Review on the contribution of the MK/PTN family to lymphocyte survival, myeloid cell phenotype, immune cell chemotaxis, and hematopoiesis.
Keywords: hematopoiesis, myeloid cells, lymphoid cells, cytokines
Abstract
Cytokines are pivotal in the generation and resolution of the inflammatory response. The midkine/pleiotrophin (MK/PTN) family of cytokines, composed of just two members, was discovered as heparin-binding neurite outgrowth-promoting factors. Since their discovery, expression of this cytokine family has been reported in a wide array of inflammatory diseases and cancer. In this minireview, we will discuss the emerging appreciation of the functions of the MK/PTN family in the immune system, which include promoting lymphocyte survival, sculpting myeloid cell phenotype, driving immune cell chemotaxis, and maintaining hematopoiesis.
Introduction
MK and PTN comprise a two-member family of heparin-binding cytokines. MK and PTN are roughly 50% homologous in amino acid sequence in mammals [1]. Mouse MK shares 87% homology in amino acid sequence with human MK, whereas human PTN shares 97% amino acid sequence identity to mouse PTN and shares 72% amino acid homology with Xenopus laevis [2]. MK was first identified as a highly expressed factor produced during the early stage of retinoic acid–driven differentiation of embryonal carcinoma cells [3]. In 1991, it was independently discovered as a growth factor in the bovine brain, which promoted the proliferation of bovine brain capillary cells in vitro [4]. PTN was first discovered as a heparin-binding, soluble protein present in the developing rat brain, which promoted neurite outgrowth in vitro [5]. In 1992, it was separately identified as a secreted growth factor produced by human breast cancer cells [6].
Fitting their respective scientific origins, much of the focus on the MK/PTN family has been on the function of MK/PTN in angiogenesis, the central nervous system, and cancer. Although PTN and MK are expressed in many different inflammatory diseases, little is known about their function in that regard. In this review, we will discuss the expression patterns of the MK/PTN family, including their putative receptors and what is currently known about their function in hematopoiesis, inflammation, and immunity.
MK/PTN: GENERAL EXPRESSION PATTERNS
MK and PTN are expressed widely during development, yet mice deficient in either MK or PTN display no overt developmental defects [7]. However, MK and PTN DKO mice exhibit a gene-dosage effect. Mice null for PTN or MK are born at expected mendelian ratios, whereas DKO mice are born at 33% of the expected ratio [7]. DKO mice also have a lower average weight and fail to gain significant weight on a high-calorie diet. In DKO males, 100% of individuals are fertile; conversely, 79% of females are sterile, which may be due to abnormal follicular maturation in ovaries and vaginal malformation. For more information on the function and expression of MK and PTN in development, see Kadomatsu et al. [8].
The expression of MK and PTN in healthy adult mice is restricted. In adults, significant PTN expression is limited largely to the central nervous system, with detectable, low-level expression in the breast epithelium, placental tissue, testis, bladder, and stomach [9, 10]. PTN is also expressed by vascular endothelial cells of the hematopoietic stem cell niche [11]. Conversely, MK is expressed in the kidney [12], gut [13], epidermis [14], and bronchial epithelium [15]. Interestingly, PTN is up-regulated significantly in multiple tissues of MK-deficient mice, but there are no detectable differences in MK expression in PTN-null mice [16]. These results suggest that PTN may compensate for the loss of MK, but the converse does not hold true.
MK/PTN FAMILY RECEPTORS
Robust heparin binding was one of the first characteristics identified in the MK/PTN family [5, 17]; thus, it comes as no surprise that the first receptors identified for MK/PTN were heparin- and chondroitin-sulfate proteoglycans. MK and PTN bind cell-surface proteoglycans, including Sdc1–4 and RPTP-β, with similar affinities. Sdc1 is primarily expressed on epithelial and malignant plasma cells, Sdc2 is expressed by fibroblasts and endothelial cells, and Sdc3 is found almost exclusively in the central nervous system, whereas Sdc4 is expressed more widely [18]. However, similar to MK and PTN, the expression of the syndecans is also modulated by tissue damage and inflammation [19–22]. In immune cells, the syndecans are mediators of migration, activation, and cell survival [20, 22–36] (see Table 1) and are expressed broadly. The only well-characterized MK/PTN/Sdc interaction is between PTN and Sdc3. Ligation of Sdc3 by PTN promotes neurite outgrowth and cell migration [37, 38] (Fig. 1). However, the biologic contexts and consequences for specific MK/PTN/Sdc interactions are largely unknown.
TABLE 1.
Expression and reported functions of MK/PTN receptors on immune cells
| Receptor | Immune cell expression pattern | Comments |
|---|---|---|
| Syndecan-1 | Expressed by immature DCs, pre-B cells, plasma cells, and thioglycollate-elicited peritoneal macrophages [23, 24, 29] | Promotes macrophage migration; expressed on macrophages in myocardial infarction [25, 35] |
| Sdc1-null mice are more susceptible to LPS-driven lethality [32] | ||
| Syndecan-2 | Monocytes and macrophages, DCs, activated T cells [20, 28, 29, 31, 34] | Expression induced by LPS, IL-1α, or TNF-α on macrophages [20] |
| Found on synovial fluid macrophages from rheumatoid arthritis patients [20] | ||
| Delayed expression on activated T cells [31, 34] | ||
| Syndecan-3 | Peritoneal and splenic macrophages [33] | Sdc3-null mice have reduced leukocyte infiltration in a preclinical model of rheumatoid arthritis [35] |
| May also be expressed by monocytes and DCs at low levels [29] | ||
| Syndecan-4 | Expressed on DCs, monocytes, and macrophages [27–29] | Down-regulated on B cells undergoing isotype switching [26] |
| Expressed by pre-B cells and most splenic macrophages [26] | Sdc4-null mice are more susceptible to LPS-mediated lethality [22] | |
| Rapidly induced on activated T cells [31, 34] | Binding to DC-HIL at the immunologic synapse perturbs T cell activation [30] | |
| Expressed on a small subset (∼10%) of splenic T cells [26] | Neutrophil migration [27] | |
| May regulate T cell proliferation [31] | ||
| RPTP-β | Found on HSCs and B cells [11, 42] | Promotes HSC maintenance and B cell survival [11, 42] |
| Lrp1 | Macrophages, neutrophils [60, 107] | Promotes PMN adhesion [107] |
| Attenuates proinflammatory macrophage activation [60] | ||
| Nucleolin | Ubiquitously expressed [65] | May be involved in macrophage recognition of early apoptotic cells [66] |
| α4β1 | T cells, B cells, DCs, monocytes, macrophages, NK cells, neutrophils, eosinophils, basophils [55, 57] | Implicated in migration; involved in B and T cell development [52] |
| α6β1 | Lymphocytes, monocytes and macrophages, granulocytes, neutrophils, eosinophils [56] | Adhesion and migration [56] |
| αvβ3 | Monocytes, macrophages, DCs, neutrophils [54] | Implicated in migration and phagocytosis [53, 54] |
Listed are the purported receptors of the MK/PTN family, the expression of those receptors on immune cells, and comments about the known functions of those receptors in immunity.
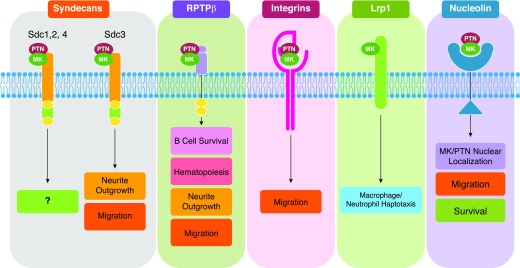
Figure 1. Known functions of MK/PTN signaling through their receptors.
Syndecans: Although MK/PTN binds to Sdc 1, 2, and 4, the downstream effectors are unknown. PTN binding to Sdc3 promotes neurite outgrowth and migration [37, 38]. RPTP-β: MK/PTN binding to RPTP-β promotes B cell survival, hematopoiesis, neurite outgrowth, and migration [39, 42]. Integrins: MK can directly interact with α4β1 and α6β1 integrins [50], whereas PTN interacts with αvβ3 [51]; those interactions promote cell migration [50, 51, 58]. Lrp1: MK interacts with α4β1 and α6β1, whereas PTN interacts with αvβ3. Lrp1 drives macrophage/neutrophil haptotaxis [140]. Nucleolin: MK/PTN interaction with nucleolin promotes MK/PTN nuclear localization, cell migration, and survival [58, 67, 68].
MK/PTN/RPTP-β interactions are characterized as driving neurite outgrowth and cell survival [39, 40] (Fig. 1); an effect mediated by activation of ALK, which is a substrate of RPTP-β. Ligation of RPTP-β by PTN prevents RPTP-β from dephosphorylating ALK, thus permitting ALK activity [41]. RPTP-β was originally thought to be restricted to the central nervous system; however, it was recently identified on B cells and HSCs, where it functions to promote cell survival [11, 42], although ALK expression by B cells remains unreported. Whether PTN or MK directly ligate ALK is disputed in the literature [43, 44], but recent evidence demonstrates that FAM150A/B are bona fide ligands for ALK [45–48]. Regardless, it is clear that, at least in the presence of RPTP-β, MK/PTN and ALK biology are intertwined [41, 49].
Nonproteoglycan receptors are also reported to interact with MK and PTN. MK can directly interact with α4β1 and α6β1 integrins [50], whereas PTN interacts with αvβ3 [51] (Fig. 1). On immune cells, α6β1, α4β1, and αvβ3 mediate cell migration and adhesion, and α4β1 is also implicated in B and T cell development [52–57]. Direct MK/PTN/integrin interactions promoting cell migration are implicated in multiple cell types, suggesting that these interactions perform similar functions on immune cells [50, 51, 58].
MK has also been identified as a high-affinity ligand for low-density Lrp1 [59]. Lrp1 is thought to attenuate proinflammatory macrophage activation [60]. MK/Lrp1 signaling also promotes cell survival and may stimulate PMN haptotaxis [59, 61, 62]. MK and PTN also bind to NCL, albeit with significantly lower affinity relative to other cell-surface receptors [63–65]. NCL is ubiquitously expressed and may be involved in recognition of early apoptotic cells by macrophages [66]. The interaction between NCL and MK/PTN promotes nuclear localization, endothelial cell migration, and cell survival [58, 67, 68] (Fig. 1).
Understanding MK/PTN signaling through their respective receptors is hampered by the fact that MK/PTN interact with numerous cell-surface proteins, and their putative receptors also interact with a variety of other ligands. Thus, elucidating the functional contribution of MK/PTN signaling requires knowledge of potential receptor expression. Table 1 provides a summary of MK/PTN receptor expression and putative function on immune cells.
REGULATION OF MK/PTN EXPRESSION
MK and PTN expression are elevated in response to inflammatory cytokines; MK induction may be driven by NF-κB because the MK promoter contains a putative NF-κB–responsive element [69]. PTN expression can be positively regulated by IFN-β and IFN-γ [70, 71]. For example, in THP-1 monocytic cells and mouse peritoneal macrophages, IFN-γ promotes PTN expression via an IFN-γ–responsive promoter element [71]. Additionally, high–molecular-weight hyaluronan, an inhibitor of TLR4 activity, decreases the expression of PTN in a murine model of Th1-type autoimmune disease, implicating PTN as a TLR4-responsive gene [72]]. Consistent with this, TNF-α can stimulate the expression of MK and PTN in monocytes and tumor cells, validating MK/PTN expression as effectors of inflammatory cytokine activity [73–75]. Lymphocytes are also reported to express MK or PTN after stimulation. For instance, MK expression is elevated in T cells after activation with anti-CD3/CD28 Abs and in peripheral blood lymphocytes in response to IL-2 or IFN-γ [76]. However, neither CD3/CD28 activation nor IL-2 or IFN-γ treatment promotes PTN expression in lymphocytes, demonstrating a differential regulation of MK and PTN expression depending on the context. Overall, these data suggest that immune cells may be a significant source of MK/PTN in inflammation and cancer.
Of the nonimmune-affiliated cytokines, MK and PTN are positively regulated by epidermal growth factor (EGF) [75, 77] and FGF-10 [77]. PTN is also up-regulated by FGF2 or basic FGF [78] and platelet-derived growth factor [79, 80] and is down-regulated by VEGF-A [81].
A variety of noncytokine-related pathways can also affect expression of MK and PTN. For example, the activation of ER-β and subsequent protein kinase Cδ activation stimulates MK expression [82, 83], whereas ER-α can negatively regulate MK expression [84]]. Further, retinoic acid stimulates MK expression but has no effect on PTN expression, even though the promoters for each gene contain putative retinoic acid–responsive elements [85]. In contrast, progesterone and testosterone augment PTN expression but have little effect on MK [86]. Additionally, MK and PTN expression are reduced in response to glucocorticoid receptor activity [85, 87]].
Downstream of MK/PTN, there is evidence that this family can influence the expression of immunomodulatory cytokines. IL-1β, IL-6, and TNF-α are up-regulated in PBMCs in response to PTN [88, 89]. Furthermore, in a murine model of peritoneal fibrosis, PTN-null mice had decreased IL-1β, TNF-α, and TGF-β expression. PTN also mediates the expression of VEGF-A in colorectal cell lines [90] and HB-EGF in primary osteoblasts [91]. In an ischemic renal injury model, MK was shown to stimulate expression of the chemokines MIP2/CXCL2 and MCP-1/CCL2, respectively [92]. This supports an immunomodulatory function of MK/PTN signaling through mediating the expression of cytokines and chemokines.
EXPRESSION OF MK/PTN FAMILY IN TISSUE DAMAGE AND INFLAMMATORY DISEASES
MK and PTN expression are elevated after injury in many tissues, including brain [93, 94], blood vessels [95], heart [96, 97], and kidney [92]. Additionally, PTN expression is increased in bone marrow endothelial cells in mice exposed to total-body irradiation [98]. In a study of the toxicity of depleted uranium on peritoneal macrophages, MK was identified as a responsive gene [99]. Furthermore, PTN is increased in response to hydrogen peroxide [100], and the expression of MK and PTN are increased after exposure to ethanol in vitro and in vivo [101, 102]. These data support the MK/PTN family as being effectors of oxidative stress.
The expression of MK/PTN is also stimulated by hypoxia. HUVECs, monocytes, and PMNs show increased MK expression under hypoxic conditions [62]. In PMNs, this effect could be reproduced using cobalt chloride, indicating that MK is a HIF-1α–responsive gene [62]. Indeed, HIF-1α directly drives MK expression via a hypoxia-responsive element in the MK promoter [103]. PTN expression is also elevated in response to hypoxia. For example, PTN expression is increased in hepatic stellate cells and cardiomyocytes in response to hypoxia in vitro, and PTN secretion is elevated by endothelial cells and macrophages in response to acute ischemic brain injury [94]. However, unlike MK, it is unknown whether PTN itself is a HIF-1α–responsive gene.
One function of MK/PTN that is widely covered in the context of tissue damage is angiogenesis. In a model of hindlimb ischemia, MK-null mice exhibited impaired angiogenesis [62]. PTN, which is often cited as a proangiogenic factor, is only weakly mitogenic for endothelial cells in vitro [104]. However, in a rat model of myocardial ischemia, PTN promoted the development of functional blood vessels [105]. Furthermore, there are multiple reports of PTN promoting tumor angiogenesis, although, in some cases, it may be angiostatic (for review, see Papadimitriou et al. [10],). It is clear that MK and PTN are not required for developmental angiogenesis because there are no apparent vascular abnormalities in MK- or PTN-null mice [10], suggesting that the proangiogenic activity of MK/PTN is not central to their function in tissue homeostasis.
MK and PTN are expressed in inflammatory conditions, including atherosclerosis [71], experimental autoimmune encephalitis [106, 107], and rheumatoid arthritis [73, 108, 109] and in models of ischemia-reperfusion injury [92, 94]. Preclinical models of these pathologic indications have yielded insight into the function of MK/PTN in the immune system, as we will discuss in the following sections.
MK/PTN EXPRESSION AND FUNCTION IN THE LYMPHOID ARM OF IMMUNITY
MK, but not PTN, is expressed by human peripheral blood lymphocytes from healthy donors, and the expression of MK is transiently and robustly increased in T cells in response to CD28 activation, IL-2, IFN-γ, or phytohemagglutinin [76]. MK appears to bind and interact with NCL on the surface of T lymphocytes [63]. Furthermore, MK colocalizes with NCL on the cell surface and in nucleoli of phytohemagglutinin-stimulated T cells, suggesting that NCL may be responsible for the internalization and nuclear localization of MK. MK binds to the C-terminal RGG domain of NCL, and this interaction is proteoglycan independent [63]. The binding of MK to NCL on T cells interferes with HIV-1 binding and subsequent infection, similar to the NCL-binding peptide, HB-19 [63, 76]. MK interference with the HIV-1 infection of T cells requires the presence of extracellular MK before HIV-1 exposure, suggesting that MK/NCL binding is the primary mechanism by which MK antagonizes HIV-1 infection. Additionally, CD4− MK-producing cells can protect CD4+ non-MK–producing cells from HIV-1 infection, demonstrating that the function of MK in blocking HIV-1 infection can be mediated in a paracrine fashion [76]. NCL-binding and HIV-blocking activities have also been validated for PTN [64]. NCL-dependent cell-surface binding of PTN was inhibited in the presence of excess HB-19 or MK, suggesting that PTN and MK recognize the same region on NCL [64].
In PBMCs, PTN is mitogenic and induces the expression of IL-6, TNF-α, and IL-1β [88]. The increase in these cytokines via PTN, however, promotes HIV-1 replication in PBMCs infected in vitro or in PBMCs derived from patients with AIDS, an effect potentiated by IL-2 [89]. When lymphocytes (B and T cells) and monocytes were treated separately, the induction of proinflammatory cytokine production by PTN was greatly reduced, and no virus replication occurred [89]. This suggests that PTN’s capacity to modulate proinflammatory cytokine expression works via an undefined mechanism in a heterogenous population of immune cells that may be mediated, in part, by NCL.
PTN is expressed highly in the lymph nodes of MRL-lpr/lpr mice, which is a model of Th1-type autoimmune disease [72]. In this model, there is lymphoaccumulation of double-negative T cells. The administration of high–molecular-weight hyaluronan, which antagonizes TLR4 activation and perturbs LPS-mediated lethality [110], greatly reduces the enlargement of submandibular lymph nodes, correlating with decreased PTN expression. Treating double-negative T cells isolated from this model with PTN attenuates apoptosis, suggesting that the presence of PTN in lymph nodes may support the accumulation of double-negative T cells, outlining a possible role for PTN in T cell survival. If surface NCL promotes double-negative T cell survival as it does in other cell types, then PTN/NCL may promote survival in this context based on the following observations: 1) the HB-19 peptide, which prevents PTN/NCL binding, decreases the proliferation and migration of HUVECs in vitro in an NCL-dependent manner [111]; 2) in the chick embryo chorioallantoic membrane assay, PTN is endogenously expressed and promotes angiogenesis mediated by NCL [68]; 3) HB-19 reduces angiogenesis in the chorioallantoic membrane assay [112]; and 4) as stated above, the MK/PTN family adheres to T cells, at least in part, through surface NCL, which is blocked by HB-19 [63, 64]. Certainly more research is required to support this hypothesis.
MK promotes B cell survival in vitro and in vivo [42]. This is mediated through RPTP-β because B cells isolated from RPTP-β–null mice fail to respond to MK. RPTP-β–null B cells also fail to respond to macrophage migration inhibitory factor or hepatocyte growth factor, which may induce B cell survival through induction of MK expression [42]. Furthermore, RPTP-β–null mice have reduced mature B cells in the bone marrow, spleen, and lymph nodes, indicating an in vivo function of MK/RPTP-β signaling in lymphoid tissue B cell survival. Given PTN’s ability to promote cell survival upon RPTP-β ligation in other cell types [40], it may also support B cell survival, underlying the importance of observing MK/PTN biology concomitantly.
MK/PTN, INFLAMMATION, AND CANCER: SCULPTING THE PHENOTYPE OF MYELOID CELLS
The MK/PTN family is highly expressed in multiple human cancers, including breast, pancreas, lung, and hematopoietic malignancies (for review, see Papadimitriou et al. [10]). Knowledge from preclinical models of cancer has yielded insight into the processes in which the MK/PTN family participates. MK/PTN is implicated in tumor angiogenesis as proliferative drivers and factors in the resistance to apoptosis. For example, MCF7 cancer cells stably transfected to express high levels of PTN grow robustly after in vivo injection in comparison to control transfected or untransfected MCF7 cells. This effect is mediated by PTN-driven extracellular matrix remodeling, increased angiogenesis, and stimulation of stromal cells in the tumor microenvironment [113]. Further, MMTV-PyMT transgenic mice engineered to overexpress PTN (MMTV-PyMT-PTN) display more rapid development of scirrhous, carcinoma-type breast-cancer foci; scirrhous carcinoma is characterized by increased desmoplasia, and it positively correlates clinically with increased lymph node metastasis [114] (although this was not assessed in MMTV-PyMT mice). MMTV-PyMT-PTN tumors also exhibit increased extracellular matrix deposition and angiogenesis [113]. Similarly, in the SW-13 adrenal carcinoma line, the ectopic expression of PTN greatly increased tumor growth and angiogenesis in vivo [115]. These data support a function of PTN in tumor progression that centers on stromal cell activity, which may include tumor-associated myeloid cells as effectors of PTN.
MK also drives tumor growth [116]. In the Lewis lung carcinoma model, MK-null mice had significantly reduced pulmonary metastasis [117]. Given MK’s function in PMN recruitment (discussed below) and the ability of MDSCs to support metastasis, it would be interesting to see if PMN-MDSC numbers are reduced in Lewis lung tumors in MK-null mice.
The effect of the MK/PTN family on angiogenesis is thought to be through the promotion of endothelial cell proliferation and tube formation [10]. However, there are conflicting reports about the angiogenic capacity of MK/PTN on primary endothelial cells in vitro, particularly in the context of how MK/PTN affect the principal angiogenic growth factor VEGF-A [10, 62, 81, 118]. Concomitant with the absence of vascular anomalies in PTN-null embryos [7], this suggests that the mechanisms behind PTN-mediated tumor angiogenesis may, in part, be due to its effects on other cell types in the tumor microenvironment. In fact, the effects of PTN on tumor angiogenesis may be mediated by its effects on myeloid cells. In 2006, Sharifi et al. [74] identified that PTN-transfected THP-1 monocytic cells down-regulate myeloid cell markers and up-regulate endothelial cell markers, including Tie-2, Vegfr2, and VE-cadherin [74]. PTN-transfected THP-1 cells also integrate into developing vasculature. In 2009, Chen et al. [119] demonstrated that treating primary CD14+ human monocytes with a combination of macrophage CSF, VEGF-A, and PTN drove expression of Tie-2, Vegfr2, and VE-cadherin [119]. In addition, a combination of macrophage CSF and PTN drove CD14+ human monocytes to form tube-like structures in vitro. Additionally, CD14+ cells cocultured with multiple myeloma cells expressed Vegfr2, Tie2, and von Willebrand factor, which was inhibited by a PTN function-blocking Ab. Lastly, Palmieri et al. [120] observed that PTN also drives Nrp-1 and VEGF-A expression in THP-1 monocytes [120], which is consistent with the report that Nrp-1 is required for PTN-mediated migration of HUVECs and prostate cancer cell lines [121].
In the context of primary monocytes, it is possible that PTN may support the expansion of the CD14+Vegfr2+ subset of myeloid-lineage endothelial progenitor cells, which normally constitute approximately 2–4% of circulating CD14+ cells [122, 123]. Interestingly, there is a population of MDSCs that is reported to express Vegfr2, and Vegfr2 may be important for MDSC expansion and chemotaxis [124, 125]. For example, the selective ligation of Vegfr2 in mice leads to the expansion of splenic MDSCs [126]. Furthermore, Vegfr2 signaling promotes MDSC and macrophage chemotaxis in preclinical models of breast and pancreatic cancer, which may be mediated by PTN-driven Vegfr2 expression by those cells [124, 125]. The influence of MK on myeloid cells in those models is unknown, but studies in preclinical models of inflammation have implicated DCs as effectors of MK.
MK suppresses tolerogenic DC development through induction of IL-12 and SHP-2 by regulatory DCs, but not conventional DCs. Further, MK reduces Stat3 activation in those cells in a SHP-2–dependent fashion. In a murine model of EAE, CD4+ and CD8+ T cells, as well as CD11b+ cells, express MK. In vitro, T cells activated with CD3 + CD28 mAbs express MK, and among T cell subsets, MK is up-regulated in Th1 cells. MK-null mice had superior clinical scores, explained by an increase in regulatory DCs and regulatory T cells [127]. However, the receptor imparting these effects was unidentified.
Mice null for the MK/PTN receptor Sdc4 were also studied in a murine model of EAE. Inverse to the MK-null mice, Sdc4-null mice had worse disease [128]. The mechanism explaining the phenotype also arrived at myeloid/T cell interactions. Binding of the DC receptor DC-HIL to Sdc4 on activated T cells reduces T cell activation. When this interaction is perturbed, either by targeting DC-HIL on myeloid cells or Sdc4 on T cells, there is an increase in T cell activation and EAE is exacerbated [128]. Furthermore, the suppressive effect of Sdc4 on T cell activation was demonstrated by direct ligation [129]. Given the opposing effects of MK and DC-HIL/Sdc4 perturbation in that model, it raises the question of the functional consequences of MK binding to Sdc4 on T cells. If MK competes with DC-HIL for binding Sdc4 and does not impair T cell activation when bound, it may function to potentiate T cell activation by disrupting DC-HIL/Sdc4 interaction, thus leading to worse disease. This would implicate a function of MK in immune checkpoint disruption, although more research is necessary to validate that.
MK/PTN FUNCTION IN MYELOID ADHESION AND CHEMOTAXIS
Evidence from multiple models of inflammation implicates MK as a key factor in inflammatory cell infiltration. In preclinical models of kidney ischemia/reperfusion injury, rheumatoid arthritis [109], atherosclerosis [95, 130], and EAE [107], MK-deficient mice exhibited significantly less tissue damage, which is associated with a reduction in leukocyte infiltration [92, 131, 132]. Specifically, in models of rheumatoid arthritis [109], kidney inflammation [92, 131, 132], and atherosclerosis [95], MK deficiency was associated with reduced neutrophil and macrophage recruitment at the site of injury. Takada et al. [108] demonstrated that MK possesses chemotactic activity toward neutrophils in vitro [133]. Sato et al. [92] postulated that the reduced neutrophil and macrophage infiltration in MK-null mice in the ischemic renal injury model was due to a concomitant decrease in the chemokines MIP-2 and MCP-1. However, in a cisplatin-induced renal injury model, neutrophil recruitment was reduced in MK-null mice, but no change in a panel of chemokines was observed [131]. In the latter model, macrophage infiltration was not significantly decreased, and no change in MCP-1 was observed, suggesting that the stimulus for inflammation or injury may affect the expression and subsequent contribution of MK to immune cell infiltration. Perhaps the fate of MK activity rests on the expression of MK receptors, which may be affected differentially based on the nature of the stimulus.
Recently, Weckbach et al. [134] gained insight into how MK may mediate neutrophil infiltration in vivo, independent of its ability to induce the expression of other chemokines [134]. Weckbach et al. [134] observed that MK deficiency reduced leukocyte adhesion to cremaster muscle venules upon TNF-α stimulation, whereas the number and velocity of rolling leukocytes was unaffected. In a hindlimb ischemia model, MK deficiency reduced PMN extravasation into inflamed tissue, which could be rescued by the introduction of exogenous MK. In an in vitro static adhesion assay, immobilized MK promoted PMN adhesion, whereas soluble MK had no effect. This corroborated the results from Takada et al. [108], which demonstrated that immobilized, not soluble MK has chemotactic activity toward neutrophils [133]. Weckbach et al. [134] observed that CD18 was not required for MK binding to PMN cells but is required for MK-mediated PMN adhesion. Lastly, Weckbach et al. [134] demonstrated that Lrp1 is required for binding and activity of MK in PMN cells, suggesting that signaling downstream of this receptor promotes a high-affinity conformation of CD18. Indeed, using an Ab that recognizes the high-affinity conformation of CD18 (mAb-24), it was demonstrated that PMNs bound to immobilized MK possessed increased high-affinity CD18. This increase was perturbed by using an inhibitor of Lrp1 [134].
Although PTN and MK share many receptors and activities, PTN was not studied in that context. Given that PTN expression is elevated in atherosclerosis [71], ischemic brain injury [135], and EAE [106], it would be interesting to determine the effects of PTN deficiency on leukocyte infiltration in preclinical models of inflammatory disease.
MK/PTN FUNCTION IN HEMATOPOIESIS
In the bone marrow, endothelial cells of the HSC niche produce PTN [11]. PTN expands long-term HSCs in vitro, and PTN-null bone marrow has significantly less long-term HSCs, suggesting that PTN is a critical HSC maintenance factor [11, 49]. Consistent with that idea, the administration of recombinant PTN helps alleviate myelosuppression and greatly improves survival of lethally irradiated mice. In that context, PTN administration increased the frequency of granulocyte-erythroid-macrophage-megakaryocyte CFUs, the multipotent progenitors of myeloid cells. Mechanistically, PTN-driven expansion of bone marrow c-Kit+Sca-1+Lin− (KSL) cells is mediated through RPTP-β, leading to Ras activation in KSL cells. The deletion of RPTP-β or Ras inhibition perturbed PTN activity in vitro and in vivo [40]. Aberrant activation of Ras in hematopoietic cells can drive myeloproliferative disorders and leukemia, but the function of PTN in that context is undetermined [136, 137]. Overall, these data underlie the ability of PTN to influence yet another component of the hematopoietic lineage.
CONCLUSIONS/FUTURE DIRECTIONS
The expression of the MK/PTN family by stimulated immune cells and in inflammatory disease posits a role for these cytokines in inflammation. From the maintenance of HSCs, B cell survival, and myeloid cell recruitment to mediators of immune modulatory cytokine expression, this cytokine family possesses pleiotropic effects on the hematopoietic lineage from top to bottom.
How the functions of MK and PTN differ from one another is a question of great biologic interest. Given the shared receptors, functions, and gene dosage effect of MK/PTN DKO mice, it appears that studying these cytokines simultaneously in preclinical models of inflammation and cancer is largely overlooked. In future studies, it will be fruitful for investigators of the MK/PTN family to study both cytokines concomitantly.
As more information emerges demonstrating immune cells as effectors of MK/PTN signaling, many questions remain: 1) which receptor or receptors are mediating MK/PTN’s effects on different immune cells, 2) what is the effect of MK/PTN expression in preclinical models of inflammation and cancer, 3) what signaling cascades mediate MK/PTN activity on immune cells, and 4) are immune cells a significant source of MK/PTN in inflammation and cancer? Answering these questions will contribute to the understanding of MK/PTN biology and may also lead to therapeutic avenues targeting this cytokine family in human disease.
AUTHORSHIP
N.S., A.T.A.D., and R.A.B. designed the concept of the review and wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported in part by grants from the U.S. National Institutes of Health (1F31 CA19603301 to N.S.) and the Effie Marie Cain Scholarship in Angiogenesis Research and the Mary Kay Foundation (to R.A.B.).
Glossary
- ALK
anaplastic lymphoma kinase
- DC
dendritic cell
- DKO
double knockout
- EAE
experimental autoimmune encephalitis
- ER
estrogen receptor
- FGF
fibroblast growth factor
- HB
heparin binding
- HIF-1α
hypoxia-inducible factor 1-α
- HSC
hematopoietic stem cell
- HUVEC
human umbilical vein endothelial cell
- Lrp1
lipoprotein receptor-related protein 1
- MCP
monocyte chemoattractant protein
- MDSC
myeloid-derived suppressor cell
- MK
midkine
- NCL
nucleolin
- PMN
polymorphonuclear cells
- PTN
pleiotrophin
- RPTP-β
receptor protein tyrosine phosphatase β
- Sdc
syndecan
- VEGF
vascular endothelial growth factor
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Muramatsu T. (2014) Structure and function of midkine as the basis of its pharmacological effects. Br. J. Pharmacol. 171, 814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu C., Maminta-Smith L. D., Guo C., Deuel T. F. (1994) Cloning and sequence of the Xenopus laevis homologue of the midkine cDNA. Gene 146, 311–312. [DOI] [PubMed] [Google Scholar]
- 3.Kadomatsu K., Tomomura M., Muramatsu T. (1988) cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochem. Biophys. Res. Commun. 151, 1312–1318. [DOI] [PubMed] [Google Scholar]
- 4.Courty J., Dauchel M. C., Caruelle D., Perderiset M., Barritault D. (1991) Mitogenic properties of a new endothelial cell growth factor related to pleiotrophin. Biochem. Biophys. Res. Commun. 180, 145–151. [DOI] [PubMed] [Google Scholar]
- 5.Rauvala H. (1989) An 18-kd heparin-binding protein of developing brain that is distinct from fibroblast growth factors. EMBO J. 8, 2933–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wellstein A., Fang W. J., Khatri A., Lu Y., Swain S. S., Dickson R. B., Sasse J., Riegel A. T., Lippman M. E. (1992) A heparin-binding growth factor secreted from breast cancer cells homologous to a developmentally regulated cytokine. J. Biol. Chem. 267, 2582–2587. [PubMed] [Google Scholar]
- 7.Muramatsu H., Zou P., Kurosawa N., Ichihara-Tanaka K., Maruyama K., Inoh K., Sakai T., Chen L., Sato M., Muramatsu T. (2006) Female infertility in mice deficient in midkine and pleiotrophin, which form a distinct family of growth factors. Genes Cells 11, 1405–1417. [DOI] [PubMed] [Google Scholar]
- 8.Kadomatsu K., Kishida S., Tsubota S. (2013) The heparin-binding growth factor midkine: the biological activities and candidate receptors. J. Biochem. 153, 511–521. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfield S. M., Bowden E. T., Cohen-Missner S., Gibby K. A., Ory V., Henke R. T., Riegel A. T., Wellstein A. (2012) Pleiotrophin (PTN) expression and function and in the mouse mammary gland and mammary epithelial cells [published correction in PLoS One (2013) 8, doi:10.1371/annotation/55d244a2-0960-4f5f-95e9-2eea136654b1] PLoS One 7, e47876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papadimitriou E., Pantazaka E., Castana P., Tsalios T., Polyzos A., Beis D. (2016) Pleiotrophin and its receptor protein tyrosine phosphatase beta/zeta as regulators of angiogenesis and cancer. Biochim. Biophys. Acta 1866, 252–265. [DOI] [PubMed] [Google Scholar]
- 11.Himburg H. A., Muramoto G. G., Daher P., Meadows S. K., Russell J. L., Doan P., Chi J. T., Salter A. B., Lento W. E., Reya T., Chao N. J., Chute J. P. (2010) Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat. Med. 16, 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadomatsu K., Huang R. P., Suganuma T., Murata F., Muramatsu T. (1990) A retinoic acid responsive gene MK found in the teratocarcinoma system is expressed in spatially and temporally controlled manner during mouse embryogenesis. J. Cell Biol. 110, 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aridome K., Tsutsui J., Takao S., Kadomatsu K., Ozawa M., Aikou T., Muramatsu T. (1995) Increased midkine gene expression in human gastrointestinal cancers. Jpn. J. Cancer Res. 86, 655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inazumi T., Tajima S., Nishikawa T., Kadomatsu K., Muramatsu H., Muramatsu T. (1997) Expression of the retinoid-inducible polypeptide, midkine, in human epidermal keratinocytes. Arch. Dermatol. Res. 289, 471–475. [DOI] [PubMed] [Google Scholar]
- 15.Nordin S. L., Andersson C., Bjermer L., Bjartell A., Mörgelin M., Egesten A. (2013) Midkine is part of the antibacterial activity released at the surface of differentiated bronchial epithelial cells. J. Innate Immun. 5, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herradon G., Ezquerra L., Nguyen T., Silos-Santiago I., Deuel T. F. (2005) Midkine regulates pleiotrophin organ-specific gene expression: evidence for transcriptional regulation and functional redundancy within the pleiotrophin/midkine developmental gene family. Biochem. Biophys. Res. Commun. 333, 714–721. [DOI] [PubMed] [Google Scholar]
- 17.Mitsiadis T. A., Salmivirta M., Muramatsu T., Muramatsu H., Rauvala H., Lehtonen E., Jalkanen M., Thesleff I. (1995) Expression of the heparin-binding cytokines, midkine (MK) and HB-GAM (pleiotrophin) is associated with epithelial-mesenchymal interactions during fetal development and organogenesis. Development 121, 37–51. [DOI] [PubMed] [Google Scholar]
- 18.Götte M. (2003) Syndecans in inflammation. FASEB J. 17, 575–591. [DOI] [PubMed] [Google Scholar]
- 19.Elenius K., Vainio S., Laato M., Salmivirta M., Thesleff I., Jalkanen M. (1991) Induced expression of syndecan in healing wounds. J. Cell Biol. 114, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clasper S., Vekemans S., Fiore M., Plebanski M., Wordsworth P., David G., Jackson D. G. (1999) Inducible expression of the cell surface heparan sulfate proteoglycan syndecan-2 (fibroglycan) on human activated macrophages can regulate fibroblast growth factor action. J. Biol. Chem. 274, 24113–24123. [DOI] [PubMed] [Google Scholar]
- 21.Sebestyén A., Gallai M., Knittel T., Ambrust T., Ramadori G., Kovalszky I. (2000) Cytokine regulation of syndecan expression in cells of liver origin. Cytokine 12, 1557–1560. [DOI] [PubMed] [Google Scholar]
- 22.Ishiguro K., Kadomatsu K., Kojima T., Muramatsu H., Iwase M., Yoshikai Y., Yanada M., Yamamoto K., Matsushita T., Nishimura M., Kusugami K., Saito H., Muramatsu T. (2001) Syndecan-4 deficiency leads to high mortality of lipopolysaccharide-injected mice. J. Biol. Chem. 276, 47483–47488. [DOI] [PubMed] [Google Scholar]
- 23.Sanderson R. D., Lalor P., Bernfield M. (1989) B lymphocytes express and lose syndecan at specific stages of differentiation. Cell Regul. 1, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeaman C., Rapraeger A. C. (1993) Post-transcriptional regulation of syndecan-1 expression by cAMP in peritoneal macrophages. J. Cell Biol. 122, 941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J., Brown L. F., Laham R. J., Volk R., Simons M. (1997) Macrophage-dependent regulation of syndecan gene expression. Circ. Res. 81, 785–796. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita Y., Oritani K., Miyoshi E. K., Wall R., Bernfield M., Kincade P. W. (1999) Syndecan-4 is expressed by B lineage lymphocytes and can transmit a signal for formation of dendritic processes. J. Immunol. 162, 5940–5948. [PubMed] [Google Scholar]
- 27.Kaneider N. C., Egger P., Dunzendorfer S., Wiedermann C. J. (2001) Syndecan-4 as antithrombin receptor of human neutrophils. Biochem. Biophys. Res. Commun. 287, 42–46. [DOI] [PubMed] [Google Scholar]
- 28.Saphire A. C., Bobardt M. D., Zhang Z., David G., Gallay P. A. (2001) Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J. Virol. 75, 9187–9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wegrowski Y., Milard A. L., Kotlarz G., Toulmonde E., Maquart F. X., Bernard J. (2006) Cell surface proteoglycan expression during maturation of human monocytes-derived dendritic cells and macrophages. Clin. Exp. Immunol. 144, 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung J. S., Dougherty I., Cruz P. D. Jr., Ariizumi K. (2007) Syndecan-4 mediates the coinhibitory function of DC-HIL on T cell activation. J. Immunol. 179, 5778–5784. [DOI] [PubMed] [Google Scholar]
- 31.Teixé T., Nieto-Blanco P., Vilella R., Engel P., Reina M., Espel E. (2008) Syndecan-2 and -4 expressed on activated primary human CD4+ lymphocytes can regulate T cell activation. Mol. Immunol. 45, 2905–2919. [DOI] [PubMed] [Google Scholar]
- 32.Hayashida K., Parks W. C., Park P. W. (2009) Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood 114, 3033–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gautier E. L., Shay T., Miller J., Greter M., Jakubzick C., Ivanov S., Helft J., Chow A., Elpek K. G., Gordonov S., Mazloom A. R., Ma’ayan A., Chua W. J., Hansen T. H., Turley S. J., Merad M., Randolph G. J.; Immunological Genome Consortium (2012) Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 13, 1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rovira-Clavé X., Angulo-Ibáñez M., Noguer O., Espel E., Reina M. (2012) Syndecan-2 can promote clearance of T-cell receptor/CD3 from the cell surface. Immunology 137, 214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kehoe O., Kalia N., King S., Eustace A., Boyes C., Reizes O., Williams A., Patterson A., Middleton J. (2014) Syndecan-3 is selectively pro-inflammatory in the joint and contributes to antigen-induced arthritis in mice. Arthritis Res. Ther. 16, R148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segaliny A. I., Brion R., Mortier E., Maillasson M., Cherel M., Jacques Y., Le Goff B., Heymann D. (2015) Syndecan-1 regulates the biological activities of interleukin-34. Biochim. Biophys. Acta 1853, 1010–1021. [DOI] [PubMed] [Google Scholar]
- 37.Raulo E., Chernousov M. A., Carey D. J., Nolo R., Rauvala H. (1994) Isolation of a neuronal cell surface receptor of heparin binding growth-associated molecule (HB-GAM). Identification as N-syndecan (syndecan-3). J. Biol. Chem. 269, 12999–13004. [PubMed] [Google Scholar]
- 38.Imai S., Kaksonen M., Raulo E., Kinnunen T., Fages C., Meng X., Lakso M., Rauvala H. (1998) Osteoblast recruitment and bone formation enhanced by cell matrix-associated heparin-binding growth-associated molecule (HB-GAM). J. Cell Biol. 143, 1113–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asai T., Watanabe K., Ichihara-Tanaka K., Kaneda N., Kojima S., Iguchi A., Inagaki F., Muramatsu T. (1997) Identification of heparin-binding sites in midkine and their role in neurite-promotion. Biochem. Biophys. Res. Commun. 236, 66–70. [DOI] [PubMed] [Google Scholar]
- 40.Himburg H. A., Yan X., Doan P. L., Quarmyne M., Micewicz E., McBride W., Chao N. J., Slamon D. J., Chute J. P. (2014) Pleiotrophin mediates hematopoietic regeneration via activation of RAS. J. Clin. Invest. 124, 4753–4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez-Pinera P., Zhang W., Chang Y., Vega J. A., Deuel T. F. (2007) Anaplastic lymphoma kinase is activated through the pleiotrophin/receptor protein-tyrosine phosphatase β/ζ signaling pathway: an alternative mechanism of receptor tyrosine kinase activation. J. Biol. Chem. 282, 28683–28690. [DOI] [PubMed] [Google Scholar]
- 42.Cohen S., Shoshana O. Y., Zelman-Toister E., Maharshak N., Binsky-Ehrenreich I., Gordin M., Hazan-Halevy I., Herishanu Y., Shvidel L., Haran M., Leng L., Bucala R., Harroch S., Shachar I. (2012) The cytokine midkine and its receptor RPTPζ regulate B cell survival in a pathway induced by CD74. J. Immunol. 188, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoica G. E., Kuo A., Aigner A., Sunitha I., Souttou B., Malerczyk C., Caughey D. J., Wen D., Karavanov A., Riegel A. T., Wellstein A. (2001) Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J. Biol. Chem. 276, 16772–16779. [DOI] [PubMed] [Google Scholar]
- 44.Stoica G. E., Kuo A., Powers C., Bowden E. T., Sale E. B., Riegel A. T., Wellstein A. (2002) Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J. Biol. Chem. 277, 35990–35998. [DOI] [PubMed] [Google Scholar]
- 45.Mathivet T., Mazot P., Vigny M. (2007) In contrast to agonist monoclonal antibodies, both C-terminal truncated form and full length form of Pleiotrophin failed to activate vertebrate ALK (anaplastic lymphoma kinase)? Cell. Signal. 19, 2434–2443. [DOI] [PubMed] [Google Scholar]
- 46.Deuel T. F. (2013) Anaplastic lymphoma kinase: “ligand independent activation” mediated by the PTN/RPTPβ/ζ signaling pathway. Biochim. Biophys. Acta 1834, 2219–2223. [DOI] [PubMed] [Google Scholar]
- 47.Guan J., Umapathy G., Yamazaki Y., Wolfstetter G., Mendoza P., Pfeifer K., Mohammed A., Hugosson F., Zhang H., Hsu A. W., Halenbeck R., Hallberg B., Palmer R. H.. (2015) FAM150A and FAM150B are activating ligands for anaplastic lymphoma kinase. Elife 4, e09811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reshetnyak A. V., Murray P. B., Shi X., Mo E. S., Mohanty J., Tome F., Bai H., Gunel M., Lax I., Schlessinger J. (2015) Augmentor α and β (FAM150) are ligands of the receptor tyrosine kinases ALK and LTK: Hierarchy and specificity of ligand-receptor interactions. Proc. Natl. Acad. Sci. USA 112, 15862–15867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Himburg H. A., Harris J. R., Ito T., Daher P., Russell J. L., Quarmyne M., Doan P. L., Helms K., Nakamura M., Fixsen E., Herradon G., Reya T., Chao N. J., Harroch S., Chute J. P. (2012) Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Reports 2, 964–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muramatsu H., Zou P., Suzuki H., Oda Y., Chen G. Y., Sakaguchi N., Sakuma S., Maeda N., Noda M., Takada Y., Muramatsu T. (2004) α4β1- and α6β1-integrins are functional receptors for midkine, a heparin-binding growth factor. J. Cell Sci. 117, 5405–5415. [DOI] [PubMed] [Google Scholar]
- 51.Mikelis C., Sfaelou E., Koutsioumpa M., Kieffer N., Papadimitriou E. (2009) Integrin αvβ3 is a pleiotrophin receptor required for pleiotrophin-induced endothelial cell migration through receptor protein tyrosine phosphatase beta/zeta. FASEB J. 23, 1459–1469. [DOI] [PubMed] [Google Scholar]
- 52.Arroyo A. G., Yang J. T., Rayburn H., Hynes R. O. (1996) Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell 85, 997–1008. [DOI] [PubMed] [Google Scholar]
- 53.Albert M. L., Kim J. I., Birge R. B. (2000) αvβ5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat. Cell Biol. 2, 899–905. [DOI] [PubMed] [Google Scholar]
- 54.Bishop G. G., McPherson J. A., Sanders J. M., Hesselbacher S. E., Feldman M. J., McNamara C. A., Gimple L. W., Powers E. R., Mousa S. A., Sarembock I. J. (2001) Selective αvβ3-receptor blockade reduces macrophage infiltration and restenosis after balloon angioplasty in the atherosclerotic rabbit. Circulation 103, 1906–1911. [DOI] [PubMed] [Google Scholar]
- 55.Jin H., Su J., Garmy-Susini B., Kleeman J., Varner J. (2006) Integrin α4β1 promotes monocyte trafficking and angiogenesis in tumors. Cancer Res. 66, 2146–2152. [DOI] [PubMed] [Google Scholar]
- 56.Voisin M. B., Nourshargh S. (2007) Role of α6β1 integrin in leukocyte adhesion and transmigration. Adhesion Molecules: Function and Inhibition (Ley K., ), Birkhäuser, Basel, Switzerland, 221–235 [Google Scholar]
- 57.Randolph G. J., Ochando J., Partida-Sánchez S. (2008) Migration of dendritic cell subsets and their precursors. Annu. Rev. Immunol. 26, 293–316. [DOI] [PubMed] [Google Scholar]
- 58.Koutsioumpa M., Polytarchou C., Courty J., Zhang Y., Kieffer N., Mikelis C., Skandalis S. S., Hellman U., Iliopoulos D., Papadimitriou E. (2013) Interplay between αvβ3 integrin and nucleolin regulates human endothelial and glioma cell migration. J. Biol. Chem. 288, 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muramatsu H., Zou K., Sakaguchi N., Ikematsu S., Sakuma S., Muramatsu T. (2000) LDL receptor-related protein as a component of the midkine receptor. Biochem. Biophys. Res. Commun. 270, 936–941. [DOI] [PubMed] [Google Scholar]
- 60.Mantuano E., Brifault C., Lam M. S., Azmoon P., Gilder A. S., Gonias S. L. (2016) LDL receptor-related protein-1 regulates NFκB and microRNA-155 in macrophages to control the inflammatory response. Proc. Natl. Acad. Sci. USA 113, 1369–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen S., Bu G., Takei Y., Sakamoto K., Ikematsu S., Muramatsu T., Kadomatsu K. (2007) Midkine and LDL-receptor-related protein 1 contribute to the anchorage-independent cell growth of cancer cells. J. Cell Sci. 120, 4009–4015. [DOI] [PubMed] [Google Scholar]
- 62.Weckbach L. T., Groesser L., Borgolte J., Pagel J. I., Pogoda F., Schymeinsky J., Müller-Höcker J., Shakibaei M., Muramatsu T., Deindl E., Walzog B. (2012) Midkine acts as proangiogenic cytokine in hypoxia-induced angiogenesis. Am. J. Physiol. Heart Circ. Physiol. 303, H429–H438. [DOI] [PubMed] [Google Scholar]
- 63.Said E. A., Krust B., Nisole S., Svab J., Briand J. P., Hovanessian A. G. (2002) The anti-HIV cytokine midkine binds the cell surface-expressed nucleolin as a low affinity receptor. J. Biol. Chem. 277, 37492–37502. [DOI] [PubMed] [Google Scholar]
- 64.Said E. A., Courty J., Svab J., Delbé J., Krust B., Hovanessian A. G. (2005) Pleiotrophin inhibits HIV infection by binding the cell surface-expressed nucleolin. FEBS J. 272, 4646–4659. [DOI] [PubMed] [Google Scholar]
- 65.Mongelard F., Bouvet P. (2007) Nucleolin: a multiFACeTed protein. Trends Cell Biol. 17, 80–86. [DOI] [PubMed] [Google Scholar]
- 66.Hirano K., Miki Y., Hirai Y., Sato R., Itoh T., Hayashi A., Yamanaka M., Eda S., Beppu M. (2005) A multifunctional shuttling protein nucleolin is a macrophage receptor for apoptotic cells. J. Biol. Chem. 280, 39284–39293. [DOI] [PubMed] [Google Scholar]
- 67.Shibata Y., Muramatsu T., Hirai M., Inui T., Kimura T., Saito H., McCormick L. M., Bu G., Kadomatsu K. (2002) Nuclear targeting by the growth factor midkine. Mol. Cell. Biol. 22, 6788–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koutsioumpa M., Drosou G., Mikelis C., Theochari K., Vourtsis D., Katsoris P., Giannopoulou E., Courty J., Petrou C., Magafa V., Cordopatis P., Papadimitriou E. (2012) Pleiotrophin expression and role in physiological angiogenesis in vivo: potential involvement of nucleolin. Vasc. Cell 4, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uehara K., Matsubara S., Kadomatsu K., Tsutsui J., Muramatsu T. (1992) Genomic structure of human midkine (MK), a retinoic acid-responsive growth/differentiation factor. J. Biochem. 111, 563–567. [DOI] [PubMed] [Google Scholar]
- 70.Satoh J., Kuroda Y. (2001) Differing effects of IFNβ vs IFNγ in MS: gene expression in cultured astrocytes. Neurology 57, 681–685. [DOI] [PubMed] [Google Scholar]
- 71.Li F., Tian F., Wang L., Williamson I. K., Sharifi B. G., Shah P. K. (2010) Pleiotrophin (PTN) is expressed in vascularized human atherosclerotic plaques: IFN-γ/JAK/STAT1 signaling is critical for the expression of PTN in macrophages. FASEB J. 24, 810–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asari A., Kanemitsu T., Kurihara H. (2010) Oral administration of high molecular weight hyaluronan (900 kDa) controls immune system via Toll-like receptor 4 in the intestinal epithelium. J. Biol. Chem. 285, 24751–24758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pufe T., Bartscher M., Petersen W., Tillmann B., Mentlein R. (2003) Expression of pleiotrophin, an embryonic growth and differentiation factor, in rheumatoid arthritis. Arthritis Rheum. 48, 660–667. [DOI] [PubMed] [Google Scholar]
- 74.Sharifi B. G., Zeng Z., Wang L., Song L., Chen H., Qin M., Sierra-Honigmann M. R., Wachsmann-Hogiu S., Shah P. K. (2006) Pleiotrophin induces transdifferentiation of monocytes into functional endothelial cells. Arterioscler. Thromb. Vasc. Biol. 26, 1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rawnaq T., Dietrich L., Wolters-Eisfeld G., Uzunoglu F. G., Vashist Y. K., Bachmann K., Simon R., Izbicki J. R., Bockhorn M., Güngör C. (2014) The multifunctional growth factor midkine promotes proliferation and migration in pancreatic cancer. Mol. Cancer Res. 12, 670–680. [DOI] [PubMed] [Google Scholar]
- 76.Callebaut C., Nisole S., Briand J. P., Krust B., Hovanessian A. G. (2001) Inhibition of HIV infection by the cytokine midkine. Virology 281, 248–264. [DOI] [PubMed] [Google Scholar]
- 77.Mitsiadis T. A., Caton J., De Bari C., Bluteau G. (2008) The large functional spectrum of the heparin-binding cytokines MK and HB-GAM in continuously growing organs: the rodent incisor as a model. Dev. Biol. 320, 256–266. [DOI] [PubMed] [Google Scholar]
- 78.Hatziapostolou M., Polytarchou C., Katsoris P., Courty J., Papadimitriou E. (2006) Heparin affin regulatory peptide/pleiotrophin mediates fibroblast growth factor 2 stimulatory effects on human prostate cancer cells. J. Biol. Chem. 281, 32217–32226. [DOI] [PubMed] [Google Scholar]
- 79.Li Y. S., Gurrieri M., Deuel T. F. (1992) Pleiotrophin gene expression is highly restricted and is regulated by platelet-derived growth factor. Biochem. Biophys. Res. Commun. 184, 427–432. [DOI] [PubMed] [Google Scholar]
- 80.Antoine M., Tag C. G., Wirz W., Borkham-Kamphorst E., Sawitza I., Gressner A. M., Kiefer P. (2005) Upregulation of pleiotrophin expression in rat hepatic stellate cells by PDGF and hypoxia: implications for its role in experimental biliary liver fibrogenesis. Biochem. Biophys. Res. Commun. 337, 1153–1164. [DOI] [PubMed] [Google Scholar]
- 81.Poimenidi E., Theodoropoulou C., Koutsioumpa M., Skondra L., Droggiti E., van den Broek M., Koolwijk P., Papadimitriou E. (2016) Vascular endothelial growth factor A (VEGF-A) decreases expression and secretion of pleiotrophin in a VEGF receptor-independent manner. Vascul. Pharmacol. 80, 11–19. [DOI] [PubMed] [Google Scholar]
- 82.Zhao G., Nie Y., Lv M., He L., Wang T., Hou Y. (2012) ERβ-mediated estradiol enhances epithelial mesenchymal transition of lung adenocarcinoma through increasing transcription of midkine. Mol. Endocrinol. 26, 1304–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang H., Okamoto M., Panzhinskiy E., Zawada W. M., Das M. (2014) PKCδ/midkine pathway drives hypoxia-induced proliferation and differentiation of human lung epithelial cells. Am. J. Physiol. Cell Physiol. 306, C648–C658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kondoh S., Inoue K., Igarashi K., Sugizaki H., Shirode-Fukuda Y., Inoue E., Yu T., Takeuchi J. K., Kanno J., Bonewald L. F., Imai Y. (2014) Estrogen receptor α in osteocytes regulates trabecular bone formation in female mice. Bone 60, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaplan F., Comber J., Sladek R., Hudson T. J., Muglia L. J., Macrae T., Gagnon S., Asada M., Brewer J. A., Sweezey N. B. (2003) The growth factor midkine is modulated by both glucocorticoid and retinoid in fetal lung development. Am. J. Respir. Cell Mol. Biol. 28, 33–41. [DOI] [PubMed] [Google Scholar]
- 86.Milhiet P. E., Vacherot F., Caruelle J. P., Barritault D., Caruelle D., Courty J. (1998) Upregulation of the angiogenic factor heparin affin regulatory peptide by progesterone in rat uterus. J. Endocrinol. 158, 389–399. [DOI] [PubMed] [Google Scholar]
- 87.Gu D., Yu B., Zhao C., Ye W., Lv Q., Hua Z., Ma J., Zhang Y. (2007) The effect of pleiotrophin signaling on adipogenesis. FEBS Lett. 581, 382–388. [DOI] [PubMed] [Google Scholar]
- 88.Achour A., M’bika J. P., Baudouin F., Caruelle D., Courty J. (2008) Pleiotrophin induces expression of inflammatory cytokines in peripheral blood mononuclear cells. Biochimie 90, 1791–1795. [DOI] [PubMed] [Google Scholar]
- 89.M’Bika J. P., Baudouin F., Courty J., Achour A. (2010) Host factor pleiotrophin induces human immunodeficiency virus type 1 replication associated with inflammatory cytokine expression. J. Gen. Virol. 91, 1346–1353. [DOI] [PubMed] [Google Scholar]
- 90.Kong Y., Bai P. S., Nan K. J., Sun H., Chen N. Z., Qi X. G. (2012) Pleiotrophin is a potential colorectal cancer prognostic factor that promotes VEGF expression and induces angiogenesis in colorectal cancer. Int. J. Colorectal Dis. 27, 287–298. [DOI] [PubMed] [Google Scholar]
- 91.Fan J. B., Liu W., Yuan K., Zhu X. H., Xu D. W., Chen J. J., Cui Z. M. (2014) EGFR trans-activation mediates pleiotrophin-induced activation of Akt and Erk in cultured osteoblasts. Biochem. Biophys. Res. Commun. 447, 425–430. [DOI] [PubMed] [Google Scholar]
- 92.Sato W., Kadomatsu K., Yuzawa Y., Muramatsu H., Hotta N., Matsuo S., Muramatsu T. (2001) Midkine is involved in neutrophil infiltration into the tubulointerstitium in ischemic renal injury. J. Immunol. 167, 3463–3469. [DOI] [PubMed] [Google Scholar]
- 93.Yoshida Y., Goto M., Tsutsui J., Ozawa M., Sato E., Osame M., Muramatsu T. (1995) Midkine is present in the early stage of cerebral infarct. Brain Res. Dev. Brain Res. 85, 25–30. [DOI] [PubMed] [Google Scholar]
- 94.Yeh H. J., He Y. Y., Xu J., Hsu C. Y., Deuel T. F. (1998) Upregulation of pleiotrophin gene expression in developing microvasculature, macrophages, and astrocytes after acute ischemic brain injury. J. Neurosci. 18, 3699–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Horiba M., Kadomatsu K., Nakamura E., Muramatsu H., Ikematsu S., Sakuma S., Hayashi K., Yuzawa Y., Matsuo S., Kuzuya M., Kaname T., Hirai M., Saito H., Muramatsu T. (2000) Neointima formation in a restenosis model is suppressed in midkine-deficient mice. J. Clin. Invest. 105, 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Obama H., Biro S., Tashiro T., Tsutsui J., Ozawa M., Yoshida H., Tanaka H., Muramatsu T. (1998) Myocardial infarction induces expression of midkine, a heparin-binding growth factor with reparative activity. Anticancer Res. 18(1A), 145–152. [PubMed] [Google Scholar]
- 97.Horiba M., Kadomatsu K., Yasui K., Lee J. K., Takenaka H., Sumida A., Kamiya K., Chen S., Sakuma S., Muramatsu T., Kodama I. (2006) Midkine plays a protective role against cardiac ischemia/reperfusion injury through a reduction of apoptotic reaction. Circulation 114, 1713–1720. [DOI] [PubMed] [Google Scholar]
- 98.Himburg H. A., Sasine J., Yan X., Kan J., Dressman H., Chute J. P. (2016) A molecular profile of the endothelial cell response to ionizing radiation. Radiat. Res. 186, 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wan B., Fleming J. T., Schultz T. W., Sayler G. S. (2006) In vitro immune toxicity of depleted uranium: effects on murine macrophages, CD4+ T cells, and gene expression profiles. Environ. Health Perspect. 114, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Polytarchou C., Hatziapostolou M., Papadimitriou E. (2005) Hydrogen peroxide stimulates proliferation and migration of human prostate cancer cells through activation of activator protein-1 and up-regulation of the heparin affin regulatory peptide gene. J. Biol. Chem. 280, 40428–40435. [DOI] [PubMed] [Google Scholar]
- 101.Vicente-Rodríguez M., Pérez-García C., Ferrer-Alcón M., Uribarri M., Sánchez-Alonso M. G., Ramos M. P., Herradón G. (2014) Pleiotrophin differentially regulates the rewarding and sedative effects of ethanol. J. Neurochem. 131, 688–695. [DOI] [PubMed] [Google Scholar]
- 102.He D., Chen H., Muramatsu H., Lasek A. W. (2015) Ethanol activates midkine and anaplastic lymphoma kinase signaling in neuroblastoma cells and in the brain. J. Neurochem. 135, 508–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reynolds P. R., Mucenski M. L., Le Cras T. D., Nichols W. C., Whitsett J. A. (2004) Midkine is regulated by hypoxia and causes pulmonary vascular remodeling. J. Biol. Chem. 279, 37124–37132. [DOI] [PubMed] [Google Scholar]
- 104.Souttou B., Raulais D., Vigny M. (2001) Pleiotrophin induces angiogenesis: involvement of the phosphoinositide-3 kinase but not the nitric oxide synthase pathways. J. Cell. Physiol. 187, 59–64. [DOI] [PubMed] [Google Scholar]
- 105.Christman K. L., Fang Q., Kim A. J., Sievers R. E., Fok H. H., Candia A. F., Colley K. J., Herradon G., Ezquerra L., Deuel T. F., Lee R. J. (2005) Pleiotrophin induces formation of functional neovasculature in vivo. Biochem. Biophys. Res. Commun. 332, 1146–1152. [DOI] [PubMed] [Google Scholar]
- 106.Liu X., Mashour G. A., Webster H. F., Kurtz A. (1998) Basic FGF and FGF receptor 1 are expressed in microglia during experimental autoimmune encephalomyelitis: temporally distinct expression of midkine and pleiotrophin. Glia 24, 390–397. [DOI] [PubMed] [Google Scholar]
- 107.Wang J., Takeuchi H., Sonobe Y., Jin S., Mizuno T., Miyakawa S., Fujiwara M., Nakamura Y., Kato T., Muramatsu H., Muramatsu T., Suzumura A. (2008) Inhibition of midkine alleviates experimental autoimmune encephalomyelitis through the expansion of regulatory T cell population. Proc. Natl. Acad. Sci. USA 105, 3915–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takada T., Toriyama K., Muramatsu H., Song X. J., Torii S., Muramatsu T. (1997) Midkine, a retinoic acid-inducible heparin-binding cytokine in inflammatory responses: chemotactic activity to neutrophils and association with inflammatory synovitis. J. Biochem. 122, 453–458. [DOI] [PubMed] [Google Scholar]
- 109.Maruyama K., Muramatsu H., Ishiguro N., Muramatsu T. (2004) Midkine, a heparin-binding growth factor, is fundamentally involved in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 50, 1420–1429. [DOI] [PubMed] [Google Scholar]
- 110.Muto J., Yamasaki K., Taylor K. R., Gallo R. L. (2009) Engagement of CD44 by hyaluronan suppresses TLR4 signaling and the septic response to LPS. Mol. Immunol. 47, 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Birmpas C., Briand J. P., Courty J., Katsoris P. (2012) The pseudopeptide HB-19 binds to cell surface nucleolin and inhibits angiogenesis. Vasc. Cell 4, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Destouches D., El Khoury D., Hamma-Kourbali Y., Krust B., Albanese P., Katsoris P., Guichard G., Briand J. P., Courty J., Hovanessian A. G. (2008) Suppression of tumor growth and angiogenesis by a specific antagonist of the cell-surface expressed nucleolin. PLoS One 3, e2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chang Y., Zuka M., Perez-Pinera P., Astudillo A., Mortimer J., Berenson J. R., Deuel T. F. (2007) Secretion of pleiotrophin stimulates breast cancer progression through remodeling of the tumor microenvironment. Proc. Natl. Acad. Sci. USA 104, 10888–10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nozoe T., Oyama T., Mori E., Uramoto H., Takenoyama M., Hanagiri T., Sugio K., Yasumoto K. (2007) Clinicopathologic significance of an immunohistochemical expression of p27 in scirrhous carcinoma of the breast. Breast Cancer 14, 277–280. [DOI] [PubMed] [Google Scholar]
- 115.Zhang N., Zhong R., Perez-Pinera P., Herradon G., Ezquerra L., Wang Z. Y., Deuel T. F. (2006) Identification of the angiogenesis signaling domain in pleiotrophin defines a mechanism of the angiogenic switch. Biochem. Biophys. Res. Commun. 343, 653–658. [DOI] [PubMed] [Google Scholar]
- 116.Hao H., Maeda Y., Fukazawa T., Yamatsuji T., Takaoka M., Bao X. H., Matsuoka J., Okui T., Shimo T., Takigawa N., Tomono Y., Nakajima M., Fink-Baldauf I. M., Nelson S., Seibel W., Papoian R., Whitsett J. A., Naomoto Y. (2013) Inhibition of the growth factor MDK/midkine by a novel small molecule compound to treat non-small cell lung cancer. PLoS One 8, e71093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Salama R. H., Muramatsu H., Zou P., Okayama M., Muramatsu T. (2006) Midkine, a heparin-binding growth factor, produced by the host enhances metastasis of Lewis lung carcinoma cells. Cancer Lett. 233, 16–20. [DOI] [PubMed] [Google Scholar]
- 118.Van der Horst E. H., Frank B. T., Chinn L., Coxon A., Li S., Polesso F., Slavin A., Ruefli-Brasse A., Wesche H. (2008) The growth factor Midkine antagonizes VEGF signaling in vitro and in vivo. Neoplasia 10, 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen H., Campbell R. A., Chang Y., Li M., Wang C. S., Li J., Sanchez E., Share M., Steinberg J., Berenson A., Shalitin D., Zeng Z., Gui D., Perez-Pinera P., Berenson R. J., Said J., Bonavida B., Deuel T. F., Berenson J. R. (2009) Pleiotrophin produced by multiple myeloma induces transdifferentiation of monocytes into vascular endothelial cells: a novel mechanism of tumor-induced vasculogenesis. Blood 113, 1992–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Palmieri D., Mura M., Mambrini S., Palombo D. (2015) Effects of Pleiotrophin on endothelial and inflammatory cells: Pro-angiogenic and anti-inflammatory properties and potential role for vascular bio-prosthesis endothelialization. Adv. Med. Sci. 60, 287–293. [DOI] [PubMed] [Google Scholar]
- 121.Elahouel R., Blanc C., Carpentier G., Frechault S., Cascone I., Destouches D., Delbé J., Courty J., Hamma-Kourbali Y. (2015) Pleiotrophin exerts its migration and invasion effect through the neuropilin-1 pathway. Neoplasia 17, 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hu T., She Q., Jiang Y., Su L., Yin Y. (2008) Level of CD14+-endothelial progenitor cells is not associated with coronary artery disease or cardiovascular risk factors. Age (Dordr.) 30, 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Krenning G., van der Strate B. W., Schipper M., van Seijen X. J., Fernandes B. C., van Luyn M. J., Harmsen M. C. (2009) CD34+ cells augment endothelial cell differentiation of CD14+ endothelial progenitor cells in vitro. J. Cell. Mol. Med. 13(8B), 2521–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dineen S. P., Lynn K. D., Holloway S. E., Miller A. F., Sullivan J. P., Shames D. S., Beck A. W., Barnett C. C., Fleming J. B., Brekken R. A. (2008) Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res. 68, 4340–4346. [DOI] [PubMed] [Google Scholar]
- 125.Roland C. L., Dineen S. P., Lynn K. D., Sullivan L. A., Dellinger M. T., Sadegh L., Sullivan J. P., Shames D. S., Brekken R. A. (2009) Inhibition of vascular endothelial growth factor reduces angiogenesis and modulates immune cell infiltration of orthotopic breast cancer xenografts. Mol. Cancer Ther. 8, 1761–1771. [DOI] [PubMed] [Google Scholar]
- 126.Huang Y., Chen X., Dikov M. M., Novitskiy S. V., Mosse C. A., Yang L., Carbone D. P. (2007) Distinct roles of Vegfr-1 and Vegfr-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood 110, 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sonobe Y., Li H., Jin S., Kishida S., Kadomatsu K., Takeuchi H., Mizuno T., Suzumura A. (2012) Midkine inhibits inducible regulatory T cell differentiation by suppressing the development of tolerogenic dendritic cells. J. Immunol. 188, 2602–2611. [DOI] [PubMed] [Google Scholar]
- 128.Chung J. S., Tamura K., Akiyoshi H., Cruz P. D. Jr., Ariizumi K. (2014) The DC-HIL/syndecan-4 pathway regulates autoimmune responses through myeloid-derived suppressor cells. J. Immunol. 192, 2576–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chung J. S., Bonkobara M., Tomihari M., Cruz P. D. Jr., Ariizumi K. (2009) The DC-HIL/syndecan-4 pathway inhibits human allogeneic T-cell responses. Eur. J. Immunol. 39, 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Banno H., Takei Y., Muramatsu T., Komori K., Kadomatsu K. (2006) Controlled release of small interfering RNA targeting midkine attenuates intimal hyperplasia in vein grafts. J. Vasc. Surg. 44, 633–641. [DOI] [PubMed] [Google Scholar]
- 131.Kawai H., Sato W., Yuzawa Y., Kosugi T., Matsuo S., Takei Y., Kadomatsu K., Muramatsu T. (2004) Lack of the growth factor midkine enhances survival against cisplatin-induced renal damage. Am. J. Pathol. 165, 1603–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kosugi T., Yuzawa Y., Sato W., Arata-Kawai H., Suzuki N., Kato N., Matsuo S., Kadomatsu K. (2007) Midkine is involved in tubulointerstitial inflammation associated with diabetic nephropathy. Lab. Invest. 87, 903–913. [DOI] [PubMed] [Google Scholar]
- 133.Takada T., Toriyama K., Muramatsu H., Song X. J., Torii S., Muramatsu T. (1997) Midkine, a retinoic acid-inducible heparin-binding cytokine in inflammatory responses: chemotactic activity to neutrophils and association with inflammatory synovitis. J. Biochem. 122, 453–458. [DOI] [PubMed] [Google Scholar]
- 134.Weckbach L. T., Gola A., Winkelmann M., Jakob S. M., Groesser L., Borgolte J., Pogoda F., Pick R., Pruenster M., Müller-Höcker J., Deindl E., Sperandio M., Walzog B. (2014) The cytokine midkine supports neutrophil trafficking during acute inflammation by promoting adhesion via β2 integrins (CD11/CD18). Blood 123, 1887–1896. [DOI] [PubMed] [Google Scholar]
- 135.Yeh H. J., He Y. Y., Xu J., Hsu C. Y., Deuel T. F. (1998) Upregulation of pleiotrophin gene expression in developing microvasculature, macrophages, and astrocytes after acute ischemic brain injury. J. Neurosci. 18, 3699–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Braun B. S., Tuveson D. A., Kong N., Le D. T., Kogan S. C., Rozmus J., Le Beau M. M., Jacks T. E., Shannon K. M. (2004) Somatic activation of oncogenic Kras in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder. Proc. Natl. Acad. Sci. USA 101, 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dorrell C., Takenaka K., Minden M. D., Hawley R. G., Dick J. E. (2004) Hematopoietic cell fate and the initiation of leukemic properties in primitive primary human cells are influenced by Ras activity and farnesyltransferase inhibition. Mol. Cell. Biol. 24, 6993–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]