Abstract
Aims
to explore the association between post return of spontaneous circulation (ROSC) hemoglobin level and survival with good neurological outcome following out-of-hospital cardiac arrest.
Methods
We studied adults with non-traumatic out-of-hospital cardiac arrest who achieved ROSC within 50 minutes of collapse. We quantified the association between post ROSC hemoglobin level and good neurological outcome (defined as Cerebral Performance Category score of 1or 2), using multivariate logistic regression analyses. The impact of Post ROSC hemoglobin level ≥ 10 g/dl and time varying hemoglobin level ≥ 10 g/dl on time to Survival with good outcome was assessed using Cox proportional hazard models.
Results
Of 931 cardiac arrest patients, 146 (16%) achieved ROSC and 30 survived to discharge with a good neurological outcome. Of those with post ROSC hemoglobin level ≥ 10 g/dl, 28% (27/98) had good outcome, whereas of those with level < 10 mg/dl only 6% (3/48) had good outcome (CPC < 3, p=0.003). The use of blood transfusions and therapeutic hypothermia were comparable in both good and bad outcome groups. An immediate post ROSC hemoglobin level ≥ 10 g/dl was significantly associated with good neurological outcome (AOR 8.31 95% CI 1.89–36.52 p=0.005). Patients with post ROSC hemoglobin ≥ 10 g/dl were more likely to achieve good outcome earlier (HR 6.02 95% CI 1.75–20.72 p=0.004).
Conclusions
Post ROSC hemoglobin level ≥ 10 g/dl is associated with survival with good neurological outcome. The importance of time to achieve such level and the role of blood transfusion warrants further investigation.
Keywords: Cardiopulmonary resuscitation, cardiac arrest, sudden death, Hemoglobin
1. Introduction
The International resuscitation committee has recommended comprehensive therapeutic strategies to improve outcome following cardiac arrest, including therapeutic hypothermia, percutaneous coronary intervention, early hemodynamic optimization and supportive care.1–5 The quintessential goal of such therapies is to achieve neurologically intact survival by maintaining adequate balance between oxygen delivery and consumption, thus alleviating the impact of ischemia and hypoxia on the brain tissue following arrest.
Cardiac arrest is a severe ischemic event that will eventually lead to the failure of organs’ defense mechanisms in the absence of adequate supportive therapies. The cerebrovascular system responds to hypoxia by increasing production of nitric oxide and stimulating sympathetic B2 receptors to achieve adequate vasodilatation that will maintain cerebral blood flow.6,7 However, in the setting of severe anemia, such increase in cerebral blood flow will eventually be insufficient to compensate for the decrease in arterial oxygen content caused by low hemoglobin levels.8 Moreover, in cardiac arrest as compared to other brain ischemic events, severe hypotension, myocardial dysfunction and lactic acidosis will further complicate the picture and decrease the chance of successful defense.
The optimal neuro-protective hemoglobin level following cardiac arrest has not yet been determined. Two studies maintained hemoglobin level above 9–10 g/dl in a post cardiac arrest protocol to improve survival.9,10 The SOS-KANTO study group demonstrated an association between higher hemoglobin level and favorable neurological outcome.11 However, there was no general agreement on the best hemoglobin level and whether such level will impact time to achieve neurologically intact survival following arrest. Hence the goal of this study was to explore the association between post arrest hemoglobin level ≥ 10 g/dl with survival with good neurological outcome, and the time to achieve good neurological outcome following arrest.
2. Methods
2.1. Setting and Study design
This study was approved by the Johns Hopkins Institutional Review Board. We performed a retrospective review of all adult patients over 18 years of age with non-traumatic out-of-hospital cardiac arrest who achieved ROSC within 50 minutes, and admitted to an academic medical center between 2004 and 2010. We excluded patients with traumatic cardiac arrest and those who died upon arrival to the emergency department. Out of 210 patients who achieved ROSC, 64 patients were excluded; 9 patients due to missing data, 10 patients were not comatose upon hospital admission and 45 did not achieve ROSC within 50 minutes. Those 45 patients were excluded as no one with ROSC > 50 minutes survived with good outcome (Fig. 1)
Fig. 1.
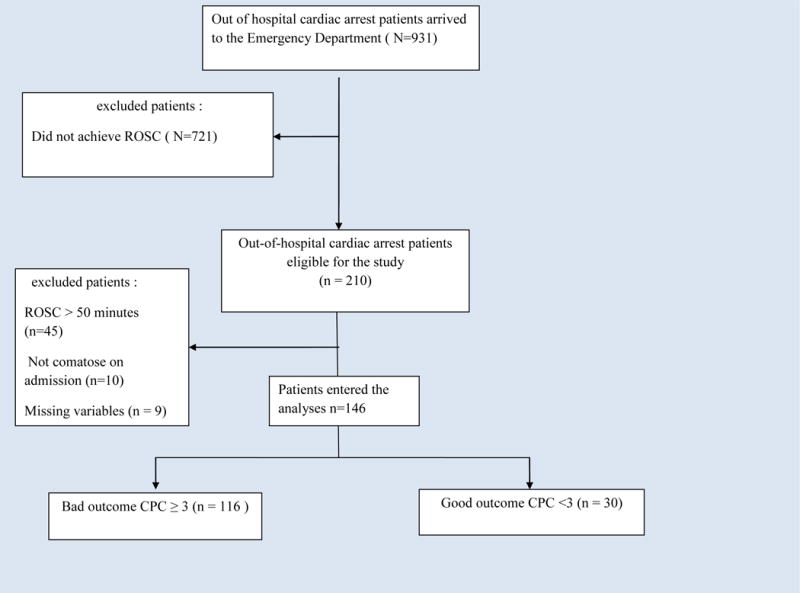
Study Flow Diagram. CPC: cerebral performance category, ROSC: return of spontaneous circulation.
2.2. Study variables and patient data
Primary outcome was survival with good neurological outcome defined as Cerebral Performance Category score (CPC) of 1 or 2, secondary outcome was time to achieve good neurological outcome in hours from the time of arrest. Patient demographics, cardiac arrest and post cardiac arrest variables were collected according to Utstein guidelines.12,13 Cardiac catheterization and percutaneous coronary intervention (PCI) were performed in select patients based on the decision of the cardiology attending. Similarly, therapeutic hypothermia was started in select patients according to a pre-defined hospital wide protocol that is based on the American Heart Association guidelines.2,3 Fluids and vasopressors were used as needed to maintain a mean arterial pressure ≥ 65 mmHg, and Propofol or Midazolam were used for sedation. Intensive care unit neurological assessments were performed by nursing staff, resident physicians, the ICU attending physician and often the consulting neurologist. Sedation was interrupted each morning to assess for recovery. In rare cases of discrepancy between observers, the report of the attending/neurologist was selected to reflect the status of the patient. Neurological outcome was assessed according to Glasgow-Pittsburgh Cerebral Performance Categories scale (CPC): CPC 1 is conscious and normal; CPC 2 conscious with moderate cerebral disability; CPC 3 conscious with severe cerebral disability; CPC 4 coma or vegetative state; and CPC 5 death.13 Patients with CPC 1 or 2 were considered to have good neurological outcome while those with CPC 3, 4 or 5 were considered to have bad neurological outcome.
2.3. Repeated measures
Hemoglobin levels were measured as follows for patients who met inclusion criteria: Post ROSC, every 6 hours for the first 24 hours and then every 24 hours until reaching good neurological outcome (CPC 1, 2) or bad outcome (CPC 3,4,5). For patients who achieved good outcome on a specific day, the immediate preceding 24 hour hemoglobin level was considered the last hemoglobin measured. Then survival analysis was performed to look at the impact of post ROSC hemoglobin level ≥ 10 g/dl and time varying hemoglobin level ≥ 10 g/dl on time to achieve good neurological outcome following cardiac arrest.
2.4. Statistical analysis
After the exclusion of patients who did not meet inclusion criteria, 146 patients entered the analyses and were divided by neurological outcome at the time of hospital discharge into patients with good (CPC 1 or 2) or bad (CPC 3, 4, or 5) neurological outcome (Fig. 1). Baseline demographics, clinical characteristics and cardiac arrest variables were studied in all 146 patients using simple statistics. Categorical variables were compared using Chi-squared test, and continuous variables were studied using unpaired t-test if normally distributed and non-parametric Wilcoxon rank-sum (Mann-Whitney) test when data were not normally distributed. The receiver operating characteristic (ROC) curve was used to evaluate the association between post ROSC hemoglobin levels and survival with good neurological outcome.
Univariate analyses were performed on all variables of interest (time to ROSC in minutes, initial rhythm VF/VT, the presence of shock requiring pressors, post ROSC hemoglobin ≥ 10 g/dl, age, the presence of multi-organ failure, post ROSC blood PH <7, post ROSC lactic acid level ≥ 10 mmol/L, congestive heart failure, cancer, ESRD on hemodialysis, brainstem reflex score within 24 ≥3) to explore association with favorable neurological outcome (CPC 1 or 2) using Chi square test. Multivariate logistic regression with stepwise backward-elimination was used to adjust for covariates that were found in univariate analyses to impact good neurological outcome with a P value of less than 0.05. Sensitivity analyses were used to evaluate the model performance following the exclusion of patients who had care withdrawal. Collinearity between variables was checked with the variation inflation factor (VIF), and the final model goodness of fit was checked using pseudo-R2 and Pearson χ2. Time to achieve good neurological outcome was assessed by Cox-proportional hazard models, censoring events were death and care withdrawal. Two different models were evaluated, one with only baseline hemoglobin as the predictor, and another with hemoglobin levels as a time-varying predictor. The proportional hazard assumption was tested based on Schoenfeld residuals (Phtest).
3. Results
3.1 Patient characteristics
Of 931 non-traumatic out of hospital cardiac arrest patients, 146 patients (16%) achieved ROSC within 50 minutes and met inclusion criteria. Of these 77 (53%) were male, 106 (73%) were Caucasian and 40 (27%) were black. The median age was 64.5 years IQR (53–77). Of 146 patients, 30 patients (21%) survived with good neurological outcome (CPC of 1 or 2), and 116 patients (79%) had bad outcome (CPC ≥ 3); of those 116 patients, 76 (65%) had care withdrawal. Therapeutic hypothermia was used in 71 patients (49%) with comparable use in patients with good and bad neurological outcome (57% vs.47% p=0.32). Among patients who underwent cardiac catheterization, only 3 patients had percutaneous coronary intervention (PCI); blood transfusion during hospital stay was performed in a total of 34 patients (23%) with comparable use in both groups (20% vs. 24% p=0.63) (Table 1). The association between different post ROSC hemoglobin levels and survival with good neurological outcome was assessed using the receiver operating characteristic (ROC) curve, AUC 0.65 95% CI (0.55–0.75). And post ROSC hemoglobin level ≥ 10 g/dl has a sensitivity of 90% and specificity of 39% (supplement table 1 and supplement figure 1).
Table 1.
Patients Characteristics
| Demographics, cardiac arrest characteristics and therapies
| ||||
|---|---|---|---|---|
| All patients N=146 |
Good Outcome N=30 |
Bad Outcome N = 116 |
P value | |
| Age, median (range) | 64.5 (53–77) | 57 (52–68) | 66.5 (56–78) | 0.03 |
| Male gender | 77 (53) | 14 (47) | 63 (54) | 0.46 |
| Race | ||||
| Caucasians | 106 (73) | 21 (70) | 85 (73) | 0.72 |
| Black | 40 (27) | 9 (30) | 31 (27) | |
| Co morbidities | ||||
| Diabetes Mellitus | 53 (36) | 10 (33) | 43 (37) | 0.70 |
| Hypertension | 110 (75) | 25 (83) | 85 (73) | 0.26 |
| Coronary artery disease | 58 (40) | 12 (40) | 46 (40) | 0.97 |
| Congestive heart failure | 55 (38) | 15 (50) | 40 (34) | 0.12 |
| CVA/TIA | 25 (17) | 4 (13) | 21 (18) | 0.54 |
| ESRD on HD | 14 (10) | 6 (20) | 8 (7) | 0.03 |
| COPD | 44 (30) | 8 (27) | 36 (31) | 0.64 |
| History of Cancer | 24 (16) | 9 (30) | 15 (13) | 0.03 |
| Co morbidity ≥2 | 105 (72) | 24 (80) | 81 (70) | 0.27 |
| Arrest characteristics | ||||
| Witnessed arrest | 113 (77) | 26 (87) | 87 (75) | 0.17 |
| Bystander CPR | 46 (32) | 9 (30) | 37 (32) | 0.84 |
| BRS ≥ 3 at 24 hours | 35 (24) | 15 (50) | 20 (17) | <0.0001 |
| Initial rhythm VF/VT | 28 (19) | 13 (40) | 16 (14) | 0.001 |
| Care withdrawal | 76 (52) | 0 (0) | 76 (65) | <0.0001 |
| Interval collapse to ROSC, min | 21.5 (12–35) | 9.5 (4–13) | 28.5 (16.5–40) | <0.0001 |
| Post arrest Hemoglobin g/dl | 11.4 (9.4–13.2) | 12.5 (11.2–13.5) | 10.8 (8.9–13.1) | 0.01 |
| Post arrest Hemoglobin ≥ 10 g/dl post arrest therapies | 98 (67) | 27 (90) | 71 (61) | 0.003 |
| Hypothermia | 71 (49) | 17 (57) | 54 (47) | 0.32 |
| Transfusion | 34 (23) | 6 (20) | 28 (24) | 0.63 |
| PCI | 3 (2) | 2 (7) | 1 (1) | 0.05 |
Data are: n (%), median (range). CVA/TIA: cerebrovascular accident/transient ischemic attack, ESRD On HD: end stage renal disease on hymodialysis, COPD: chronic obstructive lung disease, CPR: cardiopulmonary resuscitation, BRS: brain stem reflex score, VF/VT: ventricular fibrillation/ventricular tachycardia, ROSC: return of spontaneous circulation, PCI: percutaneous coronary intervention.
3.2 Univariate analysis
Good versus bad neurological outcome
Patients with good neurological outcome were more likely to have VF/VT as an initial rhythm compared to those with bad outcome (40% vs. 14%, p= 0.001). They were also more likely to have shorter time to return of spontaneous circulation [ROSC 9.5 minutes [4, 13] vs. 28.5 minutes [16.5, 40], P< 0.0001)], higher median post ROSC hemoglobin level (12.5 [11.2, 13.5] vs. 10.8 [8.9, 13.1], p= 0.01), and more patients with post ROSC hemoglobin level ≥ 10 g/dl (90% vs. 61%, p =0.003). Twenty eight percent (27/98) of patients with hemoglobin ≥10 had a good neurologic outcome while only six percent (3/48) of patients with hemoglobin <10 had a good neurologic outcome (p=0.003, Supplement table 2). In addition, patients with good neurological outcome were less likely to have shock requiring pressors (20% Vs 59% P<0.0001); they were also less likely to have post ROSC lactic acid level ≥ 10 mmol/L or PH <7 (Table 2).
Table 2.
Clinical Characteristics During Hospitalization
| All patients N=146 |
Good Outcome N=30 |
Bad Outcome N = 116 |
P value | |
|---|---|---|---|---|
| Clinical findings | ||||
| Sepsis | 70 (48) | 10 (33) | 60 (52) | 0.07 |
| Septic shock | 46 (32) | 4 (13) | 42 (36) | 0.02 |
| Cardiac shock | 38 (26) | 5 (17) | 33 (28) | 0.19 |
| Shock requiring pressors | 75 (51) | 6 (20) | 69 (59) | <0.0001 |
| MOF | 79 (54) | 11 (37) | 68 (59) | 0.03 |
| Myocardial infarction | 27 (18) | 9 (30) | 18 (15) | 0.07 |
| Pneumonia | 48 (33) | 11 (37) | 37 (32) | 0.62 |
| ARDS | 15 (10) | 2 (7) | 13 (11) | 0.47 |
| Pulmonary Embolism | 10 (7) | 1 (3) | 9 (8) | 0.39 |
| Acute renal failure | 106 (73) | 19 (63) | 87 (75) | 0.20 |
| Seizure (presentation) | 24 (16) | 3 (10) | 21 (18) | 0.29 |
| Post ROSC PH< 7 | 44 (30) | 3 (10) | 41 (35) | 0.007 |
| Post ROSC Lactic acid ≥ 10 mmol/L | 78 (53) | 8 (27) | 70 (60) | 0.001 |
Data are: n (%), median (range). MOF: multi-organ failure, ARDS: Acute respiratory distress syndrome, ROSC: return of spontaneous circulation.
3.3 Multivariable analysis
In multivariable logistic regression model with backward elimination, post ROSC hemoglobin level ≥ 10 g/dl was associated with survival with good neurological outcome (CPC of 1 or 2) (AOR 8.31, 95% CI 1.89–36.52), (Model 1-Table 3). After excluding patients who underwent care withdrawal, sensitivity analysis revealed a significant association between post ROSC hemoglobin level ≥ 10 g/dl and survival with good neurological outcome (AOR 9.19, 95% CI 1.72–49.1), (Model 2-Table 3). Sensitivity analyses also revealed similar results when we included patients who had ROSC >50 minutes.
Table 3.
Multivariable analysis for factors associated with good neurological outcome.
| Model 1: all patients N=146
| ||||
|---|---|---|---|---|
| Factors | OR | 95% Conf.interval | P value | Pseudo R2 |
| Post ROSC Hemoglobin ≥ 10 g/dl | 8.31 | 1.89–36.52 | 0.005 | 0.45 |
| Time from collapse to ROSC minutes | 0.87 | 0.81–0.92 | <0.0001 | |
| Initial Rhythm VF/VT | 6.40 | 1.72–23.87 | 0.006 | |
| Shock requiring pressors | 0.18 | 0.05–0.60 | 0.005 | |
| Model 2: Sensitivity analysis excluding patient with care withdrawal, N=70
| ||||
|---|---|---|---|---|
| Factors | OR | 95% Conf.interval | P value | Pseudo R2 |
| Post ROSC Hemoglobin ≥ 10 g/dl | 9.19 | 1.72–49.1 | 0.009 | 0.45 |
| Time from collapse to ROSC minutes | 0.91 | 0.85–0.98 | 0.009 | |
| Initial Rhythm VF/VT | 2.92 | 0.59–14.37 | 0.19 | |
| Shock requiring pressors | 0.10 | 0.03–0.42 | 0.001 | |
OR: Odds ratio, ROSC: return of spontaneous circulation, VF/VT: ventricular fibrillation/Ventricular tachycardia Goodness of fit P value tested using Pearson χ2 =0.99
OR: Odds ratio, ROSC: return of spontaneous circulation, VF/VT: ventricular fibrillation/Ventricular tachycardia Goodness of fit P value tested using Pearson χ2 =0.85
3.4 Survival analysis
Out of 30 patients who eventually achieved good neurological outcome (CPC 1 or 2), 27 patients had post ROSC hemoglobin ≥ 10 g/dl. Patients with post ROSC hemoglobin ≥ 10 g/dl were more likely to achieve good neurological outcome earlier than patients with post ROSC hemoglobin <10 g/dl (HR 6.02, 95% CI 1.75–20.72) (Table 4–Figure 2A). Further changes in hemoglobin during hospital stay were not associated with early neurological recovery (HR 1.74, 95% CI 0.78–3.88) (Table 4–Figure 2B). All 30 patients received sedation for a median of 2 days IQR (1–4).
Table 4.
Multivariable analysis for factors associated with time to survival with good neurological outcome.
| Model 1: Post ROSC hemoglobin, all patients N=146.
| ||||
|---|---|---|---|---|
| Factors | HR | 95% Conf.interval | P value | |
| Post ROSC Hemoglobin ≥ 10 | 6.02 | 1.75–20.72 | 0.004 | |
| Time from collapse to ROSC minutes | 0.92 | 0.88–0.96 | <0.0001 | |
| Active seizure on presentation | 0.22 | 0.06–0.78 | 0.02 | |
| Shock requiring pressors | 0.37 | 0.14–0.94 | 0.04 | |
| Model 2: Time varying hemoglobin, all patients N=146.
| |||
|---|---|---|---|
| Factors | HR | 95% Conf.interval | P value |
| Time varying Hemoglobin ≥ 10 | 1.74 | 0.78–3.88 | 0.18 |
| Time from collapse to ROSC minutes | 0.92 | 0.88–0.97 | 0.001 |
| Active seizure on presentation | 0.28 | 0.08–0.92 | 0.04 |
| Shock requiring pressors | 0.52 | 0.21–1.31 | 0.17 |
HR: Hazard ratio, ROSC: return of spontaneous circulation.
Fig. 2.
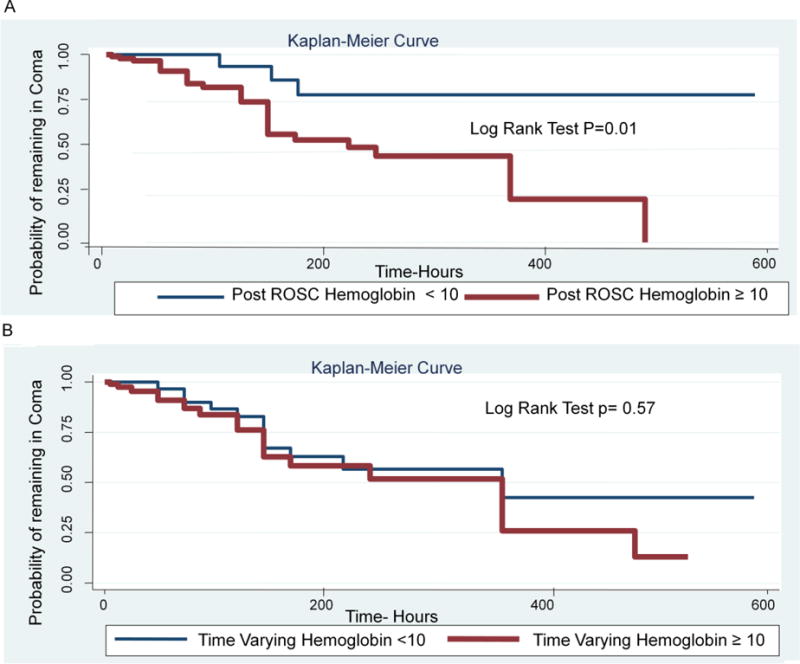
A: Post ROSC hemoglobin level and time to survival with good neurological outcome.
B: Time Varying Hemoglobin level and time to survival with good neurological outcome.
4. Discussion
In this study, we found that post ROSC hemoglobin level ≥ 10 g/dl was associated with good neurological outcome following out of hospital cardiac arrest. Patients with post ROSC hemoglobin level ≥ 10 g/dl were more likely to achieve good outcome earlier than those with hemoglobin level less than 10 g/dl. Further changes in hemoglobin during hospital stay were not associated with early favorable outcome, and this might reflect the importance of time to achieve such neuro-protective level.
Our results are consistent with literature reports of neurologically intact survival association with higher hemoglobin levels following cardiac arrest.11,14,15 The SOS-KANTO study group reported favorable short-term neurological outcome in patients with higher post arrest hemoglobin level. Study patients in the good neurological outcome group had a median hemoglobin level of 14.4 g/dl vs. 12.8 g/dl in the bad outcome group.11 Similarly, Ryu et. al. reported a significant association between favorable outcome and higher hemoglobin levels before extracorporeal cardiopulmonary resuscitation. Patients in the good outcome group had a median pre-ECMO hemoglobin level of 11.9 g/dl vs. 10.7 g/dl in the bad outcome group.15 Therefore, anemia correction might be neuroprotective.15
In similar ischemic neurologic injury models such as in animal stroke models, penumbral oxygenation in an ischemic brain had the best oxygen utilization at hematocrit of 31%.16 Inadequate oxygen utilization was shown at hemoglobin levels less than 10 g/dl,16 and with profound hemodilution to a hematocrit level of 26%.17 In addition, clinical stroke studies have also shown a U or J-shaped relationship between hemoglobin level and adverse outcome, where both high and low levels were associated with bad outcome;18,19 however, optimal oxygen delivery was achieved at a hematocrit level of 40–45%.20
Blood transfusion seems to be a reasonable treatment option to prevent further damage; however, this has never been studied following cardiac arrest. Most of the clinical trials that recommend the conservative approach of lower hemoglobin threshold for transfusion excluded patients with severe ischemic events like myocardial infarction.21,22,23 Similarly, the International Surviving Sepsis Campaign guidelines recommend maintaining a hematocrit of more than 30% (hemoglobin 10 g/dl) in the presence of hypoperfusion in the first 6 hours. After that, the transfusion threshold should be at a hemoglobin level less than 7 g/dl to achieve a level between 7 and 9 g/dl in patients who do not have myocardial ischemia, severe hypoxia, acute hemorrhage, or ischemic coronary artery disease.24 More recently, Salisbury et. al. reported lower risk of in-hospital mortality among acute myocardial infarction patients who received transfusion, and suggested that the observation of increased mortality reported in previous studies might have been influenced by selection bias, given the inability to match transfused and non-transfused patients.25
The current study also demonstrated that patients with post ROSC hemoglobin level ≥ 10 g/dl were more likely to achieve survival with good neurological outcome earlier as compared to those who had post ROSC hemoglobin <10 g/dl. This observation did not sustain with subsequent hemoglobin levels measured 6 hours later and thereafter during the hospital stay, as further changes in hemoglobin level did not associate with early neurological recovery (HR 1.74, 95% CI 0.78–3.88). This finding might fall in the same realm of the Sepsis Campaign recommendations of keeping the hematocrit level above 30% in the first 6 hours of hypoperfusion. It also raises an important question to be answered; whether there is a golden hour for transfusion following cardiac arrest. Prospective studies designed to answer this specific question are currently lacking.
5. Limitations
This study should be interpreted with the following limitations. First, this study reflects a single institution experience with a small sample size which may limit the generalizability of the results. However, our finding of the beneficial impact of higher hemoglobin level is consistent with literature reports. Second, the small number of patients with post ROSC hemoglobin level <10 g/dl who eventually achieved survival with good neurological outcome, limits the interpretation of the impact of post ROSC hemoglobin on time to achieve this outcome. Third, PCI was only performed in three patients, so we were not able to account for its impact on survival. Finally, we could not identify the impact of hemodilution on hemoglobin and hematocrit levels as it is common that post arrest patients will receive liberal amounts of fluids during resuscitation that would likely decrease these levels. However, the association of purposeful hemodilution and blood transfusion with neurologically intact survival following cardiac arrest has not been previously studied and warrants further investigations.
6. Conclusion
Post ROSC hemoglobin level ≥ 10 g/dl is associated with survival with good neurological outcome. Time to good neurologic outcome was shorter in patients with hemoglobin level ≥ 10. The importance of time to achieve such level and the role of blood transfusion warrants further investigation.
Supplementary Material
Acknowledgments
The Johns Hopkins CCHR-BEAD core.
Funding Sources: Dr. Dhananjay Vaidya was supported by NIH grant UL1TR001079 through the Johns Hopkins Institute for Clinical and Translational Research, and through the Johns Hopkins Center for Child and Community Health Research – BEAD Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: Aiham Albaeni, Shaker Eid, Bolanle Akinyele, Lekshmi narayan Kurup, and Nisha Chandra-Strobos declare that they have no conflict of interest.
Dhananjay Vaidya is a consultant for Consumable Science, Inc.
References
- 1.Neumar RW, Nolan JP, Adrie C, et al. Epidemiology, Pathophysiology, Treatment, and Prognostication A Consensus Statement From the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118(23):2452–83. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 2.Peberdy MA, Callaway CW, Neumar RW, et al. Post-cardiac arrest care: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(Suppl 3):S768–S786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 3.2005 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care, post resuscitation support. Circulation. 2005 Dec 13;112(24Suppl):IV1–203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen N, Wetterslev J, Gronberg T, et al. Targeted temperature management at 33°C versus 36° C after cardiac arrest. N Engl J Med. 2013 Dec 5;369(23):2197–206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 5.Callaway CW, Schmicker RH, Brown SP, et al. Early coronary angiography and induced hypothermia are associated with survival and functional recovery after out-of-hospital cardiac arrest. Resuscitation. 2014;85:657–663. doi: 10.1016/j.resuscitation.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hare GMT, Mazer CD, Mak W, et al. Hemodilutional anemia is associated with increased cerebral neuronal nitric oxide synthase gene expression. J Appl Physiol. 2003;94:2058–2067. doi: 10.1152/japplphysiol.00931.2002. [DOI] [PubMed] [Google Scholar]
- 7.Hare GM, Worrall JM, Baker AJ, Liu E, Sikich N, Mazer CD. Beta2 adrenergic antagonist inhibits cerebral cortical oxygen delivery after severe hemodilution in rats. Br J Anaesth. 2006;97:617–623. doi: 10.1093/bja/ael238. [DOI] [PubMed] [Google Scholar]
- 8.Kramer AH, Zygun DA. Anemia and red blood cell transfusion in neurocritical care. Crit Care. 2009;13(3):R89. doi: 10.1186/cc7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunde K, Pytte M, Jacobsen D, et al. Implementation of a standardized treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;74:227–234. doi: 10.1016/j.resuscitation.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Gaieski DF, Band RA, Abella BS, et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80(4):418–24. doi: 10.1016/j.resuscitation.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Hayashida K, Suzuki M, Shiroshita-Takeshita A, et al. Relationship between the hemoglobin level at the hospital arrival and post-cardiac arrest neurological outcome. SOS-KANTO study group. Am J Emerg Med. 2012 Jun;30(5):770–4. doi: 10.1016/j.ajem.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update and simplification of the Utstein templates for resuscitation registries: A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, heart and stroke foundation of Canada, Inter-American heart foundation, resuscitation councils of southern Africa) Circulation. 2004 Nov 23;110(21):3385–97. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 13.Cummins RO, Chamberlain DA, Abramson NS, et al. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: The Utstein style A statement for health professionals from a task force of the American Heart Association the European Resuscitation Council, the heart and stroke foundation of Canada, and the Australian Resuscitation Council. Circulation. 1991 Aug;84(2):960–75. doi: 10.1161/01.cir.84.2.960. [DOI] [PubMed] [Google Scholar]
- 14.Hayashida K, Nishiyama K, Suzuki M, et al. Estimated cerebral oxyhemoglobin as a useful indicator of neuroprotection in patients with post-cardiac arrest syndrome: a prospective, multicenter observational study. Crit Care. 2014 Aug 29;18(4):500. doi: 10.1186/s13054-014-0500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu JA, Cho YH, Sung K, et al. Predictors of neurological outcomes after successful extracorporeal cardiopulmonary resuscitation. BMC Anesthesiol. 2015 Mar 8;15:26. doi: 10.1186/s12871-015-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dexter F, Hindman BJ. Effect of haemoglobin concentration on brain oxygenation in focal stroke: a mathematical modeling study. Br J Anaesth. 1997 Sep;79(3):346–51. doi: 10.1093/bja/79.3.346. [DOI] [PubMed] [Google Scholar]
- 17.Tu YK, Kup MF, Liu HM. Cerebral oxygen transport and metabolism during graded isovolemic hemodilution in experimental global ischemia. J Neurol Sci. 1997 Sep 10;150(2):115–22. doi: 10.1016/s0022-510x(97)00111-1. [DOI] [PubMed] [Google Scholar]
- 18.Gagnon DR, Zhang TJ, Brand FN, et al. Hematocrit and the risk of cardiovascular disease–the Framingham study: a 34-year follow-up. Am Heart J. 1994 Mar;127(3):674–82. doi: 10.1016/0002-8703(94)90679-3. [DOI] [PubMed] [Google Scholar]
- 19.Diamond PT, Gale SD, Evans BA. Relationship of initial hematocrit level to discharge destination and resource utilization after ischemic stroke: a pilot study. Arch Phys Med Rehabil. 2003 Jul;84(7):964–7. doi: 10.1016/s0003-9993(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 20.Kusunoki M, Kimura K, Nakamura M, et al. Effects of hematocrit variations on cerebral blood flow and oxygen transport in ischemic cerebrovascular disease. J Cereb Blood Flow Metab. 1981;1(4):413–7. doi: 10.1038/jcbfm.1981.45. [DOI] [PubMed] [Google Scholar]
- 21.Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014 Oct 9;371(15):1381–91. doi: 10.1056/NEJMoa1406617. [DOI] [PubMed] [Google Scholar]
- 22.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 23.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 24.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salisbury AC, Reid KJ, Marso SP, et al. Blood transfusion during acute myocardial infarction: association with mortality and variability across hospitals. J Am Coll Cardiol. 2014 Aug 26;64(8):811–9. doi: 10.1016/j.jacc.2014.05.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


