Abstract
Objective
Apolipoprotein A-I (apoA-I) mimetic peptides have anti-atherogenic properties of high-density lipoprotein in vitro and have been shown to inhibit atherosclerosis in vivo. It is unclear, however, if each in vitro anti-atherogenic property of these peptides translates to a corresponding activity in vivo and if so, which of these contributes most to reduce atherosclerosis.
Approach and Results
The effect of seven apoA-I mimetic peptides, which were developed to selectively reproduce a specific component of the anti-atherogenic properties of apoA-I, on the development of atherosclerosis was investigated in apoE−/− mice fed a high fat diet for 4 or 12 weeks. The peptides include those that selectively upregulate cholesterol efflux, or are anti-inflammatory, or have anti-oxidation properties. All the peptides studied effectively inhibited the in vivo development of atherosclerosis in this model to the same extent. However, none of the peptides had the same selective effect in vivo as they had exhibited in vitro. None of the tested peptides affected plasma lipoprotein profile; capacity of plasma to support cholesterol efflux was increased modestly and similarly for all peptides.
Conclusions
There is a discordance between the selective in vitro and in vivo functional properties of apoA-I mimetic peptides and the in vivo anti-atherosclerotic effect of apoA-I-mimetic peptides is independent of their in vitro functional profile. Comparing the properties of apoA-I mimetic peptides in plasma rather than in the lipid-free state is better for predicting their in vivo effects on atherosclerosis.
Keywords: atherosclerosis, apolipoprotein A-I, mimetics, high-density lipoprotein, cholesterol
Apolipoprotein A-I (apoA-I) is the key structural element of high-density lipoprotein (HDL). While the best studied and probably the most important function of HDL is its role as a specific cholesterol acceptor in the reverse cholesterol transport pathway (RCT), HDL has many other functions including anti-inflammation, anti-oxidation, anti-proliferation and anti-thrombosis 1.
ApoA-I mimetic peptides were designed to recreate the various beneficial functions of HDL, mostly in the context of protection against atherosclerosis. Most apoA-I mimetics have no homology to the primary structure of apoA-I instead mimicking a key element of its secondary structure, namely a 22-mer amphipathic α-helix, which is believed to be responsible for lipid-acceptor properties of apoA-I. ApoA-I mimetics have been reported to be very effective acceptors of cellular cholesterol and are capable of selectively reproducing functional aspects of HDL in vitro. They have also been shown to be atheroprotective in vivo in animal models (for review see 2). ApoA-I mimetic peptides thus offer the opportunity to create new therapeutics with potentially superior properties to that of HDL.
We recently reported on a panel of apoA-I mimetic peptides where we were able to delineate the separate functional properties of each peptide in vitro 3. Some peptides were active as cholesterol acceptors with reduced anti-inflammatory and anti-oxidation capacity, while others had the inverse properties. In the current study, we tested several peptides from this panel in a mouse model of atherosclerosis to establish if differences in the in vitro capacity of each peptide translates into its corresponding effect in vivo and the ability of this to reduce atherosclerosis.
Materials and Methods
Detailed Methods section is available in the online-only Data Supplement.
Results
The effect of apoA-I mimetic peptides on early lesion development
The peptides chosen for study, together with details outlining the functional capacity of each, were previously published 3 and are summarized in Supplemental Table I. The peptides included ELK-2A2K2E, potent in cholesterol efflux, ELKA-CH2, a selective inhibitor of CD11b expression in monocytes, ELK-2A, a selective inhibitor of VCAM-1 expression in endothelial cells and 5A-CH1, which inhibits LDL oxidation. In addition, we examined the synergistic effect of ELK-2A2K2E and 5A-C1 (each peptide was added at half the concentration compared to that when tested individually). This peptide combination was selected on the basis of reproducing, at least in vitro, all four of the anti-atherogenic profiles under study (Supplemental Fig. I). Finally, the well-characterized and versatile peptide 5A was also studied under the same conditions 4, 5. The effect of each peptide on the development and characteristic of atherosclerotic plaques in apoE−/− mice fed HFD were investigated.
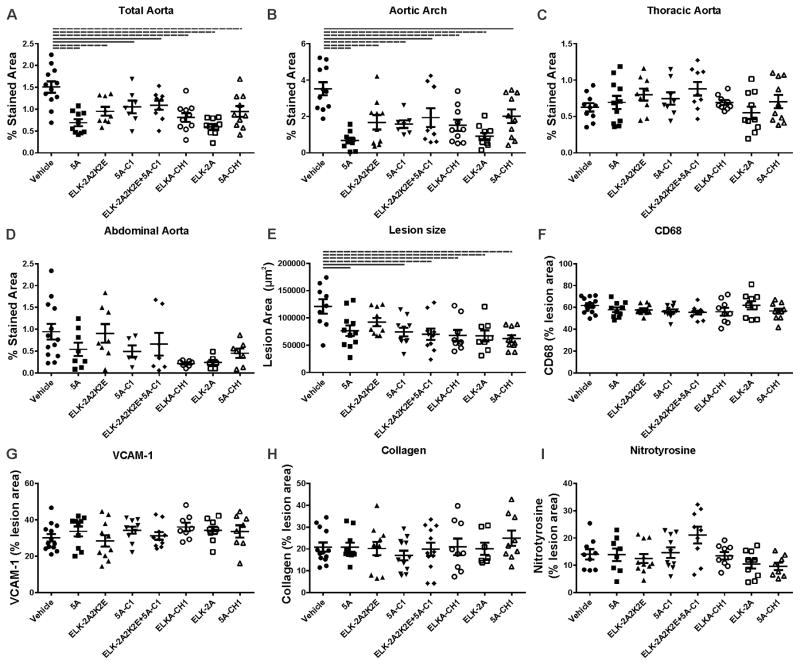
In the first set of experiments the effect of each peptide on the development of early atherosclerotic lesions in apoE−/− mice after 4 weeks feeding with HFD was examined. En face analysis of the aorta is presented in Fig. 1A–D and Supplemental Fig. IIA. All peptides have significantly reduced the abundance of atherosclerotic lesions to the same extent in total aorta (Fig 1A) and the aortic arch (Fig 1B). Few lesions were found in the thoracic (Fig. 1C) or abdominal (Fig. 1D) aorta, which were unaltered by any of the peptides. The development of atherosclerosis was then analysed in sections of the aortic sinus. Again, all peptides reduced the size of atherosclerotic lesions, and the effect was similar for all tested peptides (Fig. 1E and Supplemental Fig. IIIA).
Figure 1. The effect of apoA-I mimetic peptides on early lesion development (4 weeks HFD feeding).
The peptides were injected intraperitoneally for 4 weeks into apoE−/− mice fed HFD. The dissected aorta was stained with Sudan IV and analysed en face for abundance of atherosclerotic lesions in whole aorta (A), aortic arch (B), thoracic aorta (C) and abdominal aorta (D). The heart was dissected and the attached aortic sinus sectioned and stained to examine the lesion size and composition with: Oil Red O stain for lesion size (E), for CD68 for macrophage contents (F) anti whole and soluble VCAM-1 for lesion inflammation (G), Masson’s trichrome for collagen contents (H) and for nitrotyrosine to determine oxidative stress of the lesion. Solid lines connect pairs with p<0.05, dashed lines connect pairs with p<0.01.
To characterize the effect of the peptides on the composition of the plaques, aortic sinus sections were analysed for the abundance of CD68 positive cells (macrophages, Fig. 1F and Supplemental Fig. IIIB), VCAM-1 (soluble and cellular) (Fig. 1G and Supplemental Fig. IIIC), collagen (Fig. 1H and Supplemental Fig. IIID) and nitrotyrosine (Fig. 1I and Supplemental Fig. IIIE). None of these were different to the vehicle control. It is important to recognize that parameters of plaque composition are presented relative to the plaque size. Because treatment with the peptides reduced plaque size, the absolute values of plaque composition parameters were also reduced, however, no difference between the effects of different peptides was found (Supplemental Figure IV).
The effect of each peptide on plasma lipoprotein profile was analysed after 4 weeks of high-fat feeding. HFD caused a sharp elevation in total cholesterol (Supplemental Fig. VA) and LDL-C (Supplemental Fig. VB) levels, had no effect on TG levels (Supplemental Fig. VC) and caused a sharp reduction of HDL-C level (Supplemental Fig. VD). None of the peptides when tested 24 hours after the last dose had a major effect on these plasma lipoprotein levels (Supplemental Fig. VA–D). None of the peptides affected the weight of the animals (Supplemental Fig. VE).
To assess the effect of the peptides on systemic inflammation, we measured the levels of plasma cytokines after treatment with the peptides (Supplemental Table II and Supplemental Fig. VI). Across the panel of 13 cytokines measured, only elevation in IFN-β and IL-27 reached statistical significance and overall effects were mild with no consistent difference between the effects of different peptides.
The effect of apoA-I mimetic peptides on development of atherosclerotic plaque
The potential synergistic effect of two peptides, ELK-2A2K2E and 5A-C1, chosen since together they reproduced all the key in vitro anti-atherogenic properties, was further examined in an extended 12 weeks study.
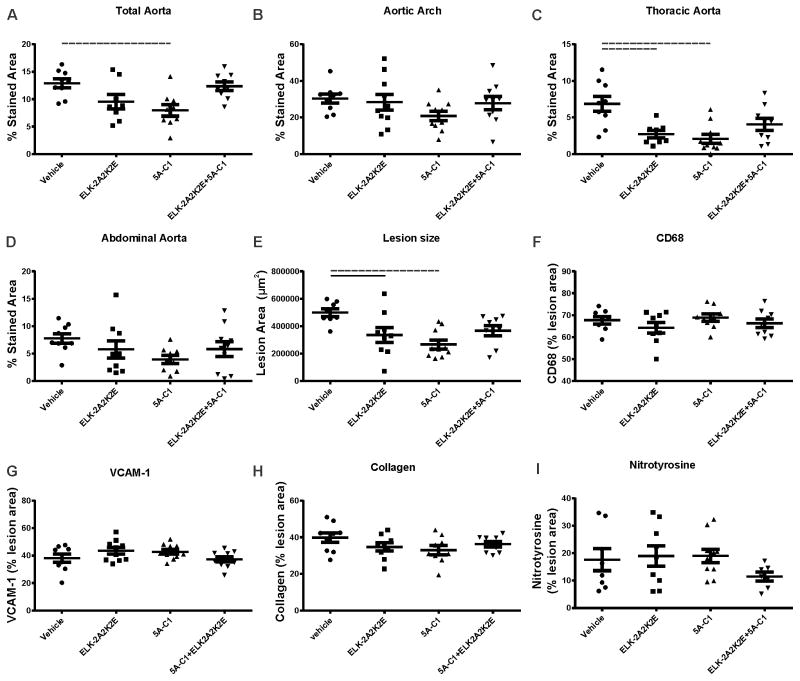
Both peptides individually reduced total aorta abundance of atherosclerotic plaques by 30–50%, while the peptide combination showed no significant effect (Fig. 2A, Supplemental Fig. IIB). A similar pattern was observed when regional differences were analysed (Fig. 2B–D). Analysis of atherosclerotic plaques in the sections of the aortic sinus confirmed inhibition of the development of atherosclerosis by the individual peptides but not by their combination (Fig. 2E, Supplemental Fig. VIIA). When plaque composition was analysed, we found no effect of the individual peptides nor peptide combination on macrophage infiltration (Fig. 2F, Supplemental Fig. VIIB), abundance of VCAM-1 (Fig. 2G, Supplemental Fig. VIIC), collagen (Fig. 2H, Supplemental Fig. VIID) or oxidized proteins (Fig. 2I, Supplemental Fig. VIIE).
Figure 2. The effect of apoA-I mimetic peptides on development of atherosclerotic plaque (12 weeks HFD feeding).
The peptides were injected intraperitoneally for 12 weeks into apoE−/− mice fed HFD. The dissected aorta was stained with Sudan IV and analysed en face for abundance of atherosclerotic lesions in whole aorta (A), aortic arch (B), thoracic aorta (C) and abdominal aorta (D). The heart was dissected and the attached aortic sinus sectioned and stained to examine the lesion size and composition with: Oil Red O stain for lesion size (E), for CD68 for macrophage contents (F) anti whole and soluble VCAM-1 for lesion inflammation (G), Masson’s trichrome for collagen contents (H) and for nitrotyrosine to determine oxidative stress of the lesion. Solid lines connect pairs with p<0.05, dashed lines connect pairs with p<0.01.
When the effect of the peptides on plasma lipoprotein profile was assessed, elevation of total cholesterol (Supplemental Fig. VIIIA), LDL-C (Supplemental Fig. VIIIB) and TGs (Supplemental Fig. VIIIC) caused by 12 weeks HFD feeding were inhibited by peptide combination, but was un-affected by the individual peptides. The sharp reduction of HDL-C level (Supplemental Fig. VIIID) induced by HFD was unaffected by peptide treatment. We found no effect of the peptides on weight of the animals (Supplemental Fig. VIIIE).
Finally, we investigated the effect of the peptides on the abundance of immune cells in lymphoid organs. The only effect found was that both individual peptides and the peptide combination caused an increase in the proportion of GR1Lo monocytes in the blood with a corresponding reduction in the lymph nodes after 12 weeks (Supplemental Fig. VIII F, G).
Pharmacokinetics and biodistribution of the peptides
We then examined whether the co-administration of the peptides affected their pharmacokinetics and biodistribution. Peptides ELK-2A2K2E and 5A-C1 were labelled with Alexa 350 and Cascade yellow respectively and injected intraperitoneally into apoE K/O mice, fed HFD for 2 weeks, either individually or as a 1:1 mixture. The pharmacokinetics of ELK-2A2K2E injected alone was similar to that observed in our previous study 6 (t1/2=2.25 h), with 5A-C1 had a longer half-life (t1/2=3.8 h) (Supplemental Fig. IXA). When the two peptides were injected together the half-life of both peptides was identical (t1/2=3.8 h) (Supplemental Fig. IXB). Both peptides were stable in vitro at 4°C for at least 7 days.
Next we investigated dynamics of distribution of both peptides among lipoprotein fractions when peptides were injected together. One hour after injection a relatively large proportion of the peptides was degraded, however, most of the intact ELK-2A2K2E was found as an unbound peptide with the remainder bound to HDL. Most of 5A-C1 was also found as an unbound peptide with only minor proportion bound to HDL and LDL fractions (Supplemental Fig. IXC). After 2 h, the distribution of ELK-2A2K2E was mostly unchanged, but relatively more 5A-C1 was found in HDL fraction (Supplemental Fig. IXD). After 6 h, most of the peptides were degraded; the intact ELK-2A2K2E was evenly divided between unbound peptide, HDL and LDL fractions while intact 5A-C1 was remained mainly unbound or in HDL fraction (Supplemental Fig. IXE). Thus, despite having been administered together, the two peptides distributed between lipoprotein fractions independently of each other.
Functionality of the peptides in plasma
We have then compared the cholesterol efflux in vitro to plasma collected from mice treated with peptides in vivo with cholesterol efflux to peptides added in lipid-free form, or after adding peptides to hyperlipidaeimic mouse plasma ex vivo. The capacity of lipid-free peptides to support cholesterol efflux in vitro was consistent with that found in our previous study 3 (Supplemental Figure XA). When the peptides were added to mouse plasma and incubated for 1 h at 37°C, they statistically significantly, albeit modestly, increased the capacity of the plasma to support cholesterol efflux in vitro (Supplemental Figure XB,C). Interestingly, the differences between the peptides moderated: the peptides that shown little cholesterol efflux capacity in lipid-free form (5A-C1, ELKA-CH1 and 5ACH1) were almost as effective as “potent” peptides when added to plasma. Similar trends were observed when plasma of mice treated with peptides in vivo was used as an acceptor, but the effects weakened and most of them did not reach statistical significance (Supplemental Figure XD). Thus, supplementation of plasma with peptides both in vitro and in vivo improved plasma cholesterol efflux capacity, but the improvement was modest and similar for all peptides.
Finally, we tested if the peptides are lytic causing haemolysis. No haemolysis of murine red blood cells was caused by any of the peptides added at concentrations up to the maximum level found in plasma (Supplemental Figure XE).
Discussion
ApoA-I mimetic peptides were designed to reproduce key elements of the atheroprotective properties of HDL (such as cholesterol efflux, anti-inflammation and anti-oxidation) and to prevent the development of atherosclerosis in vivo. Since both objectives had been achieved, we sought to define which of those properties, if any, played the larger role. Our major finding was that they were equally potent in preventing the development of atherosclerosis, but more surprisingly, that none of the specific anti-atherosclerotic elements observed using in vitro assays were observable in vivo.
There are several ways to interpret this data. It may be that all the elements examined had equal bearing on the ability of each peptide to prevent the development of atherosclerosis. However, given that none of these in vitro elements, upon which each peptide was chosen, translated into the corresponding in vivo feature, it is more likely that the atheroprotective effect of these peptides occurs independently of these features by a different mechanism altogether. One such mechanism may be the recently reported atheroprotective effects of apoA-I mimetic peptides that is unrelated to mimicking HDL in the vascular milieu, instead targeting small intestine environment. According to this hypothesis atheroprotection is achieved through the mitigation of the systemic inflammatory effects of the intestine-derived oxidized lipids 7, 8, or through modulation of transintestinal cholesterol efflux 9. Another possibility is the ability of the peptides to enhance production of natural anti-ox-LDL antibodies10. However, it is important to recognize that the peptides tested in this study were not exhaustively characterized in relation to every potential anti-atherogenic activity of HDL. For example, the ability of these peptides to stabilize ABCA1 and reduce abundance of lipid rafts or improve vascular reactivity or their potential anti-platelet and anti-apoptotic properties, were not studied. Furthermore, the differences in key anti-atherogenic property of the peptides, enhancing cholesterol efflux, levelled out when peptides were added to plasma ex vivo and in vivo, corresponding to their effect on development of atherosclerosis. This is consistent with findings of Tang et al 11 indicating that the biologic properties of the peptides are affected by their lipidation state.
Another finding of interest was that apoA-I mimetic peptides were more active in reducing the early stages of development of atherosclerosis than in mitigating a developed atherosclerotic plaque. Not only were the overall effects on atherosclerosis more pronounced after 4 weeks compared to 12 weeks of HFD feeding, but the effects were predominantly in thoracic aorta in the longer term study, where initiation of atherosclerosis was delayed when compared to the aortic arch 12. This suggests that apoA-I mimetic peptides predominantly target early events in the pathogenesis of atherosclerosis, i.e. inflammation and accumulation of cholesterol; this is consistent with findings of Wool et al.10 However, while all the peptides reduced the lesion cholesterol accumulation, no effect on local inflammation was observed. The effects on levels of plasma cytokines were minimal, but increased proportion of anti-inflammatory GR1Lo monocytes in blood and reduced proportion in lymph nodes may be an indirect indication of systemic anti-inflammatory action of the peptides.
We thus conclude that while apoA-I mimetic peptides are anti-atherogenic, there is a discordance between the in vitro and in vivo functional properties of apoA-I mimetic peptides which requires us to be cautious in our reliance on some of the standard assay systems commonly used. Determining the various in vitro functional properties of apoA-I mimetic peptide in plasma rather than in the lipid-free state most likely better reflects their physiologic effect and better correlates with the observed in vivo effects of the peptides on atherosclerosis. The in vitro assessment of future apoA-I mimetic peptides should, therefore, be based on assays that use plasma as the matrix.
Supplementary Material
Highlights.
Several apoA-I mimetic peptides that have different functional properties in vitro were equally effective in reducing atherosclerosis in animal model
There is a discordance between the selective anti-atherogenic properties of apoA-I mimetic peptides in vitro and in vivo
Acknowledgments
The authors acknowledge the use of facilities of Monash Micro Imaging, AMREP, Victoria, Australia.
Sources of Funding
This study was supported by grants GNT1003106 and GNT1036352 from the National Health and Medical Research Council of Australia.
Non-standard Abbreviations and Acronyms
- apoA-I
apolipoprotein A-I
- apoE
apolipoprotein E
- HDL
high-density lipoprotein
- HFD
high-fat diet
- LDL
low-density lipoprotein
- RCT
reverse cholesterol transport
- TG
triglyceride
Footnotes
Conflict of interests
None
References
- 1.Sviridov D, Mukhamedova N, Remaley AT, Chin-Dusting J, Nestel P. Antiatherogenic Functionality of High Density Lipoprotein: How Much versus How Good. J Atheroscler Thromb. 2008;15:52–62. doi: 10.5551/jat.e571. [DOI] [PubMed] [Google Scholar]
- 2.Sviridov D, Remaley Alan T. High-density lipoprotein mimetics: promises and challenges. Biochem J. 2015;472:249–259. doi: 10.1042/BJ20150832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Souza W, Stonik JA, Murphy A, Demosky SJ, Sethi AA, Moore XL, Chin-Dusting J, Remaley AT, Sviridov D. Structure/Function Relationships of Apolipoprotein A-I Mimetic Peptides: Implications for Antiatherogenic Activities of High-Density Lipoprotein. Circ Res. 2010;107:217–227. doi: 10.1161/CIRCRESAHA.110.216507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amar MJA, D’Souza W, Turner S, Demosky S, Sviridov D, Stonik J, Luchoomun J, Voogt J, Hellerstein M, Sviridov D, Remaley AT. 5A Apolipoprotein Mimetic Peptide Promotes Cholesterol Efflux and Reduces Atherosclerosis in Mice. J Pharmacol Experiment Ther. 2010;334:634–641. doi: 10.1124/jpet.110.167890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabet F, Remaley AT, Segaliny AI, Millet J, Yan L, Nakhla S, Barter PJ, Rye K-A, Lambert G. The 5A Apolipoprotein A-I Mimetic Peptide Displays Antiinflammatory and Antioxidant Properties In Vivo and In Vitro. Arterioscler Thromb Vasc Biol. 2010;30:246–252. doi: 10.1161/ATVBAHA.109.200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ditiatkovski M, D’Souza W, Kesani R, Chin-Dusting J, de Haan JB, Remaley A, Sviridov D. An Apolipoprotein A-I Mimetic Peptide Designed with a Reductionist Approach Stimulates Reverse Cholesterol Transport and Reduces Atherosclerosis in Mice. PLoS ONE. 2013;8:e68802. doi: 10.1371/journal.pone.0068802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navab M, Reddy ST, Van Lenten BJ, Buga GM, Hough G, Wagner AC, Fogelman AM. High-Density Lipoprotein and 4F Peptide Reduce Systemic Inflammation by Modulating Intestinal Oxidized Lipid Metabolism. Arterioscler Thromb Vasc Biol. 2012;32:2553–2560. doi: 10.1161/ATVBAHA.112.300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navab M, Reddy ST, Anantharamaiah GM, Imaizumi S, Hough G, Hama S, Fogelman AM. Intestine may be a major site of action for the apoA-I mimetic peptide 4F whether administered subcutaneously or orally. J Lipid Res. 2011;52:1200–1210. doi: 10.1194/jlr.M013144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meriwether D, Sulaiman D, Wagner A, Grijalva V, Kaji I, Williams KJ, Yu L, Fogelman S, Volpe C, Bensinger SJ, Anantharamaiah GM, Shechter I, Fogelman AM, Reddy ST. Transintestinal transport of the anti-inflammatory drug 4F and the modulation of transintestinal cholesterol efflux. J Lipid Res. 2016;57:1175–1193. doi: 10.1194/jlr.M067025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wool GD, Cabana VG, Lukens J, Shaw PX, Binder CJ, Witztum JL, Reardon CA, Getz GS. 4F Peptide reduces nascent atherosclerosis and induces natural antibody production in apolipoprotein E-null mice. FASEB J. 2011;25:290–300. doi: 10.1096/fj.10-165670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang J, Li D, Drake L, Yuan W, Deschaine S, Morin EE, Ackermann R, Olsen K, Smith DE, Schwendeman A. Influence of route of administration and lipidation of apolipoprotein A-I peptide on pharmacokinetics and cholesterol mobilization. J Lipid Res. 2017;58:124–136. doi: 10.1194/jlr.M071043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.