Abstract
Infantile spasms are the typical seizures of West syndrome, an infantile epileptic encephalopathy with poor outcomes. There is an increasing need to identify more effective and better tolerated treatments for infantile spasms. We have optimized the rat model of infantile spasms due to structural etiology, the multiple-hit rat model, for therapy discovery. Here, we test three compounds administered after spasms induction in the multiple hit model for efficacy and tolerability. Specifically, postnatal day 3 (PN3) male Sprague-Dawley rats were induced by right intracerebral injections of doxorubicin and lipopolysaccharide. On PN5 p-chlorophenylalanine was given intraperitoneally (i.p.). Daily monitoring of weights and developmental milestones was done and rats were intermittently video monitored. A blinded, randomized, vehicle-controlled study design was followed. The caspase 1 inhibitor VX-765 (50–200mg/kg i.p.) and the GABAB receptor inhibitor CGP35348 (12.5–100mg/kg i.p.) each was administered in different cohorts as single intraperitoneal injections on PN4, using a dose- and time-response design with intermittent monitoring till PN5. 17β-estradiol (40ng/g/day subcutaneously) was given daily between PN3-10 and intermittent monitoring was done till PN12. None of the treatments demonstrated acute or delayed effects on spasms, yet all were well tolerated. We discuss the implications for therapy discovery and challenges of replication trials.
Keywords: CGP35348, estradiol, VX-765, replication, epilepsy, seizure, drug resistant
Introduction
Infantile spasms (IS) are the typical seizures observed in West syndrome, an infantile epileptic encephalopathy with multiple etiologies (structural/metabolic, genetic, or unknown), often poor prognosis in regards to developmental and epilepsy outcomes and only few available treatments [1–8]. The characteristic ictal encephalographic pattern of IS is the electrodecremental response (EDR) while hypsarrhythmia (multifocally epileptic, high amplitude disorganized and slow) is the interictal EEG signature in most, but not all, infants with West syndrome [1,9]. The available treatments include adrenocorticotropic hormone (ACTH), high dose glucocorticoids, vigabatrin, which has a special indication for IS in tuberous sclerosis complex (TSC) patients, ketogenic diet as adjunctive therapy or B6 [1–6,10–12]. Other antiseizure drugs (e.g. valproic acid, zonisamide) are being used in cases when such treatments fail or cannot be administered. The efficacy of these treatments overall ranges between 39–87% depending on the study [2,5]. According to the studies reviewed in the above references, cessation of spasms and EEG normalization or resolution of hypsarrhythmia occurs between 48–87% of patients, relapses occur in a third of treated patients, while a normal or slightly subnormal cognitive outcome, defined as intelligence quotient greater than 68 occurs in approximately 22% of patients. Enduring epilepsy ensues in two thirds of the patients. Such outcomes emphasize the clinical gaps and need in finding better therapies for IS, including those infants that are less fortunate to have structural/metabolic etiologies that provide less favorable prognosis.
Most studies suggest that IS due to structural/metabolic etiologies have graver prognosis and respond less to medical treatment, although some reports differ, while infants with IS of unknown etiologies have better prognosis [1,5]. Early cessation of spasms has been proposed as an important factor in determining a better outcome, particularly in patients with IS due to unknown etiologies [13–19], although studies with different conclusions exist [20]. The currently used treatments of IS, ACTH and vigabatrin, demonstrate a lag of few days to weeks before they show the first effect on IS and therefore treatments with a more rapid mode of action would offer a significant advantage, both in regards to length of stay shortening in the hospital but, most importantly, in potentially improving the outcomes.
Tolerability and side effects is another important concern with such therapies which may lead to significant side effects in certain patients, including retinal neurotoxicity and MRI changes (vigabatrin) [21–23] or side effects of hormonal therapy such as electrolyte or metabolic abnormalities, hypertension, cortical atrophy, infections [1,24,25]. A major concern, at least in the United States, is the significant financial cost associated with the administration of ACTH [26]. All these factors warrant the identification of alternatives that will offer better efficacy and tolerability profile for IS, including the potential to improve disease course and outcomes.
Although pediatric clinical trials to test antiseizure drugs designed by extrapolation from findings in adults are an option for focal onset seizures in children over 2 years of age [27–29], they are more difficult to justify for infants with IS due to the very young age and the peculiar pharmacosensitivity of the seizures seen in this syndrome [30]. For example, phenytoin, carbamazepine or barbiturates have no effect on IS [31,32]. Therefore, the need for animal models of IS and West syndrome emerged, so as to allow for the development of syndrome-specific new treatments. The Workshop on Models of Pediatric Epilepsies (Bethesda MD, May 13–14, 2004) co-sponsored by the National Institute of Health/National Institute of Neurological Diseases and Stroke (NIH/NINDS), the American Epilepsy Society (AES), and the International League Against Epilepsy (ILAE) set the pace by defining the criteria for a model of infantile spasms and West syndrome [33], following which a growing number of models were proposed and reported [8]. In our laboratory, we have developed and optimized the multiple-hit rat model of IS, a chronic rat model induced at postnatal day 3 (PN3) by intracerebral injections of doxorubicin (DOX) and lipopolysaccharide (LPS), followed by intraperitoneal (i.p.) injection of the serotonin depleter p-chlorophenylalanine (PCPA) on PN5 (henceforth called the DLP model) [34]. The intent was to generate cytotoxic injury (via DOX) and white matter injury (via LPS), while disrupting the serotonin metabolism (PCPA), based on past studies implicating such pathologies in the pathogenesis of IS (reviewed in [7]). The DLP model exhibits clusters of spasms between PN4-13, other seizures from PN9 and on, as well as epilepsy in adulthood, as well as learning, memory, and sociability deficits [34–37]. These suggest that PCPA is not necessary for induction of spasms, yet we maintain it in our protocol to preserve the continuity and comparability of our results with the various drug studies we have conducted. Although the underlying induced lesion at the right cortical and hippocampal region and adjacent periventricular areas (see [35,38] for description of lesion at different ages) contributes to the cognitive deficits, early treatment with rapamycin (mTOR inhibitor) partially improves learning [36]. Previous characterization of this DLP model showed that it is resistant to ACTH, partially and transiently responsive to vigabatrin, while phenytoin – as expected – has no effect on IS, posing this as a model of IS due to structural lesions with drug resistant spasms. This is in agreement with the clinical experience that IS of structural etiology are more drug resistant [1]. Two of the drugs tested in our model (carisbamate and CPP-115) have already been designated orphan drugs with indication for IS by the Food and Drug Administration (FDA) in the USA (Table 1).
Table 1.
Pharmacosensitivity profile of DLP spasms and associated comorbidities
| Drug | Mechanism of action | Acute effect (latency to onset) | Sustained effect with repeat dosing | Tolerability | |
|---|---|---|---|---|---|
| Treatment period | Effect | ||||
| A. For model characterization | |||||
| ACTH 1–24 (Synacthene) [34] | Multiple ACTH signaling effects | No effect | PN4-12 | No effect on spasms | No side effects |
| Vigabatrin [34] | GABA aminotransferase inhibitor | Effective with delay (within 18–24 hours) | PN4-11 | 1 day | High mortality (sedation, poor feeding) |
| Phenytoin [45] | Sodium channel blocker | No effect | Not tested | Not tested | No side effects |
| B. Experimental drugs | |||||
| CPP-115 (vigabatrin analog) [35]§ | GABA aminotransferase inhibitor, high affinity | Effective (within the first hour) | PN4-12 | 2–3 days | No side effects |
| Rapamycin [36] | mTOR inhibitor (mTORC1 preferential) | Effective (within the first 2 hours) | PN4-6 | Suppresses spasms without rebound. Partial improvement of learning (PN16–19) | Transient deceleration of weight growth |
| Carisbamate [45]§ | Unknown (effect on spasms not via sodium channel blockade) | Effective (within the first hour) | PN4, PN6-7 | Not tested | No side effects |
| NAX 5055 (galanin analog) [38] | Galanin receptor 1 agonist | No effect | Not tested | Not tested | No side effects |
| VX-765 (this study) | Caspase 1 inhibitor | No consistent effect | Not tested | Not tested | No side effects |
| CGP35348 (this study) | GABAB receptor inhibitor | No effect | Not tested | Not tested | No side effects |
| 17β-estradiol (this study) | Estrogen signaling | No acute effect on PN3 | PN3–10 | No effect on spasms | No side effects |
Indicates drugs that have been designated by the Foods and Drug Administration orphan drugs for IS in the USA.
To develop new treatments for IS, we chose a strategy that starts by determining the acute effects of the tested treatment on behavioral spasms, given systemically and after the onset of spasms on PN4 as in clinical practice, so as to find drugs that have rapid onset of effect on spasms, within a few hours (PN4) or a day (PN5) after treatment. Although this strategy may miss some drugs that would have shown delayed effect on spasms, successful drugs may fill a gap as they would promise to act faster, and possibly improve outcomes, on IS compared to existing treatments, as discussed above. If a drug is successful, further testing is done with video EEG studies to confirm the effects on electroclinical spasms and, if effective, repeat administration of the treatment is being done to determine persistence of effect and potential for disease modification and antiepileptogenesis. Through all these tests, a parallel battery of behavioral and tolerability tests is being done to assess safety for each drug. Table 1 presents a summary of the so far published studies using this approach.
In this manuscript, we present data on 3 more drugs we have tested for efficacy and tolerability in the DLP model. The caspase 1 inhibitor VX-765 (belnacasan), which had promising antiseizure effects in preclinical studies [39–41] and had entered phase 2 clinical trials in humans for treatment-resistant focal-onset seizures, is tested here using the single injection protocol. The rationale is to test whether blockade of interleukin-1β (IL-1β) production by VX-765 may inhibit spasms in this model which is induced by the pro-inflammatory compound LPS. The second drug tested in the same manner is the GABAB receptor inhibitor CGP35348, which had induced reduction of EDR durations in the γ-butyrolactone (GBL) induced spasms in the Ts65Dn mouse model of spasms in Down syndrome [42]. The third drug, 17β-estradiol, is being tested according to the protocol proposed by Olivetti et al [43] as antiepileptogenic and disease modifying in the ARX (aristaless-related X-linked homeobox gene) knockin mouse model (ARX(GCG)10+7): repeated administration of 17β-estradiol between PN3-10. The latter was one of the replication trials proposed to us by the Citizens United for Research in Epilepsy (CURE) Infantile Spasms Initiative (http://www.cureepilepsy.org/news/story.asp?id=61) and was done with the collaboration of the Noebels’ research group that had led the ARX(GCG)10+7 study [43], which is the reason we used only one dose. Although none of these treatments showed efficacy in our model, we discuss the implications for therapy discovery that each poses both for therapy discovery in the DLP model but also for efforts to cross-validate treatments across various models of IS, as suggested by [44].
Materials and Methods
Animals and model induction
All procedures and protocols were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine and were in accordance with the ethical standards and the guidelines of the American Association for Accreditation of Laboratory Animal Care, the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Sprague Dawley male rats were used, which were the offspring of timed pregnant dams obtained from Taconic Farms (Germantown, NY, USA) and bred in litters of 10 male pups. Only male pups were used in this study. Rats were maintained in a 12hour light/12hour dark cycle schedule. The dam was fed with laboratory rodent diet 5001 (Labdiet, St Louis, MO, USA) and water was provided ad libitum.
The multiple-hit rat model was induced as described in [34–36,38,45]. Briefly, postnatal day 3 (PN3) male rats were anesthetized with isoflurane (4.5% induction, 1.5% maintenance) (Isothesia, Henry Schein, Melville, NY, USA) in 100% oxygen and doxorubicin (5μg/1.5μl/rat, right intracerebroventricularly) and lipopolysaccharide (LPS, 3μg/1.5μl/rat, right intracortically) were infused slowly. On PN5, rats received p-chlorophenylalanine (PCPA, 200mg/kg, intraperitoneally (i.p.)). The multiple-hit model is referred as the DLP model. All inducing drugs were purchased from Sigma-Aldrich (St Louis, MO, USA).
Study design and inclusion/exclusion criteria
A randomized, blinded, vehicle-controlled study design was used, where scoring of spasms, developmental milestones, and histological analysis were done blinded to treatment allocation. All rats exhibiting spasms were included in the study, provided that the following pre-set exclusion criteria were not met: (1) pre-injection frequencies of spasms on PN4-PRE <1 spasm/hour, (2) bilateral cortical lesions, (3) maternal neglect for the litter, (4) accidental death (e.g., by injection or immediate post-operative death) or death prior to drug treatment, (5) technical problems impeding intracerebral injections (e.g. due to syringe malfunction).
Drug administration and video monitoring of rats
VX-765 study
The caspase 1 inhibitor VX-765 (belnacasan, inh-vx765, Invivogen, San Diego CA) was tested in a single injection, dose-response study (50, 100, or 200 mg/kg i.p.), given at PN4 after spasms began, to evaluate its acute effects on spasms during the first 5 hours post-injection as well as on PN5 (Fig. 1). The vehicle (VEH) was 40% dimethylsulfoxide (DMSO) (Sigma-Aldrich, St Louis, MO, USA). The dose range was limited by the solubility of the drug in this vehicle.
Fig. 1. Study design (A) and effects of a single injection of VX-765 on raw (B) and normalized (C) frequencies of spasms in the multiple hit rat model of IS, when given after the onset of spasms.
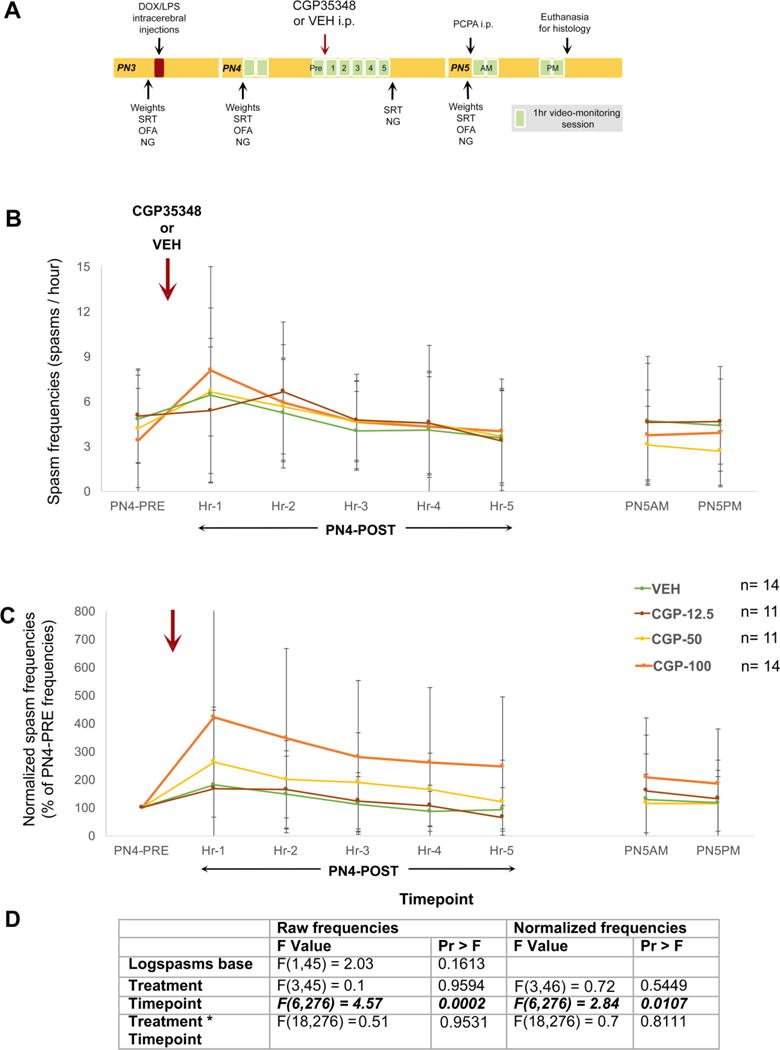
(A) PN3 male rats were induced according to the multiple hit rat model and were randomized to four treatment groups, that received a single injection of VX-765 [50 (VX-765-0 group, n=6 rats), 100 (VX-765-100 group, n=13 rats), or 200 mg/kg i.p. (VX-765-200 group, n=13 rats) or vehicle (VEH, n=12 rats) during the PN4PM monitoring session, and after the onset of spasms. The single injection of VX-765 [VX-765-100 or VX-765-200 groups) had no effect on raw (B) or normalized (C) frequencies of spasms compared to VEH. The VX-765-50 group (n=6 rats) showed significant reduction of both raw spasm frequencies (P=0.0223) and normalized spasm frequencies (P=0.0146) vs VEH at only a single timepoint, the 5th hour post-injection. Spasm scoring and histological evaluation for inclusions/exclusions were done blinded to treatment allocation. (D) The F values are given in the included table. Bolded italics indicate statistical significance at α<0.05. The yellow asterisk indicates P<0.05 between VX-765-50 and VEH at the specified timepoint. Data are represented as mean ± standard deviation.
Video monitoring was done twice daily on PN4 and PN5 for 2 hours each (AM and PM) with exception the PN4PM session that consisted of a 1hour long pre-injection period (PN4-PRE) and a 5 hours long post-injection period that started 15 min after the injection. Scoring for spasms was done during the PN4PM, PN5AM, and PN5PM sessions. The study design is given in Fig. 1.
CGP35348 study
The GABAB receptor antagonist CGP35348 (EMD Millipore Corporation, Billerica MA, USA) was tested in the single injection, dose-response study (12.5, 50, or 100 mg/kg i.p.: CGP-12.5, CGP-50, or CGP-100) versus the aqueous vehicle (VEH), given at PN4 after spasms began, and according to the same study design as VX-765 (Fig. 2). The doses were comparable to the effective doses in the Cortez et al study [42] and reached maximal solubility at this vehicle.
Fig. 2. Study design (A) and effects of a single injection of CGP35348 on raw (B,D) and normalized (C–D) frequencies of spasms in the multiple hit rat model of IS.
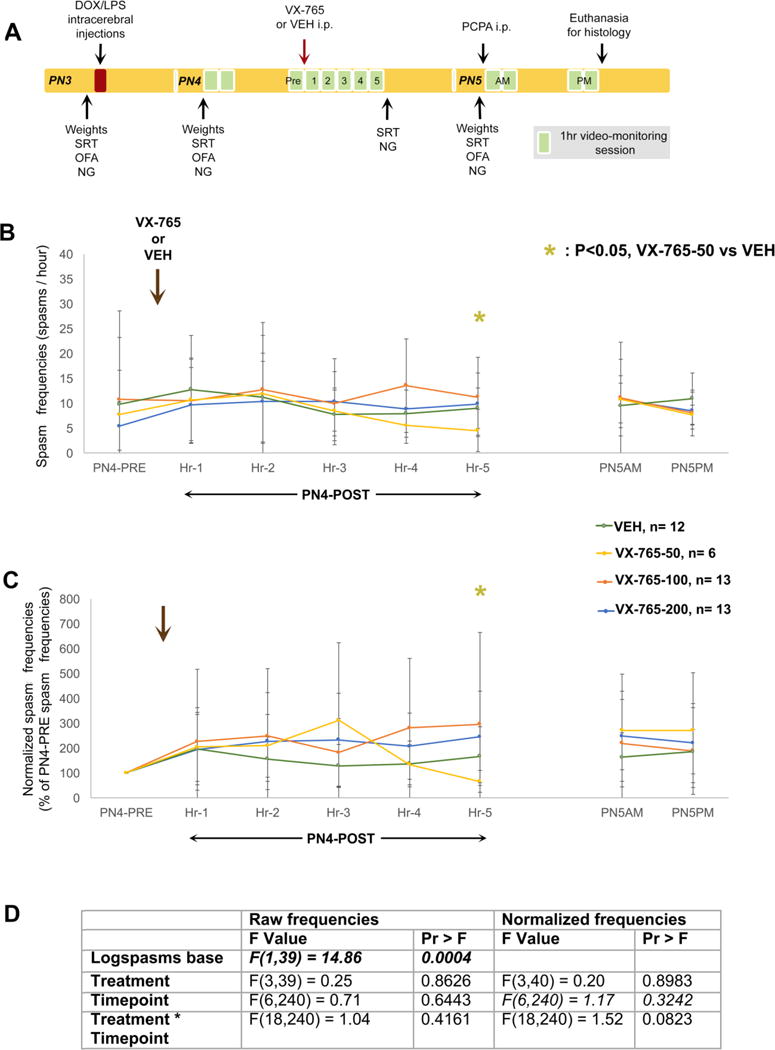
(A) PN3 male rats were induced according to the multiple hit rat model and were randomized to four treatment groups, that received a single injection of CGP35348 [12.5 (CGP-12.5 group, n=11 rats), 50 (CGP-50 group, n=11 rats), or 100 mg/kg i.p. (CGP-100 group, n=14 rats)] or vehicle (VEH, n=14 rats) during the PN4PM monitoring session, and after the onset of spasms. Spasm scoring and histological evaluation for inclusions/exclusions were done blinded to treatment allocation. The single injection of CGP35348 had no effect on raw (B) or normalized (C) frequencies of spasms compared to VEH. (D) The F values are given in the included table. Bolded italics indicate statistical significance at α<0.05. Data are represented as mean ± standard deviation.
Estradiol study
The 1,3,5(10-estratrien-3, 17β-diol compound (17β-estradiol, catalog number E0950-000, Steraloids, Inc, Newport RI) was tested as repeat administration of a single dose (40ng/g, subcutaneously (SC)) vs vehicle (sesame oil, Acros Organics, Fisher Scientific, Pittsburgh PA, USA) given between PN3-10, according to the early treatment protocol described in [43] (Fig. 3). The daily injections were given in the afternoon, either after the induction surgery on PN3 or just prior to the PM monitoring session. One dose was only selected as the goal was to replicate the experimental design of the Olivetti et al study [43].
Fig. 3. Study design (A) and effects of daily estradiol (40ng/g per day) given SC between PN3-10 on behavioral spasms (B, C) and weights (D) in the multiple hit rat model of IS.
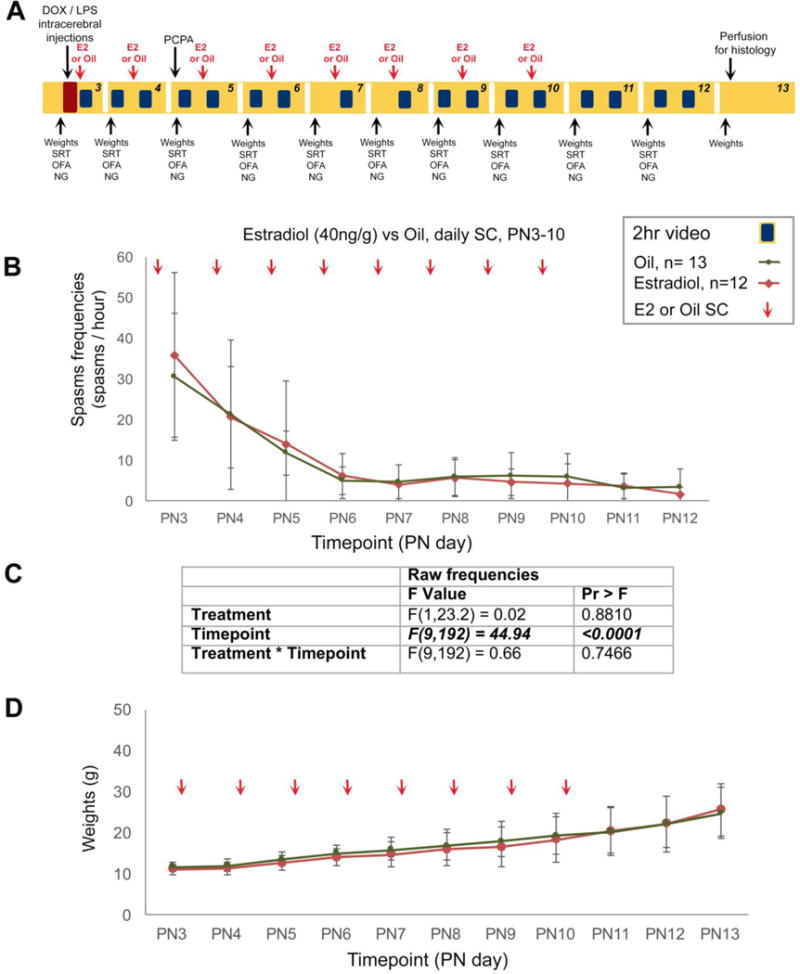
(A) PN3 male rats were induced according to the multiple hit rat model and were randomized to two treatment groups, that received either repeat daily injections of either estradiol (40ng/g per day, n=12) given SC between PN3-10 or equal volume of vehicle (oil, n=13). Spasm scoring was done daily from 2hour long PM sessions and histological evaluation for inclusions/exclusions were done blinded to treatment allocation. Linear mixed model analysis considering repeated values was done on log-transformed daily spasm frequencies. (B) 17β-Estradiol had no significant effect on spasms. (C) The F values for the drug effects on spasms are given in the table. Bolded italics indicate statistical significance at α<0.05. (D) Weights were not affected by estradiol. Data are represented as mean ± standard deviation.
Video monitoring (2 hour sessions) started after the first estradiol injection, post-operatively. One or two daily 2hour sessions were done as shown in Figure 2A, with the single daily video sessions done on the induction day and during weekend days. Scoring for spasms was done during the PM sessions.
Assessment of milestones
Daily weights, surface righting time (SRT), open field activity (OFA), negative geotaxis (NG) were done daily in the morning of each day in both studies. Protocols are described in [34–36,38,45] and 60sec were set as endpoint if goal was not reached (i.e., 60 sec were interpreted as failure). An additional assessment of SRT and NG was done at the end of the PN4PM session in the VX-765 study to assess for sedation and/or toxicity. Mortality was scored when rats were either found dead or met pre-set euthanasia criteria, according to our approved animal protocol.
Statistics
We used the SAS 9.3 software (SAS Institute Inc, Cary NC, USA). Normalized frequencies of spasms represent the percent of pre-injection PN4-PRE spasm frequencies. Because frequencies followed a log-normal distribution, both raw and normalized frequencies of spasms were log-transformed prior to analysis according to the Ln(1+frequency) formula so that “0” frequencies could be represented by “0”. Linear mixed models analysis was done considering repeated measures from each animal. ANOVA test was used to compare weights across the groups at each timepoint. Linear mixed models test was used to compare weight gain rates across treatments. Kruskal-Wallis test was used to analyze the reaction times in the milestones. Failure rates in the milestones were compared by Pearson chi-square test or Fisher’s exact test when n in any cell was ≤5. Statistical significance was pre-set at α=0.05.
Power analysis
Power analyses were based on earlier experiments that we had done using either single or repeat administration experiments [35,36,38,45]. The rat numbers used in the single injection experiments were estimated to be such that a minimum of approximately 12 rats would be included in each group, after the exclusions, to reach 84% power to detect 0.22 standard deviations difference in spasms frequencies, on the log scale between treatments [38]. For the repeat drug administration experiments, 1000 simulations of a mixed linear model were used considering data from five timepoints over 9 days post-injection, with the rate of spasms decreasing to ¼ of initial spasm frequencies. Twelve rats per group provided 87% power to show a difference of 0.22 standard deviations on the log scale between groups or 11% difference in normalized rates of spasms.
Results
Effects of a single VX-765 injection after spasms’ onset on spasms in the DLP model
PN3 male rats were induced according to the multiple hit rat model and were randomized to four treatment groups, that received a single injection of VX-765 [50 (VX-765-0 group, n=6 rats), 100 (VX-765-100 group, n=13 rats), or 200 mg/kg i.p. (VX-765-200 group, n=13 rats)] or vehicle (VEH, n=12 rats) during the PN4PM monitoring session, and after the onset of spasms. The number of rats reflect those included in the study after the pre-set exclusions. From a total of 55 male rats, 7 VX-765-50 rats, one VX-765-100 and one VX-765-200 rat were excluded due to bilateral lesions, while one VEH and one VX-765-50 rat were excluded due to lack of spasms prior to the drug injection.
The tolerability of the selected doses of VX-765 was excellent. We observed no mortality in VX-765 or VEH treated pups till PN5. Pre-treatment weights did not differ among groups (P=0.60 on PN3 and P=0.24 on PN4) and treatment did not affect weights on PN5AM (P= 0.55). In terms of weight gain rates, linear mixed model results (random intercept and random slope) show that the average weight gain rate was 0.41 g/day for the VEH group, 0.62 g/day for the VX-765-50 group, 0.28 g/day for the VX-765-100 group, and 0.50 g/day for the VX-765-200 group although these weight gain rates did not significantly differ from one another [F(3,40)treatment * timepoint=1.51, P=0.23]. In addition, no significant treatment effects were found for the SRT, OFA, and NG scores.
There was also no overall effect of VX-765 on spasm raw or normalized spasm frequencies, with only exception an observed reduction in spasm frequencies at the 5th post-injection timepoint of the VX-765-50 group compared to the VEH group. No differences were also found in the % of spasm-free rats per timepoint. The results and statistics are shown in Fig. 1. Because of the single delayed timepoint of significance at the lowest of the 3 tested doses, further testing with video-EEG monitoring was deferred.
Effects of single CGP35348 injection after spasms’ onset in the DLP model
A total of 60 male rats were randomized in the 4 treatment groups. From these, one CGP-12.5 and two CGP-50 rats were excluded because of bilateral lesions; three CGP-12.5, three CGP-50, and one VEH rat were excluded because of lack of spasms prior to injection.
The tolerability of CGP35348 was excellent. We saw no mortality till the end of PN5 (terminal timepoint). Weights were similar prior to treatment (P=0.95 on PN3 and P=0.77 on PN4) and following treatment (P=0.56). Weight gain rates were similar across groups (F(3,46) treatment * timepoint=1.1, P=0.36, linear mixed model, random intercept and slope): VEH= 0.46g/day, CGP-12.5=0.34g/day, CGP-50=0.29g/day, CGP-100=0.52g/day). We saw no group differences in failure rates or reaction scores for SRT, OFA, or NG.
There was no overall effect of CGP-348 on the raw or normalized frequencies of spasms. Results and statistics are shown in Fig. 2. The percent of spasm-free rats per timepoint did not differ among groups (Fisher’s exact test). These findings show that although a single injection of CGP35348 is well tolerated, CGP35348 has no effect on DLP spasms when given after spasms onset.
Effects of repeated estradiol administration in the DLP model
A total of 55 male rats were randomized into an estradiol-treated (n=12) and oil-treated (n=13) group. All rats were included in the study. 17β-Estradiol was well tolerated and no significant difference in mortality was observed till PN13: 3/12 estradiol-treated and 2/13 oil-treated rats died prior to terminal endpoint. There was no significant difference in SRT, NG, or OFA failure rates between the two groups. Repetitive 17β-estradiol treatment had no effect on spasm frequencies in the DLP model. The results and statistics are shown in Fig. 3.
Discussion
All drugs tested in this study (VX-765, CGP35348, 17β-estradiol) were well tolerated but had no consistent efficacy on behavioral spasms, even if used at doses that had shown efficacy in other models. As a result, further testing using video-EEG was not done.
There are multiple reasons to suspect neuroinflammatory cascades in the pathogenesis of IS both from the clinical literature but also from the DLP model, in which LPS is a key inducing pro-inflammatory trigger (reviewed in [8,46]). In preliminary studies, we also observed a significant upregulation of inflammatory markers, such as IL-1β, in the acute phase of spasms, which prompted the selection of VX-765 (Galanopoulou, unpublished data). We observed only a single timepoint of spasm reduction with the lower dose of VX-765 which was not replicated by the higher doses. This may represent a random effect since this group had more excluded rats than the other groups and the study was therefore not pursued further. The possibility that the doses we used are not effective is unlikely, since these compare to the VX-765 doses used in other studies in adult rodents, which typically require larger doses [39–41]. The lack of overall acute therapeutic effects of VX-765 in our model does not preclude the possibility that repetitive administration of the drug could be effective in the long run on spasms and this is something that will be explored in the future. In fact, VX-765 had delayed effects on seizures in previous studies [47]. Our data however establish a good tolerability window for these doses in the very young ages when spasms are observed.
We used CGP35348 based on the previous report that it acutely reduces extensor spasms in the GBL/Ts65Dn mouse model of IS, when given prior to GBL spasm induction [42]. GBL is a pro-drug of the GABAB receptor agonist γ-hydroxybutyrate and therefore the efficacy of CGP35348 in this model, given prior to GBL, only strengthens the authors’ postulate that GABAB receptor signaling activation is involved in the generation of GBL spasms in their model. The GBL/Ts65Dn mouse model is an acute model that is sensitive to both ACTH1–24 and vigabatrin pre-treatment [42]. The lack of CGP35348 efficacy in our model may be due to the more severe underlying pathology that renders it more drug-resistant, as is also the case with ACTH1–24. Even though vigabatrin and its analog CPP-115 have shown some efficacy in the DLP model, GABAB receptor inhibition does not seem to play a role in the acute management of DLP spasms. Cortez et al have reported GABAB receptor overexpression in the thalamus and medulla of their Ts65Dn model which may contribute to the increased sensitivity to GABAB receptor modulation. We have not pursued these studies in the DLP model, due to the lack of effect of CGP35348. However, other possibilities for the difference in the effects is that the two models are developed in different species (rat vs mouse) and different ages (PN4 vs adult) while the genetic background of the Ts65Dn may be an additional factor determining the differing pharmacosensitivity of the spasms in that model. Finally, the two studies also differ in the timing of drug administration (post-treatment vs pre-treatment), which may significantly influence the results.
Neonatal (PN3-10) 17β-estradiol has been shown to prevent spasms and epilepsy in the ARX(GCG)10+7 mouse model through its ability to rescue the observed calbindin and NPY (neuropeptide Y) interneuronopathy [43]. The interneuronopathy in the ARX(GCG)10+7 mouse model is of genetic etiology attributed to the ARX mutant protein dysfunction that reduces its nuclear localization and diminishes the migration of calbindin and NPY GABAergic interneurons in the neocortex, hippocampus, thalamus, and striatum, although it does not affect parvalbumin interneurons [48]. The DLP model also exhibits interneuronopathy contralateral to the infusions cortex, but this is probably due to the induced lesion and affects predominantly parvalbumin interneurons [49]. Unlike the study of Olivetti et al, we saw no difference in spasm frequencies in our rats, probably suggesting that the target mechanism of 17β-estradiol is not relevant in DLP male rats, due to the different etiology and probably different type of interneuronal dysfunction. Alternatively, it is possible that secondary effects of 17β-estradiol may account for the divergent results. For example, we have previously shown that overactivation of the mTOR pathway is important for the generation of DLP spasms [36]. Interestingly, 17β-estradiol has been shown to activate mTOR pathway [50–53], which may therefore counteract any other beneficial effects on DLP spasms. The relevance of mTOR pathway in the ARX(GCG)10+7 mouse model is currently unknown. A recent report in the prenatal betamethasone/postnatal NMDA acute model of spasms also did not report any effects of 17β-estradiol early treatment (PN3-10) on NMDA spasms induced on PN12, PN13, and PN15 male and female rats [54]. We did not observe any differences in the developmental milestones or weights between 17β-estradiol or oil-treated male rats till PN13, although it was previously reported that weight gain is increased in estradiol-treated mixed male and female PN15 rats in the prenatal betamethasone/postnatal NMDA model [54]. In addition, the differences in species (mouse vs rats) and neurodevelopmental effects of the altered genetic background in ARX(GCG)10+7 mouse model may contribute to the differences in efficacy.
What are the implications for the human West/IS syndrome? Characterization of the DLP model with treatments that may work in human IS (e.g., ACTH1–24 or vigabatrin) or do not treat IS (e.g., phenytoin) indicates that the DLP model is a model of drug-resistant IS due to structural lesions (Table 1). Validation of a model of IS for predicting human response would require validation of the preclinical results in pediatric clinical trials in a population of infants with IS. Most of the chronic models of IS have been published over the last 8 years and it is therefore too early to be able to draw conclusions on model validation. There are currently no clinical trials for IS for the other drugs that have been tested in our model, yet some promising case reports have started appearing. CPP-115, a high affinity vigabatrin analog with lower risk for retinal neurotoxicity based on animal studies [55] demonstrated better efficacy and tolerability than vigabatrin in the DLP model, and at significantly lower doses, even though complete spasm suppression was not achieved [35]. A case report of an infant with IS who was switched from vigabatrin to CPP-115 showed a similar better efficacy and tolerability profile, compared to vigabatrin, and at significantly lower doses [56]. We have also shown a therapeutic effect of rapamycin on DLP spasms [36]. Although rapamycin has not been tested in human patients with IS, Samueli et al reported on a patient with IS and TSC who responded with 58% reduction in seizures with everolimus, an mTOR inhibitor, while the patient’s EEG converted from hypsarrhythmia to multifocal spikes [57]. Unfortunately, the large clinical studies testing the effects of everolimus on seizures in patients with TSC exclude patients younger than 2 years with active IS and therefore it is premature to draw any definitive conclusions on efficacy in human IS [58].
Overall, the experience with the extrapolation (CGP35348) or replication (neonatal estradiol) of treatments that show efficacy on other models illustrates the challenges in relying on cross-validation of therapies across the existing models of IS. Beyond the methodological differences, species, age, and genetic background differences that exist among the existing models, the significant differences in the methods of induction and underlying pathologies create significant obstacles in relying on a replication trial done in a different model to validate a treatment. Interestingly, the impressive heterogeneity of etiologies of human IS may also be a similar challenge, except that in humans “lack of replication of efficacy in other patients with IS” may, sometimes, be interpreted as “failed treatment in drug-resistant individuals”. Lack of replication of a treatment in a different model might suggest that this may not be a common pathway but does not necessarily indicate lack of validity for a specific cohort of subjects (animal models or human patients) for which this target mechanism may be operative. Therefore, recognition of factors that can serve as biomarkers for the selection of the likely to benefit population becomes paramount in deciding whether a treatment should go forward.
Acknowledgments
ASG reports grants from NINDS-R01NS91170, NINDS-1U54NS100064, Department of Defense (W81XWH-13-1-0180), and the Infantile Spasms Initiative from CURE (Citizens United for Research in Epilepsy), and acknowledges also research funding from the Heffer Family and the Segal Family Foundations and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. WBM reports grant support from NINDS-1U54NS100064, Department of Defense (W81XWH-13-1-0180), the Infantile Spasms Initiative from CURE (Citizens United for Research in Epilepsy), and the Rett Syndrome Research Trust. SLM is the Charles Frost Chair in Neurosurgery and Neurology. He reports grants NINDS-NS020253, NINDS-NS43209, NINDS-NS45911, NINDS-1U54NS100064 and a grant from the US Department of Defense (W81XWH-13-1-0180), CURE Infantile Spasms Initiative grant and donations from the Heffer Family and the Segal Family Foundations and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. SLM holds a US patent for the multiple hit rat model (#7863499); no financial profits or conflicts have ensued as a result of this patent. We would like to thank Charles B. Hall, PhD (Division of Biostatistics, Albert Einstein College of Medicine) who performed the power analysis on the repeat administration treatment studies. We also wish to acknowledge the excellent technical assistance of Mrs Hong Wang.
Footnotes
ORCID IDs:
Aristea S. Galanopoulou: 0000-0002-0472-2903
Wenzhu B. Mowrey: 0000-0002-7407-7014
Solomon L. Moshé: 0000-0001-9427-9476
None of the authors has any conflicts of interest in regards to this article.
References
- 1.Pellock JM, Hrachovy R, Shinnar S, Baram TZ, Bettis D, Dlugos DJ, Gaillard WD, Gibson PA, Holmes GL, Nordl DR, O’Dell C, Shields WD, Trevathan E, Wheless JW. Infantile spasms: a U.S. consensus report. Epilepsia. 2010;51(10):2175–2189. doi: 10.1111/j.1528-1167.2010.02657.x. [DOI] [PubMed] [Google Scholar]
- 2.Riikonen R. Recent advances in the pharmacotherapy of infantile spasms. CNS Drugs. 2014;28(4):279–290. doi: 10.1007/s40263-014-0139-5. [DOI] [PubMed] [Google Scholar]
- 3.Knupp KG, Coryell J, Nickels KC, Ryan N, Leister E, Loddenkemper T, Grinspan Z, Hartman AL, Kossoff EH, Gaillard WD, Mytinger JR, Joshi S, Shellhaas RA, Sullivan J, Dlugos D, Hamikawa L, Berg AT, Millichap J, Nordli DR, Jr, Wirrell E, Pediatric Epilepsy Research C Response to treatment in a prospective national infantile spasms cohort. Ann Neurol. 2016;79(3):475–484. doi: 10.1002/ana.24594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knupp KG, Leister E, Coryell J, Nickels KC, Ryan N, Juarez-Colunga E, Gaillard WD, Mytinger JR, Berg AT, Millichap J, Nordli DR, Jr, Joshi S, Shellhaas RA, Loddenkemper T, Dlugos D, Wirrell E, Sullivan J, Hartman AL, Kossoff EH, Grinspan ZM, Hamikawa L, Pediatric Epilepsy Research C Response to second treatment after initial failed treatment in a multicenter prospective infantile spasms cohort. Epilepsia. 2016;57(11):1834–1842. doi: 10.1111/epi.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riikonen R. Combination therapy for treatment of infantile spasms. Lancet Neurol. 2017;16(1):19–20. doi: 10.1016/S1474-4422(16)30276-9. [DOI] [PubMed] [Google Scholar]
- 6.Go CY, Mackay MT, Weiss SK, Stephens D, Adams-Webber T, Ashwal S, Snead OC, 3rd, Child Neurology S, American Academy of N Evidence-based guideline update: medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2012;78(24):1974–1980. doi: 10.1212/WNL.0b013e318259e2cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lado FA, Moshe SL. Role of subcortical structures in the pathogenesis of infantile spasms: what are possible subcortical mediators? Int Rev Neurobiol. 2002;49:115–140. doi: 10.1016/s0074-7742(02)49010-1. [DOI] [PubMed] [Google Scholar]
- 8.Galanopoulou AS, Moshe SL. Pathogenesis and new candidate treatments for infantile spasms and early life epileptic encephalopathies: A view from preclinical studies. Neurobiol Dis. 2015;79:135–149. doi: 10.1016/j.nbd.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dulac O, Bast T, Dalla Bernardina B, Gaily E, Neville B. Infantile spasms: toward a selective diagnostic and therapeutic approach. Epilepsia. 2010;51(10):2218–2219. doi: 10.1111/j.1528-1167.2010.02736.x. author reply 2221. [DOI] [PubMed] [Google Scholar]
- 10.Darke K, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Lux AL, Newton RW, O’Callaghan FJ, Verity CM, Osborne JP, trial steering committee on behalf of participating i Developmental and epilepsy outcomes at age 4 years in the UKISS trial comparing hormonal treatments to vigabatrin for infantile spasms: a multi-centre randomised trial. Arch Dis Child. 2010;95(5):382–386. doi: 10.1136/adc.2009.160606. [DOI] [PubMed] [Google Scholar]
- 11.O’Callaghan FJ, Edwards SW, Alber FD, Hancock E, Johnson AL, Kennedy CR, Likeman M, Lux AL, Mackay M, Mallick AA, Newton RW, Nolan M, Pressler R, Rating D, Schmitt B, Verity CM, Osborne JP, participating i Safety and effectiveness of hormonal treatment versus hormonal treatment with vigabatrin for infantile spasms (ICISS): a randomised, multicentre, open-label trial. Lancet Neurol. 2017;16(1):33–42. doi: 10.1016/S1474-4422(16)30294-0. [DOI] [PubMed] [Google Scholar]
- 12.Hancock EC, Osborne JP, Edwards SW. Treatment of infantile spasms. Cochrane Database Syst Rev. 2013;(6):CD001770. doi: 10.1002/14651858.CD001770.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riikonen R. A long-term follow-up study of 214 children with the syndrome of infantile spasms. Neuropediatrics. 1982;13(1):14–23. doi: 10.1055/s-2008-1059590. [DOI] [PubMed] [Google Scholar]
- 14.Lombroso CT. A prospective study of infantile spasms: clinical and therapeutic correlations. Epilepsia. 1983;24(2):135–158. doi: 10.1111/j.1528-1157.1983.tb04874.x. [DOI] [PubMed] [Google Scholar]
- 15.Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, O’Callaghan FJ, Verity CM, Osborne JP, United Kingdom Infantile Spasms S The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol. 2005;4(11):712–717. doi: 10.1016/S1474-4422(05)70199-X. [DOI] [PubMed] [Google Scholar]
- 16.O’Callaghan FJ, Lux AL, Darke K, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, Verity CM, Osborne JP. The effect of lead time to treatment and of age of onset on developmental outcome at 4 years in infantile spasms: evidence from the United Kingdom Infantile Spasms Study. Epilepsia. 2011;52(7):1359–1364. doi: 10.1111/j.1528-1167.2011.03127.x. [DOI] [PubMed] [Google Scholar]
- 17.Kivity S, Lerman P, Ariel R, Danziger Y, Mimouni M, Shinnar S. Long-term cognitive outcomes of a cohort of children with cryptogenic infantile spasms treated with high-dose adrenocorticotropic hormone. Epilepsia. 2004;45(3):255–262. doi: 10.1111/j.0013-9580.2004.30503.x. [DOI] [PubMed] [Google Scholar]
- 18.Auvin S, Hartman AL, Desnous B, Moreau AC, Alberti C, Delanoe C, Romano A, Terrone G, Kossoff EH, Del Giudice E, Titomanlio L. Diagnosis delay in West syndrome: misdiagnosis and consequences. Eur J Pediatr. 2012;171(11):1695–1701. doi: 10.1007/s00431-012-1813-6. [DOI] [PubMed] [Google Scholar]
- 19.Cohen-Sadan S, Kramer U, Ben-Zeev B, Lahat E, Sahar E, Nevo Y, Eidlitz T, Zeharia A, Kivity S, Goldberg-Stern H. Multicenter long-term follow-up of children with idiopathic West syndrome: ACTH versus vigabatrin. Eur J Neurol. 2009;16(4):482–487. doi: 10.1111/j.1468-1331.2008.02498.x. [DOI] [PubMed] [Google Scholar]
- 20.Mohamed BP, Scott RC, Desai N, Gutta P, Patil S. Seizure outcome in infantile spasms–a retrospective study. Epilepsia. 2011;52(4):746–752. doi: 10.1111/j.1528-1167.2010.02963.x. [DOI] [PubMed] [Google Scholar]
- 21.Hussain K, Walsh TJ, Chazen JL. Brain MRI findings with vigabatrin therapy: case report and literature review. Clin Imaging. 2016;40(1):180–182. doi: 10.1016/j.clinimag.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Willmore LJ, Abelson MB, Ben-Menachem E, Pellock JM, Shields WD. Vigabatrin: 2008 update. Epilepsia. 2009;50(2):163–173. doi: 10.1111/j.1528-1167.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- 23.Riikonen R. Long-term outcome in children with infantile spasms treated with vigabatrin: A cohort of 180 patients. Epilepsia. 2015;56(5):807–809. doi: 10.1111/epi.12953. [DOI] [PubMed] [Google Scholar]
- 24.Riikonen R, Donner M. ACTH therapy in infantile spasms: side effects. Arch Dis Child. 1980;55(9):664–672. doi: 10.1136/adc.55.9.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konishi Y, Hayakawa K, Kuriyama M, Saito M, Fujii Y, Sudo M. Effects of ACTH on brain midline structures in infants with infantile spasms. Pediatr Neurol. 1995;13(2):134–136. doi: 10.1016/0887-8994(95)00122-v. [DOI] [PubMed] [Google Scholar]
- 26.Wray CD, Benke TA. Effect of price increase of adrenocorticotropic hormone on treatment practices of infantile spasms. Pediatr Neurol. 2010;43(3):163–166. doi: 10.1016/j.pediatrneurol.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Committee for Medicinal Products for Human Use. Guideline on clinical investigation of medicinal products in the treatment of epileptic disorders. European Medicines Agency; 2010. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070043.pdf. Accessed March 4 2017. [Google Scholar]
- 28.Pellock JM, Carman WJ, Thyagarajan V, Daniels T, Morris DL, D’Cruz O. Efficacy of antiepileptic drugs in adults predicts efficacy in children: a systematic review. Neurology. 2012;79(14):1482–1489. doi: 10.1212/WNL.0b013e31826d5ec0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunne J, Rodriguez WJ, Murphy MD, Beasley BN, Burckart GJ, Filie JD, Lewis LL, Sachs HC, Sheridan PH, Starke P, Yao LP. Extrapolation of adult data and other data in pediatric drug-development programs. Pediatrics. 2011;128(5):e1242–1249. doi: 10.1542/peds.2010-3487. [DOI] [PubMed] [Google Scholar]
- 30.Galanopoulou AS, Mowrey WB. Not all that glitters is gold: A guide to critical appraisal of animal drug trials in epilepsy. Epilepsia Open. 2016;1(3–4):86–101. doi: 10.1002/epi4.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haines ST, Casto DT. Treatment of infantile spasms. Ann Pharmacother. 1994;28(6):779–791. doi: 10.1177/106002809402800616. [DOI] [PubMed] [Google Scholar]
- 32.Mackay MT, Weiss SK, Adams-Webber T, Ashwal S, Stephens D, Ballaban-Gill K, Baram TZ, Duchowny M, Hirtz D, Pellock JM, Shields WD, Shinnar S, Wyllie E, Snead OC, 3rd, American Academy of N, Child Neurology S Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004;62(10):1668–1681. doi: 10.1212/01.wnl.0000127773.72699.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stafstrom CE, Moshe SL, Swann JW, Nehlig A, Jacobs MP, Schwartzkroin PA. Models of pediatric epilepsies: strategies and opportunities. Epilepsia. 2006;47(8):1407–1414. doi: 10.1111/j.1528-1167.2006.00674_1.x. [DOI] [PubMed] [Google Scholar]
- 34.Scantlebury MH, Galanopoulou AS, Chudomelova L, Raffo E, Betancourth D, Moshe SL. A model of symptomatic infantile spasms syndrome. Neurobiol Dis. 2010;37(3):604–612. doi: 10.1016/j.nbd.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Briggs SW, Mowrey W, Hall CB, Galanopoulou AS. CPP-115, a vigabatrin analogue, decreases spasms in the multiple-hit rat model of infantile spasms. Epilepsia. 2014;55(1):94–102. doi: 10.1111/epi.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raffo E, Coppola A, Ono T, Briggs SW, Galanopoulou AS. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiol Dis. 2011;43(2):322–329. doi: 10.1016/j.nbd.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akman O, Briggs SW, Galanopoulou AS. Long-term follow up of the multiple-hit model of symptomatic infantile spasms. Epilepsy Curr. 2012;13:147. [Google Scholar]
- 38.Jequier Gygax M, Klein BD, White HS, Kim M, Galanopoulou AS. Efficacy and tolerability of the galanin analog NAX 5055 in the multiple-hit rat model of symptomatic infantile spasms. Epilepsy Res. 2014;108(1):98–108. doi: 10.1016/j.eplepsyres.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noe FM, Polascheck N, Frigerio F, Bankstahl M, Ravizza T, Marchini S, Beltrame L, Bandero CR, Loscher W, Vezzani A. Pharmacological blockade of IL-1beta/IL-1 receptor type 1 axis during epileptogenesis provides neuroprotection in two rat models of temporal lobe epilepsy. Neurobiol Dis. 2013;59:183–193. doi: 10.1016/j.nbd.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 40.Ravizza T, Lucas SM, Balosso S, Bernardino L, Ku G, Noe F, Malva J, Randle JC, Allan S, Vezzani A. Inactivation of caspase-1 in rodent brain: a novel anticonvulsive strategy. Epilepsia. 2006;47(7):1160–1168. doi: 10.1111/j.1528-1167.2006.00590.x. [DOI] [PubMed] [Google Scholar]
- 41.Ravizza T, Noe F, Zardoni D, Vaghi V, Sifringer M, Vezzani A. Interleukin Converting Enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1beta production. Neurobiol Dis. 2008;31(3):327–333. doi: 10.1016/j.nbd.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Cortez MA, Shen L, Wu Y, Aleem IS, Trepanier CH, Sadeghnia HR, Ashraf A, Kanawaty A, Liu CC, Stewart L, Snead OC., 3rd Infantile spasms and Down syndrome: a new animal model. Pediatr Res. 2009;65(5):499–503. doi: 10.1203/PDR.0b013e31819d9076. [DOI] [PubMed] [Google Scholar]
- 43.Olivetti PR, Maheshwari A, Noebels JL. Neonatal estradiol stimulation prevents epilepsy in Arx model of X-linked infantile spasms syndrome. Sci Transl Med. 2014;6(220):220ra212. doi: 10.1126/scitranslmed.3007231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galanopoulou AS, Buckmaster PS, Staley KJ, Moshe SL, Perucca E, Engel J, Jr, Loscher W, Noebels JL, Pitkanen A, Stables J, White HS, O’Brien TJ, Simonato M, American Epilepsy Society Basic Science C, The International League Against Epilepsy Working Group On Recommendations For Preclinical Epilepsy Drug D Identification of new epilepsy treatments: issues in preclinical methodology. Epilepsia. 2012;53(3):571–582. doi: 10.1111/j.1528-1167.2011.03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ono T, Moshe SL, Galanopoulou AS. Carisbamate acutely suppresses spasms in a rat model of symptomatic infantile spasms. Epilepsia. 2011;52(9):1678–1684. doi: 10.1111/j.1528-1167.2011.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pardo CA, Nabbout R, Galanopoulou AS. Mechanisms of epileptogenesis in pediatric epileptic syndromes: Rasmussen encephalitis, infantile spasms, and febrile infection-related epilepsy syndrome (FIRES) Neurotherapeutics. 2014;11(2):297–310. doi: 10.1007/s13311-014-0265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maroso M, Balosso S, Ravizza T, Iori V, Wright CI, French J, Vezzani A. Interleukin-1beta biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics. 2011;8(2):304–315. doi: 10.1007/s13311-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price MG, Yoo JW, Burgess DL, Deng F, Hrachovy RA, Frost JD, Jr, Noebels JL. A triplet repeat expansion genetic mouse model of infantile spasms syndrome, Arx(GCG)10+7, with interneuronopathy, spasms in infancy, persistent seizures, and adult cognitive and behavioral impairment. J Neurosci. 2009;29(27):8752–8763. doi: 10.1523/JNEUROSCI.0915-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katsarou A, Moshé SL. Galanopoulou AS Interneuronopathy as a Non-Genetic Etiology of Infantile Spasms. In: American Epilepsy Society Basic Science C, editor. American Epilepsy Society annual meeting. Houston, TX: American Epilepsy Society; 2016. p. #3.002. [Google Scholar]
- 50.Tao Y, Sun H, Sun H, Qiu X, Xu C, Shi C, Du J. 17beta-estradiol activates mTOR in chondrocytes by AKT-dependent and AKT-independent signaling pathways. Int J Clin Exp Pathol. 2015;8(12):15911–15918. [PMC free article] [PubMed] [Google Scholar]
- 51.Yang WR, Wang Y, Wang Y, Zhang JJ, Zhang JH, Lu C, Wang XZ. mTOR is involved in 17beta-estradiol-induced, cultured immature boar Sertoli cell proliferation via regulating the expression of SKP2, CCND1, and CCNE1. Mol Reprod Dev. 2015;82(4):305–314. doi: 10.1002/mrd.22473. [DOI] [PubMed] [Google Scholar]
- 52.Kazi AA, Molitoris KH, Koos RD. Estrogen rapidly activates the PI3K/AKT pathway and hypoxia-inducible factor 1 and induces vascular endothelial growth factor A expression in luminal epithelial cells of the rat uterus. Biol Reprod. 2009;81(2):378–387. doi: 10.1095/biolreprod.109.076117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu J, Henske EP. Estrogen-induced activation of mammalian target of rapamycin is mediated via tuberin and the small GTPase Ras homologue enriched in brain. Cancer Res. 2006;66(19):9461–9466. doi: 10.1158/0008-5472.CAN-06-1895. [DOI] [PubMed] [Google Scholar]
- 54.Chachua T, Di Grazia P, Chern CR, Johnkutty M, Hellman B, Lau HA, Shakil F, Daniel M, Goletiani C, Veliskova J, Velisek L. Estradiol does not affect spasms in the betamethasone-NMDA rat model of infantile spasms. Epilepsia. 2016;57(8):1326–1336. doi: 10.1111/epi.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan Y, Gerasimov MR, Kvist T, Wellendorph P, Madsen KK, Pera E, Lee H, Schousboe A, Chebib M, Brauner-Osborne H, Craft CM, Brodie JD, Schiffer WK, Dewey SL, Miller SR, Silverman RB. (1S, 3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115), a potent gamma-aminobutyric acid aminotransferase inactivator for the treatment of cocaine addiction. J Med Chem. 2012;55(1):357–366. doi: 10.1021/jm201231w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doumlele K, Conway E, Hedlund J, Tolete P, Devinsky O. A case report on the efficacy of vigabatrin analogue (1S, 3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115) in a patient with infantile spasms. Epilepsy Behav Case Rep. 2016;6:67–69. doi: 10.1016/j.ebcr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samueli S, Abraham K, Dressler A, Groppel G, Muhlebner-Fahrngruber A, Scholl T, Kasprian G, Laccone F, Feucht M. Efficacy and safety of Everolimus in children with TSC - associated epilepsy - Pilot data from an open single-center prospective study. Orphanet J Rare Dis. 2016;11(1):145. doi: 10.1186/s13023-016-0530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.French JA, Lawson JA, Yapici Z, Ikeda H, Polster T, Nabbout R, Curatolo P, de Vries PJ, Dlugos DJ, Berkowitz N, Voi M, Peyrard S, Pelov D, Franz DN. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388(10056):2153–2163. doi: 10.1016/S0140-6736(16)31419-2. [DOI] [PubMed] [Google Scholar]


