Abstract
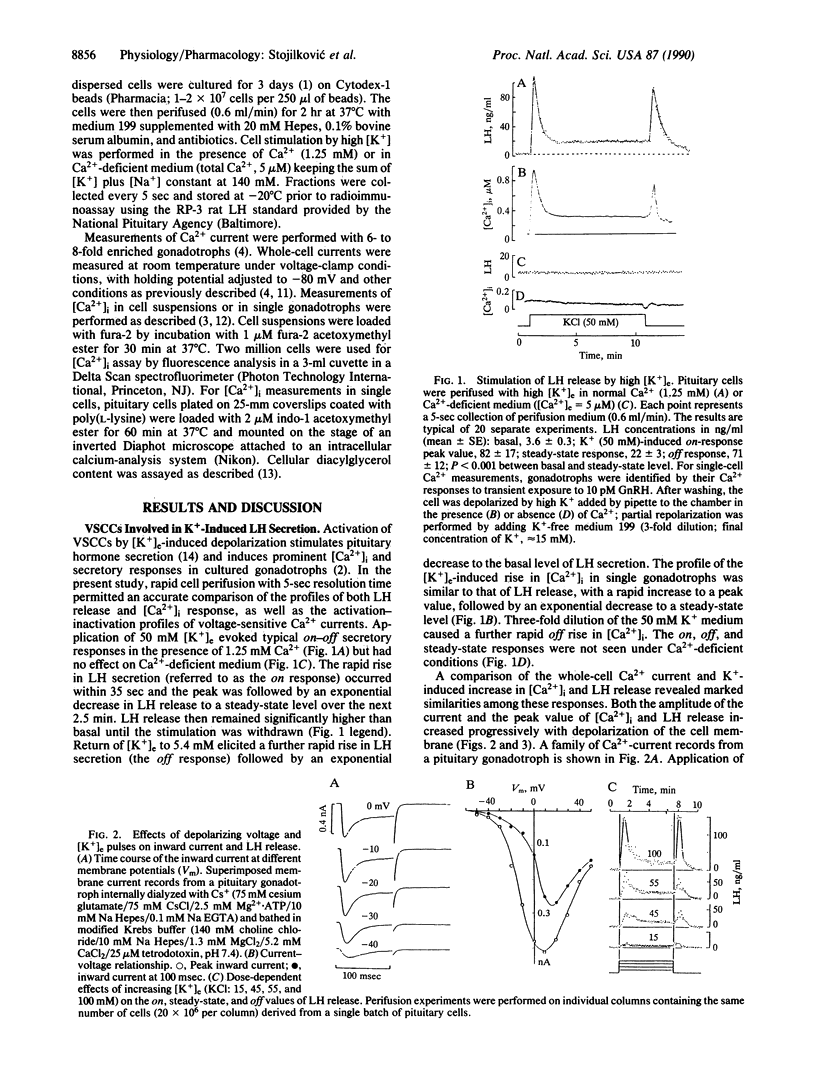
The relationships between the activation status of voltage-sensitive Ca2+ channels and secretory responses were analyzed in perfused rat gonadotrophs during stimulation by high extracellular K+ concentration ([K+]e) or the physiological agonist, gonadotropin-releasing hormone (GnRH). Increase of [K+]e to 50 mM evokes an on-off secretory response, with a rapid rise in luteinizing hormone (LH) secretion to a peak at 35 sec (on response) followed by an exponential decrease to the steady-state level. Cessation of K+ stimulation elicits a transient (off) response followed by an exponential decrease to the basal level. The LH response to high [K+]e is nifedipine-sensitive and its amplitude depends on membrane potential. There is a close relationship between the LH secretory response to high [K+]e and the amplitude of the inward Ca2+ current measured at 100 msec in whole-cell patch clamp experiments. In addition, the profile of the LH secretory response is similar to that of the response of intracellular Ca2+ concentration ([Ca2+]i) in K(+)-stimulated cells. In Ca2(+)-deficient medium, the effect of high [K+]e is abolished; subsequent elevation of [Ca2+]e during the K+ pulse is followed by restoration of the on response, but with reduced magnitude. Agonist stimulation during the steady-state phase of the [K+]e pulse or after repetitive stimulation by high [K+]e elicited biphasic [Ca2+]i and secretory responses with a significantly reduced plateau phase; conversely, K(+)-induced LH release was reduced in cells treated with desensitizing doses of GnRH. These findings indicate that depolarization-induced changes in the status of voltage-sensitive Ca2+ channels determine the profiles of [Ca2+]i and LH responses to stimulation by high [K+]e; the initial activation of dihydropyridine-sensitive Ca2+ channels is clearly dependent on membrane potential, whereas their subsequent inactivation depends on increased [Ca2+]i. Such inactivation of voltage-sensitive Ca2+ channels also occurs during GnRH action and may represent an additional regulatory mechanism to limit the entry of extracellular Ca2+ during prolonged or frequent agonist stimulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catt K. J., Stojilković S. S. Calcium signaling and gonadotropin secretion. Trends Endocrinol Metab. 1989 SepâOct;1(1):15–20. doi: 10.1016/1043-2760(89)90024-6. [DOI] [PubMed] [Google Scholar]
- Chang J. P., Stojilković S. S., Graeter J. S., Catt K. J. Gonadotropin-releasing hormone stimulates luteinizing hormone secretion by extracellular calcium-dependent and -independent mechanisms. Endocrinology. 1988 Jul;123(1):87–97. doi: 10.1210/endo-123-1-87. [DOI] [PubMed] [Google Scholar]
- Collins C. A., Rojas E., Suarez-Isla B. A. Activation and inactivation characteristics of the sodium permeability in muscle fibres from Rana temporaria. J Physiol. 1982 Mar;324:297–318. doi: 10.1113/jphysiol.1982.sp014114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxton T. L., Ben-Jonathan N., Armstrong W. M. Gonadotropin-releasing hormone induces oscillatory membrane currents in rat gonadotropes. Endocrinology. 1988 Oct;123(4):1783–1791. doi: 10.1210/endo-123-4-1783. [DOI] [PubMed] [Google Scholar]
- Doroshenko P. A., Kostyuk P. G., Martynyuk A. E. Intracellular metabolism of adenosine 3',5'-cyclic monophosphate and calcium inward current in perfused neurones of Helix pomatia. Neuroscience. 1982;7(9):2125–2134. doi: 10.1016/0306-4522(82)90124-5. [DOI] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Hunyady L., Baukal A. J., Bor M., Ely J. A., Catt K. J. Regulation of 1,2-diacylglycerol production by angiotensin-II in bovine adrenal glomerulosa cells. Endocrinology. 1990 Feb;126(2):1001–1008. doi: 10.1210/endo-126-2-1001. [DOI] [PubMed] [Google Scholar]
- Izumi S., Stojilković S. S., Catt K. J. Calcium mobilization and influx during the biphasic cytosolic calcium and secretory responses in agonist-stimulated pituitary gonadotrophs. Arch Biochem Biophys. 1989 Dec;275(2):410–428. doi: 10.1016/0003-9861(89)90388-3. [DOI] [PubMed] [Google Scholar]
- Kalman D., O'Lague P. H., Erxleben C., Armstrong D. L. Calcium-dependent inactivation of the dihydropyridine-sensitive calcium channels in GH3 cells. J Gen Physiol. 1988 Oct;92(4):531–548. doi: 10.1085/jgp.92.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C., Childs G. V., Brown A. M. Membrane currents of identified isolated rat corticotropes and gonadotropes. Am J Physiol. 1987 Mar;252(3 Pt 1):E340–E346. doi: 10.1152/ajpendo.1987.252.3.E340. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Schilling W. P., Rajan L., Strobl-Jager E. Characterization of the bradykinin-stimulated calcium influx pathway of cultured vascular endothelial cells. Saturability, selectivity, and kinetics. J Biol Chem. 1989 Aug 5;264(22):12838–12848. [PubMed] [Google Scholar]
- Shangold G. A., Murphy S. N., Miller R. J. Gonadotropin-releasing hormone-induced Ca2+ transients in single identified gonadotropes require both intracellular Ca2+ mobilization and Ca2+ influx. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6566–6570. doi: 10.1073/pnas.85.17.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simasko S. M., Weiland G. A., Oswald R. E. Pharmacological characterization of two calcium currents in GH3 cells. Am J Physiol. 1988 Mar;254(3 Pt 1):E328–E336. doi: 10.1152/ajpendo.1988.254.3.E328. [DOI] [PubMed] [Google Scholar]
- Stojilković S. S., Chang J. P., Ngo D., Catt K. J. Evidence for a role of protein kinase C in luteinizing hormone synthesis and secretion. Impaired responses to gonadotropin-releasing hormone in protein kinase C-depleted pituitary cells. J Biol Chem. 1988 Nov 25;263(33):17307–17311. [PubMed] [Google Scholar]
- Stojilković S. S., Izumi S., Catt K. J. Participation of voltage-sensitive calcium channels in pituitary hormone release. J Biol Chem. 1988 Sep 15;263(26):13054–13061. [PubMed] [Google Scholar]
- Stojilković S. S., Merelli F., Iida T., Krsmanović L. Z., Catt K. J. Endothelin stimulation of cytosolic calcium and gonadotropin secretion in anterior pituitary cells. Science. 1990 Jun 29;248(4963):1663–1666. doi: 10.1126/science.2163546. [DOI] [PubMed] [Google Scholar]
- Stojilković S. S., Rojas E., Stutzin A., Izumi S., Catt K. J. Desensitization of pituitary gonadotropin secretion by agonist-induced inactivation of voltage-sensitive calcium channels. J Biol Chem. 1989 Jul 5;264(19):10939–10942. [PubMed] [Google Scholar]
- Stojilković S. S., Stutzin A., Izumi S., Dufour S., Torsello A., Virmani M. A., Rojas E., Catt K. J. Generation and amplification of the cytosolic calcium signal during secretory responses to gonadotropin-releasing hormone. New Biol. 1990 Mar;2(3):272–283. [PubMed] [Google Scholar]
- Stutzin A., Stojilković S. S., Catt K. J., Rojas E. Characteristics of two types of calcium channels in rat pituitary gonadotrophs. Am J Physiol. 1989 Nov;257(5 Pt 1):C865–C874. doi: 10.1152/ajpcell.1989.257.5.C865. [DOI] [PubMed] [Google Scholar]
- Tan K. N., Tashjian A. H., Jr Voltage-dependent calcium channels in pituitary cells in culture. I. Characterization by 45Ca2+ fluxes. J Biol Chem. 1984 Jan 10;259(1):418–426. [PubMed] [Google Scholar]
- Wakabayashi K., Kamberi I. A., McCann S. M. In vitro response of the rat pituitary to gonadotrophin-releasing factors and to ions. Endocrinology. 1969 Dec;85(6):1046–1056. doi: 10.1210/endo-85-6-1046. [DOI] [PubMed] [Google Scholar]






