and Supplemental Figs. 1 and 2).
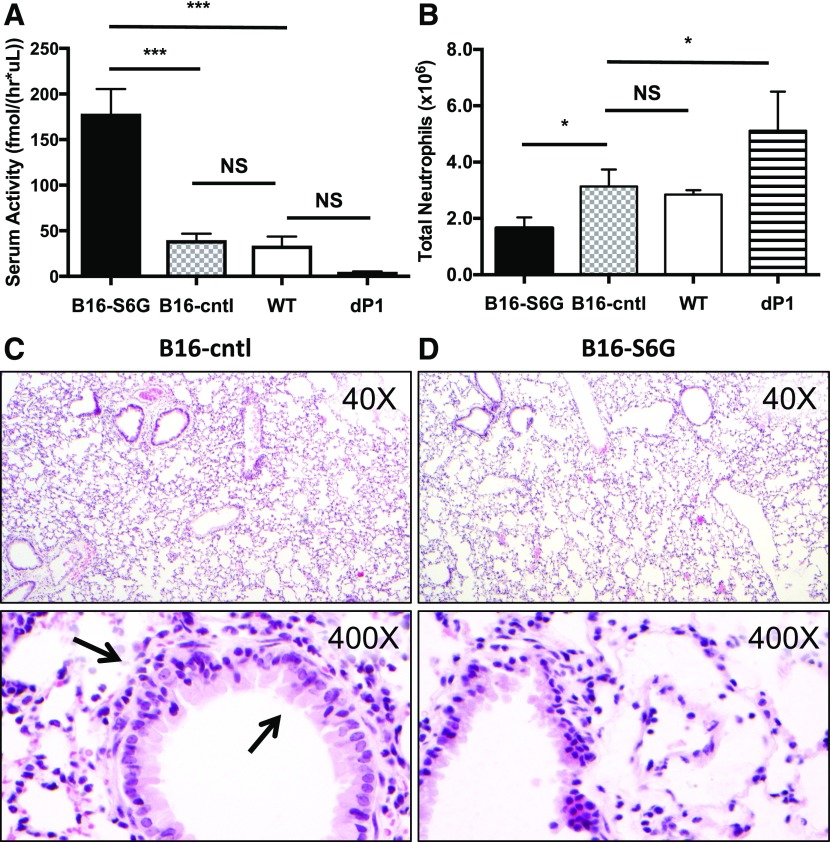
The LPS-induced model of acute lung inflammation was used to test the idea that in vivo supplementation of circulatory ST6Gal-1 will limit induced inflammation by attenuating the production of neutrophils, as intratracheal instillation of LPS elicits a pronounced influx of neutrophils into the airway [46, 47]. Mice bearing B16-S6G and B16-cntl were compared to WT and dP1 mice, a strain with a genetic deficiency resulting in low circulatory ST6Gal-1 previously shown to have increased neutrophil counts in the lung upon LPS instillation [35]. Inflammatory cell infiltration in the BALF was monitored as the primary readout, with neutrophils being the dominant cell type (>85%) in the infiltrates of all animals examined (Supplemental Fig. 3). At baseline, mice with B16-S6G had ∼4-fold greater circulatory ST6Gal-1 activity (Fig. 1A). No significant change in serum enzymatic activity was observed between WT and B16-cntl animals. dP1 values trended low and were consistent with previously reported levels, although our data did not reach statistical significance because of the small sample size [48]. Elevating circulatory ST6Gal-1 levels had a pronounced impact in suppressing LPS-induced acute airway inflammation; the B16-S6G mice had almost a 2-fold reduction in neutrophils recoverable in the BALF compared to the BALF of B16-cntl or WT lungs (Fig. 1B). The dP1 mouse—as expected because of its circulatory ST6Gal-1 deficiency—had an overabundance of neutrophil infiltration, resulting in a ∼2-fold increase in cell counts recovered in the BALF when compared with B16-S6G. Blinded pathologic evaluation by a board-certified pathologist revealed minimal airway damage from the LPS insult, presumably because of the short time (18 h) between challenge and analysis. Nevertheless, histopathology (Fig. 1C and D) revealed increased neutrophils at low power (×40) in the B16-cntl mice when compared with the B16-S6G, appearing as a diffuse and subtle thickening of alveolar walls without formation of discrete nodules. Increased cellularity was more obvious at higher power (×400) in both alveolar walls and around bronchioles. The neutrophils formed a rim around the top half of a bronchiole, with a few entering the bronchiolar epithelium (Fig. 1C).
Figure 1. Neutrophil lung infiltration and inflammation correlates inversely with circulatory ST6Gal-1.
WT, dP1, B16-cntl, and B16-S6G mice were subjected to 50 μg of LPS by oropharyngeal instillation. At 18 h, the BALF was collected and the neutrophils counted. Serum samples were collected for enzyme analysis. (A) Sialyltransferase activities in the sera were measured by following the transfer of CMP-[3H]Sia to Galβ1–4GlcNAc-O-Bn (LacNAc). The α2,6-Sia product formed by ST6Gal-1 is shown (n = 3). ***P < 0.001. (B) Total number of neutrophils recovered from the BALF of LPS-induced animals (n = 3). *P < 0.05. (C and D) B16-cntl (C) and B16-S6G (D) show a decreased number of infiltrated neutrophils at 18 h. Arrows: infiltrating neutrophils around the bronchiole and within the bronchiolar epithelium.
Elevation of systemic ST6Gal-1 levels attenuates mature neutrophil production in the marrow
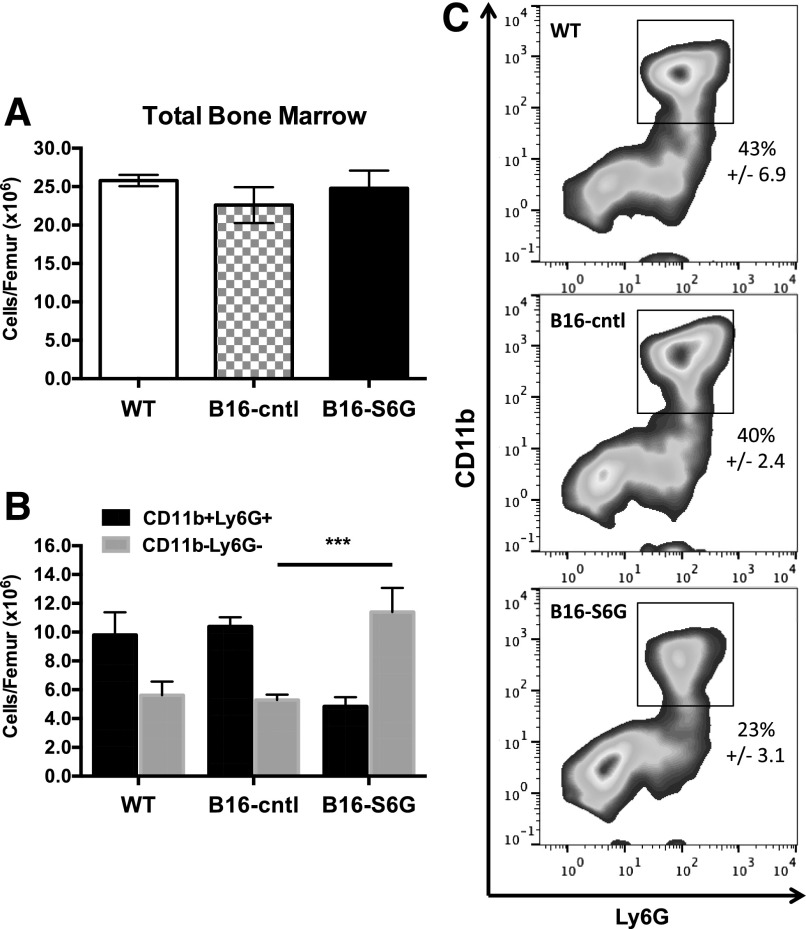
Previous observations suggested that altered recruitment is an unlikely cause of diminished inflammatory cell influx, and we therefore hypothesized that elevated blood ST6Gal-1 affects neutrophil availability [34]. No overt differences were observed in circulating blood counts between B16-S6G bearing, B16-cntl, and native WT animals (Supplemental Fig. 4), and the overall marrow cellularity was essentially unchanged (Fig. 2A). However, there was a striking depletion (by <50%) of the differentiated neutrophils (CD11b+Ly6G+) in the B16-S6G marrow (Fig. 2B and C). Presumably the depleted neutrophil pool was related to impeded neutrophil production, but perturbations to the individual hematopoietic progenitor compartments to account for this shift were not apparent between B16-S6G- and B16-cntl-bearing animals at baseline (Supplemental Table 1).
Figure 2. Depleted marrow neutrophil reservoir in B16-S6G mice.
(A) Total marrow cellularity per femur. (B) Total cells per femur found to express PMN surface phenotype (CD11b+Ly6G+) vs. non-PMNs (CD11−Ly6G−) (n = 3). ***P < 0.001. (C) Representative flow cytometry plots depicting the population shift quantified in (B).
Identification of an ST6Gal-1 checkpoint within the bipotent GMP population
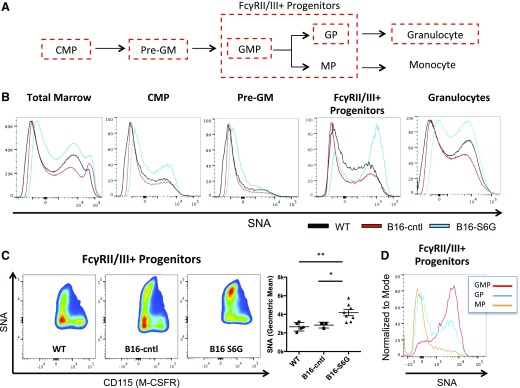
To understand how circulating ST6Gal-1 alters the replenishment of the neutrophil compartment in vivo, we sought to identify the target cell population of systemic ST6Gal-1 action. We used SNA, a lectin that recognizes the cell surface α2,6-linked sialic acids constructed by ST6Gal-1. A modified 15-color flow cytometry panel based on established phenotypic markers was used to examine the hematopoietic progenitors that eventually give rise to neutrophils [49–51]. For a complete list of populations examined, please see Materials and Methods. Of particular interest were the subpopulations within the LK pool that were the precursors of myeloid lineage development, specifically the CMPs and the Pre-GM, and FcγRII/III+ progenitors (Fig. 3A; Supplemental Figs. 5 and 6).
Figure 3. ST6Gal-1 modulates surface α2,6 sialylation levels on GPs in vivo.
WT, B16-cntl, and B16-S6G marrow was isolated, and both the α2,6 surface sialylation and the specific bone marrow subsets were analyzed by flow cytometry. (A) Lineage commitment of progenitors upstream of granulopoiesis. (B) SNA profiles of total bone marrow, CMPs, Pre-GMs, FcγRII/III+ progenitors, and mature granulocytes. (C) Quantification of SNA staining on cells expressing FcγRII/III+ progenitor markers and distribution by CD115 (M-CSFR) expression (n = 4). *P < 0.05; **P < 0.01. (D) WT marrow from the GMP, GP, and MP subsets were assessed for SNA staining.
An increase in cell surface SNA reactivity was observed in the FcγRII/III+ progenitors of the B16-S6G marrow, relative to WT and B16-cntl marrows (Fig. 3B and C). Modest increases in SNA reactivity were seen within other myeloid populations on a per-sample basis, but none reached statistical significance (Fig. 3B; Supplemental Fig. 7). Further dissection of the FcγRII/III+ progenitor compartment with recently published phenotypic markers (Supplemental Fig. 6A) revealed that the increased SNA reactivity was restricted to those cells negative for the monocyte lineage marker, CD115 (M-CSF receptor; Fig. 3C) [52]. Similarly, extensive α2,6-sialylation was detected on bipotent GMPs (LK/CD41− CD150− FcγRII/III+ CD115− Ly6C−) and GPs (LK/CD41− CD150− FcγRII/III+ CD115− Ly6C+) to a lesser degree, whereas the MPs (LK/CD41− CD150− FcγRII/III+ CD115+ Ly6C+) had little or no cell surface sialylation in WT mice at baseline (Fig. 3D). In all, the data implicate extrinsic ST6Gal-1 in the remodeling of cell surface glycans at the GMP level.
ST6Gal-1 inhibits GMP-to-GP transition, G-CSF signaling, and MPO production
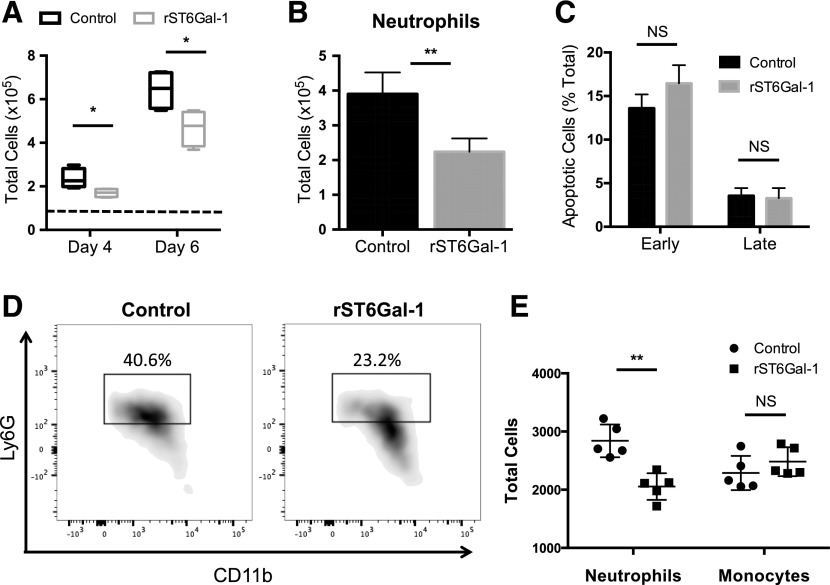
To determine whether extrinsic ST6Gal-1 impedes neutrophil production by causing death in the progenitor cells, particularly in the bipotent GMP compartment, purified WT FcγRII/III+ progenitors were placed in ex vivo culture in the presence of cytokines that induce granulocyte differentiation. The addition of soluble ST6Gal-1 resulted in both diminished overall cell proliferation and production of CD11bhiGr-1hi neutrophils (Fig. 4A and B). There was no observable difference in apoptosis (Fig. 4C), discounting cell death as a likely mechanism of action by ST6Gal-1. These observations were duplicated with purified GMPs as the starting population (Fig. 4D and E; Supplemental Fig. 8). Monocyte differentiation was unaltered by ST6Gal-1 treatment of GMPs in the presence of M-CSF, indicating specificity for granulocytic fate decisions (Fig. 4E).
Figure 4. ST6Gal-1 suppresses neutrophilic, but not monocytic, differentiation of GMPs.
FcγRII/III+ progenitors (0.75 × 105) enriched from pooled WT marrow were expanded in culture in the presence of murine G-CSF, SCF, and IL-3. ST6Gal-1-treated cells were subjected to 3 h of ex vivo sialylation before expansion. At days 4 and 6, samples were counted and analyzed by flow cytometry for lineage markers. Cells undergoing apoptosis were enumerated on d 7, with annexin V and PI staining. In a separate experiment, GMPs enriched from pooled WT marrow were expanded in culture in the presence of murine G-CSF, M-CSF, SCF, and IL-3. After 3 d, samples were analyzed by flow cytometry for lineage markers. (A) Total cells per well recovered after 4 and 6 d in culture. Dashed line: day 0 cell counts (n = 4). *P < 0.05. (B) Quantification of total CD11b+ Gr-1+ neutrophils per well recovered after 6 d in culture (n = 4). **P < 0.01. (C) Apoptotic index of treated vs. untreated cells. Early apoptotic cells were defined as annexin VhiPIlo; late apoptotic cells were defined as annexin VhiPIhi. NS, not significant. (D) Representative flow plots showing GMP neutrophil differentiation. Gate indicates CD11b+ Ly6C low Ly6G+ neutrophils. Percentages are of total cells recovered. (E) Quantification of total CD11b+ Ly6C low Ly6G+ neutrophils and CD11b+ Ly6C+ monocytes per well (n = 5). **P < 0.01.
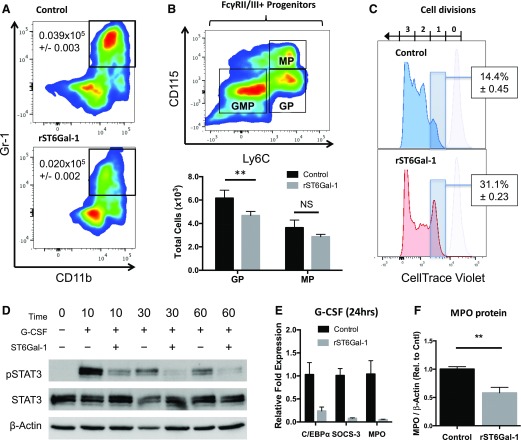
To ascertain further the mechanism of extrinsic ST6Gal-1, LK cells from WT marrow were cultured with granulocyte differentiating cytokines (SCF, G-CSF, and IL-3), and treated with soluble ST6Gal-1 or with PBS as control. Four days after introduction of soluble ST6Gal-1, the treated LK cells failed to produce as many CD11b+Gr-1+ neutrophils as control cells cultured in the absence of ST6Gal-1 (Fig. 5A). Gr-1+ cells generated in the presence of ST6Gal-1 also expressed less MPO, the primary granule protein and one of the earliest markers of terminal neutrophilic differentiation (Fig. 5F). The presence of added soluble ST6Gal-1 resulted in a suppression of GP production without altering the MP pool (Fig. 5B), indicating inhibited transition of the bipotent GMPs into committed GPs. Dye dilution experiments revealed that 85% of control LK cells completed more than 1 cell division, compared with <70% of ST6Gal-1-treated cells after 7 d in culture (Fig. 5C).
Figure 5. ST6Gal-1 inhibits G-CSF signaling and GP formation.
LK cells enriched from pooled WT marrow were expanded in culture in the presence of murine G-CSF, SCF, and IL-3. ST6Gal-1-treated cells were subjected to 3 h of ex vivo sialylation before expansion. After 4 d, samples were collected and analyzed by flow cytometry for lineage and FcγRII/III+ progenitor surface markers. Proliferation was assessed as a function of CellTrace Violet dye dilution after 7 d in culture. LK cells treated and expanded as above were purified by Gr-1 expression and analyzed by Western blot for MPO content. In a separate experiment, we measured STAT-3 phosphorylation and downstream gene expression in ST6Gal-1-treated and control LK exposed to 100 ng/ml G-CSF. (A) Quantification of total number of CD11bhiGr-1hi neutrophils per milliliter recovered after 4 d in culture (n = 6). P < 0.0001. (B) Representative plot of myeloid progenitor composition, with total GPs and MPs recovered after 4 d in culture. (C) Effects of rST6Gal-1 on cell proliferation. Light purple peaks represent initial intensity of CellTrace Violet staining. Percentages denote proportion of cells that have undergone only 1 cell division (n = 3). P < 0.0001. (D) Representative p-STAT-3 (Tyr705) blot after G-CSF treatment of LK cells. (E) Expression of several genes downstream of the G-CSF pathway was assessed by RT-qPCR analysis relative to β-2 microglobulin after 24 h cytokine treatment. (F) MPO protein content of Gr-1+ cells isolated from 7 d LK culture, normalized to β-actin. Values shown are relative to highest control sample (n = 3). **P < 0.01.
G-CSF rapidly induces a signal transduction cascade in myeloid precursors, of which STAT-3 is particularly crucial for triggering G-CSF-driven demand granulopoiesis [53, 54]. Bone marrow cells enriched for cKit+ cells demonstrated a strong induction of p-STAT-3 starting at 10 min after G-CSF treatment alone. In contrast, pretreatment of cells with rST6Gal-1 reduced STAT-3 phosphorylation by 3.5–4-fold at all time points assessed (Fig. 5D). Furthermore, ST6Gal-1-treated cells down-regulated the expression of G-CSF-responsive genes and myeloid transcription factors 24 h after G-CSF exposure, including Cebpa, Socs3, and Mpo (Fig. 5E) [55, 56]. Cebpb expression was not considerably altered by ST6Gal-1 treatment at this time point (data not shown).
Attenuated granulocytic development from human CD34+ cells by extrinsic ST6Gal-1
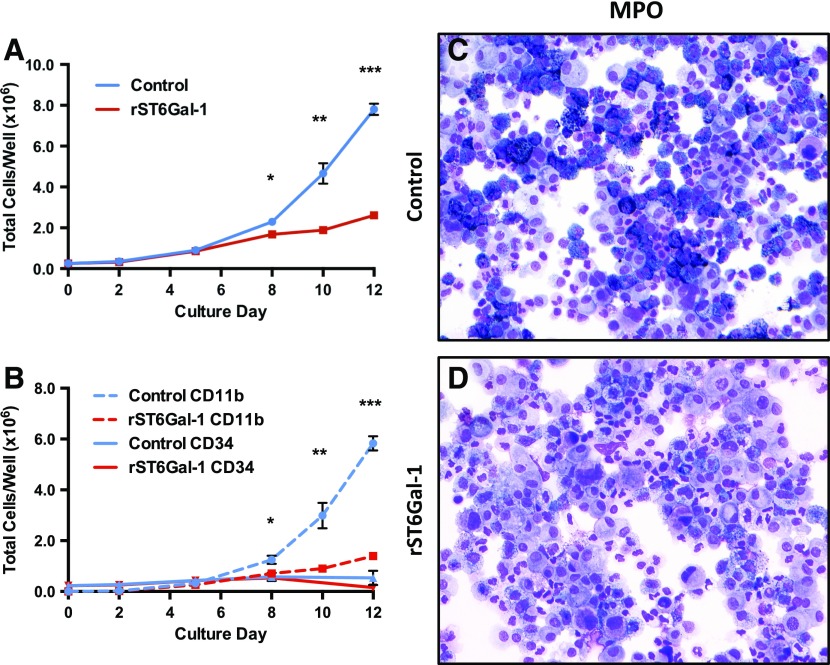
Human bone marrow CD34+ cells, which are functional HSPCs, were induced ex vivo to undergo proliferation and differentiation into myeloid effector lineages upon stimulation by a regiment of SCF, IL-3, and G-CSF. This process was monitored by cell count and the appearance of CD11b, a surface integrin strongly associated in humans with myeloid development past the promyelocyte stage [57]. The addition of recombinant, soluble ST6Gal-1 into the culture resulted in 4-fold fewer total cells and 5.6-fold fewer CD11b+ cells after 13 d in culture, but did not strikingly alter the number of remaining CD34+ cells (Fig. 6A and B). By day 12, CD11b+ cells comprised >75% of the sham-treated sample but only 50% of the ST6Gal-1-treated sample. CD34+ cells were <7% of the total cells in both samples (Supplemental Fig. 9A).
Figure 6. ST6Gal-1 suppresses differentiation of human CD34+ cells in vitro.
Mobilized human CD34+ bone marrow was plated at a density of 0.5 × 106 cells/ml and expanded in vitro in the presence of rhSCF, IL-3, and G-CSF. Before expansion, ST6Gal-1-treated cells were subjected to 3 h of ex vivo sialylation. Aliquots were removed approximately every 2 d and analyzed for cellularity and surface phenotype. (A) Total cellularity in wells receiving rST6Gal-1 vs. control (n = 5). (B) Total cells per well found to express either CD11b or CD34 surface markers as assessed by flow cytometry (n = 5). *P < 0.05; **P < 0.01; ***P < 0.001. (C and D) MPO-stained cytospins of cells collected at the termination of the experiment.
There was an overall diminished intensity of MPO, the hallmark of terminal neutrophilic differentiation, in the presence of ST6Gal-1 (Fig. 6C and D). Because the expression of human MPO is largely restricted to early promyelocytes, the result further validates the role of ST6Gal-1 observed in murine progenitors [57, 58]. Blinded reading by a board-certified pathologist revealed a concomitant 4-fold increased incidence of blasts, along with diminished frequency (from 40 to 25%) of cells staining positively for MPO. A representative field of Wright-Giemsa-stained samples used for the reading is shown in Supplemental Fig. 9B and C. Staining for NSE, a marker for myeloid monocytic differentiation, revealed diffuse staining with no qualitative or quantitative differences between control and ST6Gal-1-treated samples (Supplemental Fig. 9D and E). Together, these observations demonstrate a role for circulatory ST6Gal-1 in regulating early granulocytic development.
DISCUSSION
The presence of ST6Gal-1 and other glycosyltransferases in systemic circulation was noted as early as the 1980s, but their functions in the extracellular milieu were never clear. Elevated release of ST6Gal-1 into the blood is a component of the hepatic acute phase response [59, 60]. Genetically altered deficiency in circulatory ST6Gal-1 led to an overly exuberant response to inflammatory challenges that was attributed to excessive production of inflammatory leukocytes [33–36], leading to the idea that manipulating ST6Gal-1 levels in the blood may have value in controlling the availability of inflammatory cells for clinically desirable outcomes. We used an s.c. implantable B16-F10 melanoma system to affect a 5-fold elevation of circulatory ST6Gal-1 levels. Although depleted neutrophil reserves were observed in the marrow, there were no alterations to the baseline number of marrow progenitors or circulating blood counts. Likely, multiple overlapping signaling pathways collaborate to maintain blood homeostasis and multipotent progenitor populations to obscure the effects of ST6Gal-1 at baseline. However, during an event with an acute demand for inflammatory cells, raising systemic ST6Gal-1 levels was effective in imposing a check on new neutrophil production. Together, these observations suggest a role for circulatory ST6Gal-1 in modulating new neutrophil production during emergency-driven demand granulopoiesis and a lesser role in the maintenance of the baseline number of neutrophils.
Recent evidence linking the preservation of pluripotency in human pluripotent stem cells with α2,6-sialylation suggests that ST6Gal-1 has an important role in the suppression of differentiation [14]. We hypothesized that cells at the GMP stage of hematopoietic development were particularly susceptible to extrinsic ST6Gal-1, leading to attenuated differentiation/proliferation of GMPs and decreased granulopoiesis. The data show that FcγRII/III+ progenitors were a specific HSPC target of cell surface sialylation by extrinsic ST6Gal-1, suggesting a causal relationship between increased sialylation within this population and the depleted neutrophil reservoir in the B16-S6G mice. In vitro data demonstrating that purified FcγRII/III+ progenitors and GMPs failed to produce as many neutrophils after treatment with ST6Gal-1 corroborates this observation. M-CSFR low FcγRII/III+ progenitors underwent the largest changes in SNA staining in our overexpression model compared to baseline, and most α2,6 sialylation in WT FcγRII/III+ progenitors was found on GMPs.
Exposure to extrinsic ST6Gal-1 blunted the capacity to differentiate into granulocytic lineage in response to cytokines, as indicated by diminished acquisition of the neutrophil markers Gr-1 and MPO. Monocyte production by GMPs was unaffected. Differentiation of GMPs into committed granulocyte progenitors is largely driven by the engagement of the cytokine G-CSF to its cognate receptor to initiate the STAT-3-driven intracellular signaling cascade [8, 53, 54]. The data show the immediate blunting of STAT-3 phosphorylation by extrinsic ST6Gal-1, followed by diminished expression of downstream Cebpa and Socs3. C/EBPα cooperates with PU.1 to active MPO expression, and we observed the blunted expression of both Cebpa and Mpo [56]. Similar to our own observations, a moderate (3-fold) knockdown of Cebpa was sufficient to abrogate granulopoiesis without compromising monocyte production at the GMP stage and beyond [61]. Expression of SOCS3, another transcription factor frequently identified as downstream in the STAT-3 cascade, was likewise suppressed [62]. Taken together, the data infer strongly that extrinsic ST6Gal-1 interferes with the G-CSF signaling in GMPs, thereby interrupting the STAT-3 driven cascade that orchestrates the instructive cues for granulopoietic differentiation.
The idea that cell surface sialylation can affect signal transduction of cell surface receptors has precedence. The Siglec CD22 is a well-known inhibitor of BCR signaling via its cytosolic ITIM. The mechanism of signal inhibition appears to rely on the recognition by CD22 of α2,6-sialic acid ligands present on the B-cell antigen receptor [63]. Competitive inhibition of this interaction, mimicking loss of BCR sialylation, resulted in increased calcium response after anti-IgM stimulation [64]. Cytokine signaling may be similarly affected, as sialylation of epidermal growth factor receptor inhibited downstream signaling by attenuating receptor dimerization and possibly ligand binding [65]. The novelty in the current report is that extracellular ST6Gal-1, produced from distal sources and released into the extracellular milieu, acts by extrinsic modification of GMP level hematopoietic progenitors to regulate granulopoiesis.
The traditional view of glycosylation is that of an exclusively cell-autonomous event mediated by intrinsic expression of glycosyltransferases modifying nascent glycoconjugates as they transit the intracellular secretory apparatus. However, it has been known for some time that α2,6-sialylation cannot always be explained by the presence of cell-intrinsic ST6Gal-1 [66]. Extensive surface α2,6-sialylation on marrow HSPC populations lacking native expression of ST6Gal-1 sialyltransferase indicated that our understanding of glycoconjugate construction was incomplete [36]. In other studies, we proposed that extracellular ST6Gal-1 could remodel glycans on target cell surfaces in a novel, non–cell-autonomous, extrinsic process [32, 36]. This represents a significant departure from the traditional cell-autonomous paradigm of Golgi-ER-mediated glycosylation.
One potential translational application of these studies is that manipulating blood ST6Gal-1 levels may have therapeutic value in the management of inflammatory conditions. The benefit may extend to other conditions affecting granulopoiesis, such as in severe myeloablative procedures when more rapid recovery of neutrophil numbers can be highly desirable. This approach leverages the natural function of a naturally occurring circulatory enzyme, and targets the novel axis of extrinsic glycosylation.
AUTHORSHIP
C.W.L.D. designed the research, performed the experiments, and wrote the paper; A.B. designed the research, performed the experiments, and wrote the paper; M.J.N. interpreted the data; M.N. designed the research; E.E.I. performed the experiments; P.N.B. interpreted the data; and J.T.Y.L. designed the research, coordinated project activities, and wrote the paper.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in whole or in part by U.S. National Institutes of Health (NIH), National Heart and Lung Institute Grant P01HL107146 and NIH, National Institute of Allergy and Infectious Diseases Grant R01AI056082 (to J.T.Y.L.). The core facilities of Roswell Park Cancer Institute used in this work were supported in part by NIH, National Cancer Institute Cancer Center Support Grant CA15056.
Glossary
- B16-cntl
mice implanted with B16-F10 endogenous ST6Gal-1 knockdown
- B16-S6G
mice implanted with B16-cntl further modified with rat ST6Gal-1 vector
- BALF
bronchial alveolar lavage fluid
- CAE-NSE
chloroacetate esterase and nonspecific esterase
- CD
cluster of differentiation
- CMP
common myeloid progenitor
- dP1
St6gal1-dP1 mouse
- GMP
granulocyte–monocyte progenitor
- GP
granulocyte progenitor
- hHSC
human hemopoietic stem cell
- HSPC
hematopoietic stem and progenitor cell
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- LK
Lin−Sca-1−c-Kit+
- M-CSFR
M-CSF receptor
- MkP
megakaryocyte progenitor
- MP
monocyte progenitor
- MPO
myeloperoxidase
- NSE
nonspecific esterase
- Pre-GMP
pregranulocyte monocyte progenitor
- rh
recombinant human
- rm
recombinant murine
- SCF
stem cell factor
- shRNA
short hairpin RNA
- SNA
Sambucus nigra lectin
- WT
C57BL/6 wild-type mouse
Footnotes
The online version of this paper, found at www.jleukbio.org, contains supplemental information.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Malech H. L., Nauseef W. M. (1997) Primary inherited defects in neutrophil function: etiology and treatment. Semin. Hematol. 34, 279–290. [PubMed] [Google Scholar]
- 2.Hoenderdos K., Condliffe A. (2013) The neutrophil in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 48, 531–539. [DOI] [PubMed] [Google Scholar]
- 3.Segel G. B., Halterman M. W., Lichtman M. A. (2011) The paradox of the neutrophil’s role in tissue injury. J. Leukoc. Biol. 89, 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright H. L., Moots R. J., Bucknall R. C., Edwards S. W. (2010) Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 49, 1618–1631. [DOI] [PubMed] [Google Scholar]
- 5.Lim K., Hyun Y. M., Lambert-Emo K., Capece T., Bae S., Miller R., Topham D. J., Kim M. (2015) Neutrophil trails guide influenza-specific CD8+ T cells in the airways. Science 349, aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassatella M. A. (1999) Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 73, 369–509. [DOI] [PubMed] [Google Scholar]
- 7.Scapini P., Lapinet-Vera J. A., Gasperini S., Calzetti F., Bazzoni F., Cassatella M. A. (2000) The neutrophil as a cellular source of chemokines. Immunol. Rev. 177, 195–203. [DOI] [PubMed] [Google Scholar]
- 8.Rieger M. A., Hoppe P. S., Smejkal B. M., Eitelhuber A. C., Schroeder T. (2009) Hematopoietic cytokines can instruct lineage choice. Science 325, 217–218. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C. C., Lodish H. F. (2008) Cytokines regulating hematopoietic stem cell function. Curr. Opin. Hematol. 15, 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marth J. D., Grewal P. K. (2008) Mammalian glycosylation in immunity. Nat. Rev. Immunol. 8, 874–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkler I. G., Barbier V., Nowlan B., Jacobsen R. N., Forristal C. E., Patton J. T., Magnani J. L., Lévesque J. P. (2012) Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat. Med. 18, 1651–1657. [DOI] [PubMed] [Google Scholar]
- 12.Alisson-Silva F., de Carvalho Rodrigues D., Vairo L., Asensi K. D., Vasconcelos-dos-Santos A., Mantuano N. R., Dias W. B., Rondinelli E., Goldenberg R. C., Urmenyi T. P., Todeschini A. R. (2014) Evidences for the involvement of cell surface glycans in stem cell pluripotency and differentiation. Glycobiology 24, 458–468. [DOI] [PubMed] [Google Scholar]
- 13.Sackstein R. (2012) Glycoengineering of HCELL, the human bone marrow homing receptor: sweetly programming cell migration. Ann. Biomed. Eng. 40, 766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y. C., Stein J. W., Lynch C. L., Tran H. T., Lee C. Y., Coleman R., Hatch A., Antontsev V. G., Chy H. S., O’Brien C. M., Murthy S. K., Laslett A. L., Peterson S. E., Loring J. F. (2015) Glycosyltransferase ST6GAL1 contributes to the regulation of pluripotency in human pluripotent stem cells. Sci. Rep. 5, 13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee M., Lee H. J., Bae S., Lee Y. S. (2008) Protein sialylation by sialyltransferase involves radiation resistance. Mol. Cancer Res. 6, 1316–1325. [DOI] [PubMed] [Google Scholar]
- 16.Schultz M. J., Swindall A. F., Wright J. W., Sztul E. S., Landen C. N., Bellis S. L. (2013) ST6Gal-I sialyltransferase confers cisplatin resistance in ovarian tumor cells. J. Ovarian Res. 6, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultz M. J., Holdbrooks A. T., Chakraborty A., Grizzle W. E., Landen C. N., Buchsbaum D. J., Conner M. G., Arend R. C., Yoon K. J., Klug C. A., Bullard D. C., Kesterson R. A., Oliver P. G., O’Connor A. K., Yoder B. K., Bellis S. L. (2016) The tumor-associated glycosyltransferase ST6Gal-I regulates stem cell transcription factors and confers a cancer stem cell phenotype. Cancer Res. 76, 3978–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dabelic S., Flögel M., Maravić G., Lauc G. (2004) Stress causes tissue-specific changes in the sialyltransferase activity. Z. Naturforsch., C, J. Biosci. 59, 276–280. [DOI] [PubMed] [Google Scholar]
- 19.Sage A. P., Mallat Z. (2014) Sialyltransferase activity and atherosclerosis. Circ. Res. 114, 935–937. [DOI] [PubMed] [Google Scholar]
- 20.Gracheva E. V., Samovilova N. N., Golovanova N. K., Il’inskaya O. P., Tararak E. M., Malyshev P. P., Kukharchuk V. V., Prokazova N. V. (2002) Sialyltransferase activity of human plasma and aortic intima is enhanced in atherosclerosis. Biochim. Biophys. Acta 1586, 123–128. [DOI] [PubMed] [Google Scholar]
- 21.Stibler H., Borg S. (1991) Glycoprotein glycosyltransferase activities in serum in alcohol-abusing patients and healthy controls. Scand. J. Clin. Lab. Invest. 51, 43–51. [PubMed] [Google Scholar]
- 22.Gong M., Castillo L., Redman R. S., Garige M., Hirsch K., Azuine M., Amdur R. L., Seth D., Haber P. S., Lakshman M. R. (2008) Down-regulation of liver Galbeta1, 4GlcNAc alpha2, 6-sialyltransferase gene by ethanol significantly correlates with alcoholic steatosis in humans. Metabolism 57, 1663–1668. [DOI] [PubMed] [Google Scholar]
- 23.Lu J., Isaji T., Im S., Fukuda T., Hashii N., Takakura D., Kawasaki N., Gu J. (2014) β-Galactoside α2,6-sialyltranferase 1 promotes transforming growth factor-β-mediated epithelial-mesenchymal transition. J. Biol. Chem. 289, 34627–34641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu J., Gu J. (2015) Significance of beta-galactoside alpha2,6 sialyltranferase 1 in cancers. Molecules 20, 7509–7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park J. J., Lee M. (2013) Increasing the α 2, 6 sialylation of glycoproteins may contribute to metastatic spread and therapeutic resistance in colorectal cancer. Gut Liver 7, 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiser M. M., Wilson J. R. (1981) Serum levels of glycosyltransferases and related glycoproteins as indicators of cancer: biological and clinical implications. Crit. Rev. Clin. Lab. Sci. 14, 189–239. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan H. A., Woloski B. M., Hellman M., Jamieson J. C. (1983) Studies on the effect of inflammation on rat liver and serum sialyltransferase. Evidence that inflammation causes release of Gal beta 1 leads to 4GlcNAc alpha 2 leads to 6 sialyltransferase from liver. J. Biol. Chem. 258, 11505–11509. [PubMed] [Google Scholar]
- 28.Lammers G., Jamieson J. C. (1986) Studies on the effect of experimental inflammation on sialyltransferase in the mouse and guinea pig. Comp. Biochem. Physiol. B 84, 181–187. [DOI] [PubMed] [Google Scholar]
- 29.Thorne-Tjomsland G., Hosfield T., Jamieson J. C., Liu B., Nickerson P., Gough J. C., Rush D. N., Jeffery J. R., McKenna R. M. (2000) Increased levels of GALbeta1-4GLCNACalpha2-6 sialyltransferase pretransplant predict delayed graft function in kidney transplant recipients. Transplantation 69, 806–808. [DOI] [PubMed] [Google Scholar]
- 30.Gessner P., Riedl S., Quentmaier A., Kemmner W. (1993) Enhanced activity of CMP-neuAc:Gal beta 1-4GlcNAc:alpha 2,6-sialyltransferase in metastasizing human colorectal tumor tissue and serum of tumor patients. Cancer Lett. 75, 143–149. [DOI] [PubMed] [Google Scholar]
- 31.Kessel D., Allen J. (1975) Elevated plasma sialyltransferase in the cancer patient. Cancer Res. 35, 670–672. [PubMed] [Google Scholar]
- 32.Lee M. M., Nasirikenari M., Manhardt C. T., Ashline D. J., Hanneman A. J., Reinhold V. N., Lau J. T. (2014) Platelets support extracellular sialylation by supplying the sugar donor substrate. J. Biol. Chem. 289, 8742–8748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nasirikenari M., Chandrasekaran E. V., Matta K. L., Segal B. H., Bogner P. N., Lugade A. A., Thanavala Y., Lee J. J., Lau J. T. (2010) Altered eosinophil profile in mice with ST6Gal-1 deficiency: an additional role for ST6Gal-1 generated by the P1 promoter in regulating allergic inflammation. J. Leukoc. Biol. 87, 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nasirikenari M., Segal B. H., Ostberg J. R., Urbasic A., Lau J. T. (2006) Altered granulopoietic profile and exaggerated acute neutrophilic inflammation in mice with targeted deficiency in the sialyltransferase ST6Gal I. Blood 108, 3397–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Appenheimer M. M., Huang R. Y., Chandrasekaran E. V., Dalziel M., Hu Y. P., Soloway P. D., Wuensch S. A., Matta K. L., Lau J. T. (2003) Biologic contribution of P1 promoter-mediated expression of ST6Gal I sialyltransferase. Glycobiology 13, 591–600. [DOI] [PubMed] [Google Scholar]
- 36.Nasirikenari M., Veillon L., Collins C. C., Azadi P., Lau J. T. (2014) Remodeling of marrow hematopoietic stem and progenitor cells by non-self ST6Gal-1 sialyltransferase. J. Biol. Chem. 289, 7178–7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Y. P., Dalziel M., Lau J. T. (1997) Murine hepatic beta-galactoside alpha 2,6-sialyltransferase gene expression involves usage of a novel upstream exon region. Glycoconj. J. 14, 407–411. [DOI] [PubMed] [Google Scholar]
- 38.Buffone A. Jr., Nasirikenari M., Manhardt C. T., Lugade A., Bogner P. N., Sackstein R., Thanavala Y., Neelamegham S., Lau J. T. (2017) Leukocyte-borne α(1,3)-fucose is a negative regulator of β2-integrin-dependent recruitment in lung inflammation. J. Leukoc. Biol. 101, 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee-Sundlov M. M., Ashline D. J., Hanneman A. J., Grozovsky R., Reinhold V. N., Hoffmeister K. M., Lau J. T. (2017) Circulating blood and platelets supply glycosyltransferases that enable extrinsic extracellular glycosylation. Glycobiology. 27, 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mondal N., Buffone A. Jr., Stolfa G., Antonopoulos A., Lau J. T., Haslam S. M., Dell A., Neelamegham S. (2015) ST3Gal-4 is the primary sialyltransferase regulating the synthesis of E-, P-, and L-selectin ligands on human myeloid leukocytes. Blood 125, 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hino M., Suzuki K., Yamane T., Sakai N., Kubota H., Koh K. R., Ohta K., Hato F., Kitagawa S., Tatsumi N. (2000) Ex vivo expansion of mature human neutrophils with normal functions from purified peripheral blood CD34+ haematopoietic progenitor cells. Br. J. Haematol. 109, 314–321. [DOI] [PubMed] [Google Scholar]
- 42.Mondal N., Stolfa G., Antonopoulos A., Zhu Y., Wang S. S., Buffone A. Jr., Atilla-Gokcumen G. E., Haslam S. M., Dell A., Neelamegham S. (2016) Glycosphingolipids on human myeloid cells stabilize E-selectin-dependent rolling in the multistep leukocyte adhesion cascade. Arterioscler. Thromb. Vasc. Biol. 36, 718–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simmons A. D., Li B., Gonzalez-Edick M., Lin C., Moskalenko M., Du T., Creson J., VanRoey M. J., Jooss K. (2007) GM-CSF-secreting cancer immunotherapies: preclinical analysis of the mechanism of action. Cancer Immunol. Immunother. 56, 1653–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curran M. A., Allison J. P. (2009) Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res. 69, 7747–7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buffone A. Jr, Mondal N., Gupta R., McHugh K. P., Lau J. T. Y., Neelamegham S. (2013) Silencing α1,3-fucosyltransferases in human leukocytes reveals a role for FUT9 enzyme during E-selectin-mediated cell adhesion. J. Biol. Chem. 288, 1620–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reutershan J., Basit A., Galkina E. V., Ley K. (2005) Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L807–L815. [DOI] [PubMed] [Google Scholar]
- 47.Beck-Schimmer B., Schwendener R., Pasch T., Reyes L., Booy C., Schimmer R. C. (2005) Alveolar macrophages regulate neutrophil recruitment in endotoxin-induced lung injury. Respir. Res. 6, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones M. B., Nasirikenari M., Feng L., Migliore M. T., Choi K. S., Kazim L., Lau J. T. (2010) Role for hepatic and circulatory ST6Gal-1 sialyltransferase in regulating myelopoiesis. J. Biol. Chem. 285, 25009–25017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson A., Laurenti E., Oser G., van der Wath R. C., Blanco-Bose W., Jaworski M., Offner S., Dunant C. F., Eshkind L., Bockamp E., Lió P., Macdonald H. R., Trumpp A. (2008) Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129. [DOI] [PubMed] [Google Scholar]
- 50.Oguro H., Ding L., Morrison S. J. (2013) SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell 13, 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pronk C. J., Rossi D. J., Månsson R., Attema J. L., Norddahl G. L., Chan C. K., Sigvardsson M., Weissman I. L., Bryder D. (2007) Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell 1, 428–442. [DOI] [PubMed] [Google Scholar]
- 52.Yáñez A., Ng M. Y., Hassanzadeh-Kiabi N., Goodridge H. S. (2015) IRF8 acts in lineage-committed rather than oligopotent progenitors to control neutrophil vs monocyte production. Blood 125, 1452–1459. [DOI] [PubMed] [Google Scholar]
- 53.Panopoulos A. D., Zhang L., Snow J. W., Jones D. M., Smith A. M., El Kasmi K. C., Liu F., Goldsmith M. A., Link D. C., Murray P. J., Watowich S. S. (2006) STAT3 governs distinct pathways in emergency granulopoiesis and mature neutrophils. Blood 108, 3682–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H., Nguyen-Jackson H., Panopoulos A. D., Li H. S., Murray P. J., Watowich S. S. (2010) STAT3 controls myeloid progenitor growth during emergency granulopoiesis. Blood 116, 2462–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panopoulos A. D., Watowich S. S. (2008) Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine 42, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang W., Wang X., Ward A. C., Touw I. P., Friedman A. D. (2001) C/EBPalpha and G-CSF receptor signals cooperate to induce the myeloperoxidase and neutrophil elastase genes. Leukemia 15, 779–786. [DOI] [PubMed] [Google Scholar]
- 57.Mora-Jensen H., Jendholm J., Fossum A., Porse B., Borregaard N., Theilgaard-Mönch K. (2011) Technical advance: immunophenotypical characterization of human neutrophil differentiation. J. Leukoc. Biol. 90, 629–634. [DOI] [PubMed] [Google Scholar]
- 58.Theilgaard-Mönch K., Jacobsen L. C., Borup R., Rasmussen T., Bjerregaard M. D., Nielsen F. C., Cowland J. B., Borregaard N. (2005) The transcriptional program of terminal granulocytic differentiation. Blood 105, 1785–1796. [DOI] [PubMed] [Google Scholar]
- 59.Jamieson J. C., McCaffrey G., Harder P. G. (1993) Sialyltransferase: a novel acute-phase reactant. Comp. Biochem. Physiol. B 105, 29–33. [DOI] [PubMed] [Google Scholar]
- 60.Baumann H., Gauldie J. (1994) The acute phase response. Immunol. Today 15, 74–80. [DOI] [PubMed] [Google Scholar]
- 61.Ma O., Hong S., Guo H., Ghiaur G., Friedman A. D. (2014) Granulopoiesis requires increased C/EBPα compared to monopoiesis, correlated with elevated Cebpa in immature G-CSF receptor versus M-CSF receptor expressing cells. PLoS One 9, e95784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hörtner M., Nielsch U., Mayr L. M., Johnston J. A., Heinrich P. C., Haan S. (2002) Suppressor of cytokine signaling-3 is recruited to the activated granulocyte-colony stimulating factor receptor and modulates its signal transduction. J. Immunol. 169, 1219–1227. [DOI] [PubMed] [Google Scholar]
- 63.Nitschke L., Tsubata T. (2004) Molecular interactions regulate BCR signal inhibition by CD22 and CD72. Trends Immunol. 25, 543–550. [DOI] [PubMed] [Google Scholar]
- 64.Kelm S., Gerlach J., Brossmer R., Danzer C. P., Nitschke L. (2002) The ligand-binding domain of CD22 is needed for inhibition of the B cell receptor signal, as demonstrated by a novel human CD22-specific inhibitor compound. J. Exp. Med. 195, 1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yen H. Y., Liu Y. C., Chen N. Y., Tsai C. F., Wang Y. T., Chen Y. J., Hsu T. L., Yang P. C., Wong C. H. (2015) Effect of sialylation on EGFR phosphorylation and resistance to tyrosine kinase inhibition. Proc. Natl. Acad. Sci. USA 112, 6955–6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaneko Y., Yamamoto H., Colley K. J., Moskal J. R. (1995) Expression of Gal beta 1,4GlcNAc alpha 2,6-sialyltransferase and alpha 2,6-linked sialoglycoconjugates in normal human and rat tissues. J. Histochem. Cytochem. 43, 945–954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.