Abstract
Distress tolerance (DT), defined as the ability to persist in goal-directed behavior while experiencing psychological distress, is associated with greater frequency of substance use and poor treatment outcomes. To examine a potential causal role substance use may play in DT, we developed a rodent model of DT in which rats had to press a lever within a continuously decreasing time window for reward, while receiving negative feedback on failure trials. DT was defined as the time rats continued to seek reward before quitting the task. We assessed the relationship of DT with cocaine seeking/taking by measuring DT before cocaine self-administration (SA), and after one week and one month of drug abstinence. We found that DT prior to cocaine SA did not predict cocaine seeking/taking, yet DT measured after one month abstinence significantly predicted subsequent high levels of early session cocaine taking. Additionally, high DT measured after abstinence protected against high cocaine seeking, but this protective effect was blocked in rats with high impulsivity. Finally, while a decrease in one month-abstinent DT was observed following SA across treatment conditions, among cocaine-exposed rats, greater cocaine self-administration correlated with a steeper decrease in DT. These results show that low DT after drug abstinence is associated with heightened levels of cocaine seeking and taking behavior, and that impulsivity influences this effect. Collectively, these results support the validity of our rodent DT model while extending the human literature, and set the foundation for future animal studies designed to determine neural mechanisms underlying DT.
Keywords: Abstinence, Cocaine, Distress Tolerance, Impulsivity, Rat, Self-Administration
Introduction
Negative reinforcement models of addiction indicate that a motivational basis of drug use is the reduction or avoidance of physical and/or psychological distress (Baker et al., 2004; Koob, 2013). As a proxy for negative reinforcement behavior, laboratory paradigms assess distress tolerance (DT), defined behaviorally as the ability to persist in goal-directed behavior while experiencing psychological distress (Magidson et al., 2013). Behavioral measures indicate that low DT is associated with a greater frequency of substance use, and among treatment seeking substance users, greater likelihood of treatment dropout and relapse (Brown et al., 2002; Brandon et al., 2003; Daughters et al., 2005a,b; Brown et al., 2009; Strong et al., 2012).
In addition to negative reinforcement models of substance use, established literature emphasizes the role of motor impulsivity, defined broadly as an inability to inhibit behavioral responses, as a risk factor for substance use (Steinberg et al., 2008). Importantly, evidence indicates that one’s ability to persist in goal-directed behavior while experiencing affective distress is influenced by top-down neural mechanisms (Daughters et al., 2016), which overlap with those implicated in impulsivity (Hu et al., 2015). As such, an examination of the unique and interactive role of DT and impulsivity on substance use is important to consider.
The ability of DT to predict subsequent relapse makes it a promising behavioral target, and a number of studies have developed DT-based treatment strategies (Brown et al., 2008; Bornalova et al., 2012). Although studies have begun to examine the neurobiology of DT (Daughters et al., 2016), the underlying mechanisms of DT remain poorly understood. Furthermore, the exact role of DT in addiction itself is not clear. For example, although studies on drug naive adolescents provide evidence suggesting a link between low DT and risk behavior, including alcohol use (Daughters et al., 2009; Cummings et al., 2013), it is unknown whether drug-naïve DT influences subsequent substance use, or, conversely, if substance use lowers DT. Indeed, long-term longitudinal studies are required to answer these questions within a human population.
Here, we took a translational approach to address these issues by developing a rodent model of DT. Our model is based on behavioral tasks used to measure psychological DT in humans, namely the Computerized Paced Auditory Serial Attention Task (PASAT-C, Lejeuz et al., 2003). Human participants engage in an increasingly difficult task, characterized by forced failure and negative feedback, with the belief that task performance is associated with a reward. Following a controlled amount of exposure to the task, the participant is then provided the option to continue engaging in the task to improve performance, or to quit, with DT measured as the duration of time the participants spend on this last level before quitting. In our model, we designed a reaction time procedure in which rats learn to respond within a brief time window to receive a reward. On test day, the time window narrows significantly, causing the animal to encounter repeated forced failure and negative feedback (white noise burst), similar to the human DT tasks. Our dependent measure is also the amount of time it takes each rat to quit responding in the task. Guided by the findings relating DT and motor impulsivity to substance use, we sought to determine the relationship between DT, motor impulsivity and a model of substance use in rodents, namely 6-hour access to cocaine self-administration (Ahmed & Koob, 1998).
Materials and Methods
Subjects
Twenty-one male Long Evans rats (Charles River, Raleigh, NC) initially aged 8–10 weeks and weighing 300–325 g were used. Rats were individually housed with a 12h/12h light-dark cycle (lights on at 7 PM) and initially food restricted to no less than 85% free feed weight. Rats were then subsequently fed 15–20 g of Purina laboratory chow each day. Procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and in accordance with the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee.
Apparatus
Eight behavioral chambers (43 x 43 x 53 cm) housed in sound-attenuating cubicles (Med Associates, St. Albans, VT) were used. One side of each chamber contained two retractable levers with a cue-light above each and a food/water receptacle positioned equal distance between the levers. The opposite wall contained a nosepoke device with a houselight and speakers for tones and white noise mounted above it. The floor of each chamber was comprised of evenly spaced stainless steel bars (0.5cm dia, 1.5 cm apart). Cocaine infusion was controlled via a motor-driven syringe pump (Med Associates), and tubing was tethered using a counterweighted arm to provide for animal mobility. During tasks that involved food pellets, the nosepoke was covered with a plate to restrict access to it. During self-administration, the plate was removed and the levers were retracted. Operant chamber function and data input were controlled by MED-PC (Med Associates, St. Albans, VT).
Surgery
Rats were anesthetized with a mixture of 100 mg/kg ketamine hydrochloride and 10 mg/kg xylazine and implanted with a chronic indwelling catheter (Access Technologies, Skokie, IL) into their right jugular vein under aseptic conditions. Implantation of catheters into the jugular vein is routinely completed in our laboratory and described previously (Carelli et al., 2000; Saddoris et al., 2016).
Behavioral Procedures
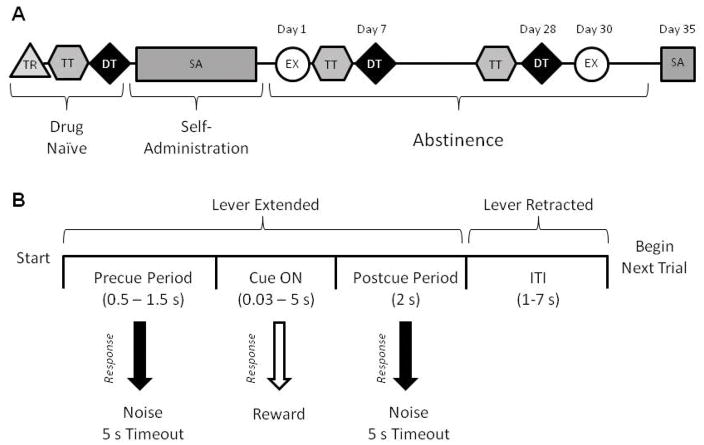
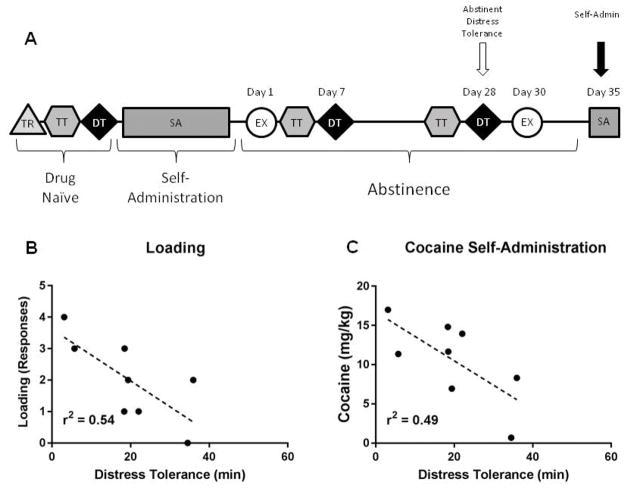
All rats underwent extensive behavioral procedures conducted in consecutive phases. The timeline for behavioral training and description of the requirements of each phase are summarized in Fig. 1a, and described in detail below.
Figure 1.
Experimental timeline and schematic overview of distress tolerance task. A) Experimental timeline. TR = Initial Training; TT = Titration Task, DT = Distress Tolerance Task, SA = Cocaine Self-Administration, EX = Cocaine Extinction. See main text for details of task design. B) Schematic of a single distress tolerance trial. Responses during the cue period (Cue ON) were rewarded with a single sucrose pellet. Responses during the Precue or Postcue periods were considered ‘errors’ and were signaled by a noise and a 5 s timeout. The duration of the cue ON period titrated according to task performance in the Titration task, and progressively decreased in the Distress Tolerance task.
Initial Behavioral Training (TR)
Drug naïve rats were initially trained to press a lever for a sucrose pellet (45 mg, Purina TestDiet, Richmond, IN) with levers counterbalanced as left or right between animals. Upon obtaining 50 pellets for two consecutive days, rats were trained to lever press during a 5 s cue period in order to obtain reward; pressing outside of the 5 s cue window resulted in an error. After animals completed 2 consecutive sessions with at least 50% accuracy, they began the titration task.
Titration Task (TT)
The TT task (100 trials; depicted in Fig. 1b) is composed of a pre-cue period, cue period, post-cue period, and intertrial interval (ITI; variable time, VT 4 s). The lever was extended for the duration of the trial. Once the lever was pressed (or if the ITI began without the animal making a response), it was retracted until the beginning of the next trial. If the animal pressed during the cue period (when the cue light was illuminated), the lever retracted and a sucrose pellet was delivered. If the subject pressed the lever either before or after the cue light illuminated, no sugar pellet was given. These ‘errors’ were signaled by a short white noise burst and a five second timeout. During each trial, the pre-cue period lasted VT 1s and the post-cue period was 2s. The duration of the cue period varied depending on the individual’s past performance on the task. If the previous trial was correct, the cue duration became 10% shorter. If the previous trial was incorrect, the cue duration became 10% longer. If the rat omitted a trial, the duration remained the same. For each session, the initial cue duration was set to the previous session’s final cue duration. The TT phase lasted until the animal either made 100 responses or 1 hour elapsed. To ensure that animals had sufficiently learned the TT before beginning the DT task, they had to have between 40 – 60% correct responses and had to meet criteria for stability in task performance. Stability was defined as no significant changes in cue duration or percent of correct responses over three consecutive days.
Distress Tolerance Task (DT)
Following acquisition of TT, the same drug naïve rats began the DT task. This task had similar structure to the TT, but was designed so that the cue duration became progressively shorter until it was almost impossible for the animals to obtain a correct response. The initial cue duration was tailored towards each animal’s ability in the TT, and equal to the average response latency on correct trials in the TT for each rat multiplied by two. Early in the DT task, incorrect responses had no effect on cue duration while correct responses rapidly and progressively decreased it according to the formula: Cue Duration = Previous Cue Duration * 0.9n, where n = the number of correct responses obtained thus far by the rat. Rats could only obtain pellets during the first 20 responses (by the end of which the cue duration was typically < 250 ms) and were allowed a maximum of 5 pellets in the task. After 20 responses or 5 correct responses (whichever came first), all responses (correct or incorrect) decreased cue duration by 10%. Omissions did not affect cue duration.
Our goal with the DT task was to determine each rat’s ability to persist in lever pressing in the presence of ‘psychological’ distress (forced failure, negative feedback). Rats were considered to have quit the task (our dependent measure) when they obtained 5 consecutive omissions directly preceded by 3 omissions out of 5 trials (thus 8 omissions out of 10 trials total). This time was capped at 1 hr if the animal did not ever reach criterion.
Self-administration (SA)
After the DT task was completed, animals underwent surgery for intravenous catheterization and began the self-administration task one week later. Here, a nosepoke led to an intravenous infusion of 0.33 mg of cocaine (n = 14) or an equivalent volume of both saline (i.v.) and water (delivered to the water receptacle) (n = 7) coupled with a 30s houselight and tone compound stimulus. Nosepokes during this 30s period did not result in additional reinforcement. The rationale for water training was to keep the degree of operant responding (# of nosepokes) for cocaine versus control rats similar. The self-administration task lasted for 6 hr/session for 14 days. A subset of the controls only underwent 2 hr access for water/saline. Other than the amount of water/saline administered, there were no significant differences between 2 and 6 hr water/saline rats; thus both groups were collapsed for all analyses unrelated to self-administration. Cocaine hydrochloride was obtained from the National Institute on Drug Abuse and dissolved in 0.9% saline.
Extinction (EX; Day 1)
After completion of the self-administration phase, rats underwent a 34 day drug abstinence period during which a number of additional behavioral measurements were obtained. On day 1 of abstinence, rats underwent a 2 hr extinction task which was identical to the self-administration procedure described above, but no reinforcers were delivered.
Distress Tolerance (DT; Days 7 & 28)
On days 7 and 28 of abstinence, rats were again tested on the DT task, using identical procedures as described above. Each DT test was preceded by 3 days on the TT.
Extinction (EX; Day 30)
On day 30 of abstinence rats underwent an additional 2 hr extinction phase, as described above.
Self-Administration (SA; Day 35)
Finally, 5 days following the day 30 extinction session, an additional 2 hr self-administration session was conducted, identical to the original self-administration sessions described above.
Exclusions
Some rats had to be excluded from part of the analysis. Two cocaine rats lost patency during the initial self-administration period and were excluded from all subsequent analyses. One cocaine rat died for reasons unrelated to the experiment during the abstinence phase. Additionally, three cocaine rats lost patency during abstinence and could not be tested on the final self-administration session. Five of the water/saline rats were not tested on the day 7 DT test or any of the extinction or self-administration behavior during abstinence. Finally, due to a program error, latencies to the first nosepoke were not recorded in seven of the cocaine rats during extinction day 1.
Data Analysis
Distress Tolerance (Drug Naïve)
To establish that the DT task was more challenging than the TT we used t-tests to examine task differences in percent of correct responses and number of omissions.
Relationship of DT to Motor Impulsivity
To determine if DT was related to any other behavioral phenotype, Pearson correlations were calculated between DT and measures in the TT (cue duration, omissions, latency, and impulsivity; averaged over the 3 stable days before the DT task). Impulsivity was defined as number of early errors/(number of early errors + number of late errors) reflecting the proportion of early errors.
Self-Administration
The primary dependent measures for self-administration were mg/kg of cocaine self-administered (or ml of water for controls), latency to first nosepoke, and loading behavior. Loading behavior was defined as the number of rapid consecutive responses early in the session that were less than half of the inter-reinforcer interval for the entire session; this measure has been previously correlated with measures of motivation to acquire drug in our earlier work (Carelli and Deadwyler, 1996; Wheeler et al,. 2008). To determine if 6 hr access to cocaine resulted in an escalation of cocaine intake as previously reported, we ran a 1-way ANOVA on the amount of cocaine consumed by rats across the 14 days of self-administration followed by Bonferroni post hoc tests. Pearson correlations examined if drug-naïve DT or cue duration predicted any behavior during self-administration (amount consumed, loading, and latency to first press), including the last 3 days when escalation of intake occurred. Finally, we determined if impulsivity interacted with the ability of DT to predict self-administration. Here, we used the moderation analysis macro PROCESS (Hayes, 2013), with drug naïve DT as the independent variable, self-administration behavior (amount consumed, loading, or latency to first press) as the dependent variables, and drug naïve impulsivity as the moderator.
Distress Tolerance (Abstinent)
To confirm that the DT task remained more challenging than the TT following abstinence, we examined task differences in percent of correct responses and number of omissions using t-tests. To determine test-retest reliability, Pearson correlations compared abstinent DT with drug naïve DT. A 2 x 2 mixed design ANOVA with Bonferroni post hoc tests was completed with Time (Drug-Naïve, 28 d of abstinence) and Drug (Cocaine, Water/Saline) as factors to examine the effect of cocaine on DT.
Extinction
The dependent measures for extinction were the number of nosepokes and the latency to first nosepoke. A paired sample t-test was completed to compare the number of nosepokes during extinction after 1 d and 30 d of abstinence. To determine if DT predicted extinction behavior, Pearson correlations on these data were calculated. Finally, to determine if impulsivity influenced the ability of DT to predict extinction, we conducted moderation analyses, using DT (drug naïve, day 7, or day 28) as the independent variable, extinction behavior (number of nosepokes or latency after 1 or 30 d of abstinence) as the dependent variable, and impulsivity (drug naïve, day 7, or day 28) as the moderator.
Self-administration (Day 35)
Pearson correlations were calculated to determine if DT (drug naïve, day 7, or day 28) predicted self-administration. To determine if impulsivity influenced the ability of DT to predict self-administration behavior, we conducted moderation analyses, using DT (drug naïve, day 7, or day 28) as the independent variable, self-administration (amount consumed, loading, and latency to first press) as the dependent variable, and impulsivity (drug naïve, day 7, or day 28) as the moderator.
All statistical analyses were completed with SPSS version 23 and Graphpad Prism.
Results
Initial Behavioral Training
Rats learned to lever press for a sucrose pellet over 6.14 ± 0.49 days, then to respond within a 5 s cue period with at least 50% accuracy after 4.43 ± 0.98 days.
Titration Task
Rats made 49.61 ± 0.49% correct responses following 14.81 ± 0.87 days of titration training. During the last 3 training days, responding was stable as indicated by consistent: 1) cue duration periods (1.75 ± 0.17s), 2) percent correct responses (49.61 ± 0.49%) and, 3) number of omissions (21.95 ± 6.29).
Distress Tolerance (Drug Naïve)
Next, rats entered the DT phase, in which the cue duration became progressively shorter over the course of the session. Behavior reflected this increased difficulty, as rats had significantly fewer percent correct responses (t(20)=72.99, p<0.001; Fig. 2A) and more omissions (t(20)=14.33, p<0.001; Fig. 2B) than during the TT. All rats stopped responding before one hour had elapsed, with some quitting quickly and others taking almost the entire hour (range of time to quit: 16.63–59.32 min, Fig. 2C).
Figure 2.
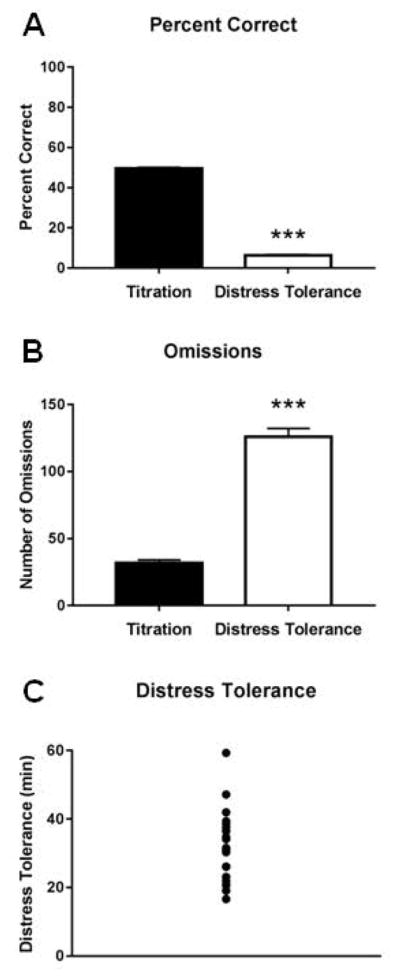
Behavior in the distress tolerance task. Rats exhibited significantly fewer percent correct responses (A) and more omissions (B) in the distress tolerance task than the titration task. C) Rats exhibited a wide range in how quickly they stopped responding (i.e., time to quit) in the distress tolerance task.
Importantly, since baseline omission levels during the TT could directly influence our DT measure, we ran a correlation between baseline omissions in the TT and subsequent DT, but found no relationship (r2 = 0.07, p=0.242). Thus, baseline motivation for sucrose likely had little impact on DT. Finally, we found no relationships between DT and baseline cue duration (r2=0.08, p=0.212), latency (r2=0.05, p=0.318), or proportion of early errors (r2=0.04, p=0.413), suggesting that DT was unrelated to task ability, reaction speed, or impulsivity.
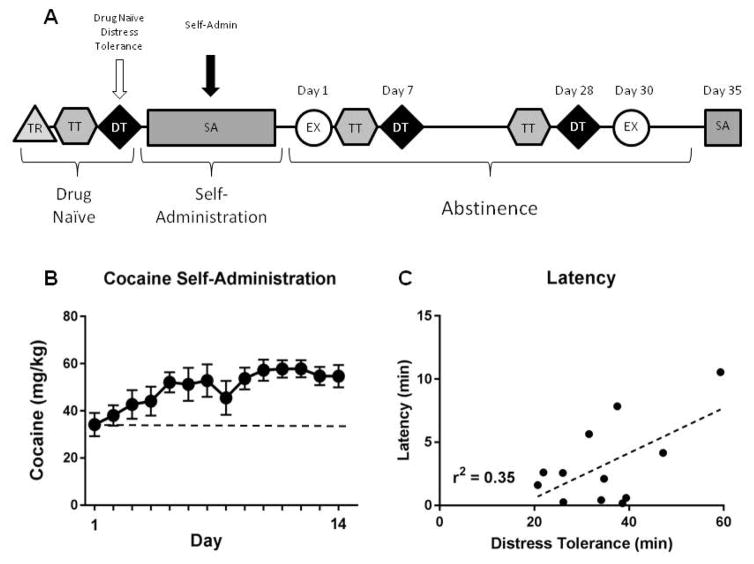
Self-Administration (SA)
Next, animals underwent surgery and, one week later, SA training. Across the last three days of training, rats (n=12) showed stable responding, completing 74.55 ± 5.29 nosepokes with a mean ITI of 5.18 ± 0.47 min (total amount of cocaine consumed was 55.78 ± 3.57 mg/kg/d). Characteristic of 6 hr access to cocaine, rats escalated intake across the 14 days of SA (F(13,143)=3.796, p<0.001; Bonferroni post hoc tests show days 9–12 significantly greater than day 1; Fig. 3B). Animals self-administering water and saline (n=7) also showed stable behavior, completing 25.67 ± 3.09 nosepokes (2 hr session: mean ITI; 7.52 ± 2.44 min), and 83.50 ± 8.50 nosepokes (6 hr session; mean ITI; 3.91 ± 0.27 min). A major goal of this study was to examine if drug naïve DT predicted cocaine seeking or taking behavior. Figure 3A shows where comparisons were made in our procedural timeline. Regression analysis showed that drug naïve DT did not predict cocaine intake or loading across all sessions, or over the last 3 days of SA (all p’s > 0.05). However, drug naïve low DT did predict shorter latency to first response over the last 3 days of SA (r2=0.35, p=0.041; Fig. 3C). Additionally, drug naïve impulsivity and cue duration both did not predict any element of SA, and no interactions between DT and impulsivity were significant (all p’s > 0.05). Finally, neither drug naïve DT nor impulsivity predicted escalation of intake (p’s > 0.05). Collectively, these data suggest that rats with low basal DT may have increased motivation to initiate drug-taking behavior following escalation of cocaine SA.
Figure 3.
Relationship between drug naïve distress tolerance and self-administration behavior. A) Experimental timeline highlighting analysis time points. B) Animals significantly escalated cocaine intake over the 14 days of self-administration access. C) Drug naïve distress tolerance significantly predicted the latency to the first response of the last 3 days of cocaine self-administration.
Extinction (Day 1)
To determine if DT predicted drug-seeking, cocaine rats underwent a 2 hr extinction session on the first day of abstinence, making 29.82 ± 3.96 responses with 1.61 ± 0.56 min latency to the first response. Drug naïve DT did not predict the number of responses during the task (r2=0.18, p=0.176), nor was there an interaction with drug naïve impulsivity, indicating that drug naïve DT does not predict drug-seeking at the beginning of abstinence.
Distress Tolerance (Days 7 & 28)
DT was again measured on days 7 & 28 of abstinence, and each DT test was preceded by three days on the TT. Similar to drug naïve TT and DT, rats showed evidence of the increased difficulty on the DT task, with the DT task associated with significantly fewer percent correct responses (day 7: 6.33 ± 0.49% vs. 46.39 ± 1.57%, t(11)=29.13, p<0.001; day 28: 8.62 ± 0.89% vs. 49.54 ± 0.45%, t(15)=52.22, p<0.001) and more omissions (day 7: 119.83 ± 14.34 omissions vs. 16.67 ± 7.27 omissions, t(11)=10.00, p<0.001; day 28: 167.94 ± 10.83 omissions vs. 21.99 ± 6.05 omissions, t(15)=10.72, p<0.001). Animals again exhibited high individual variability during both test days (day 7, range of time to quit: 12.41 – 59.80 min; day 28, range of time to quit: 3.11 – 42.29 min). Additionally, the DT task displayed good test-retest reliability, since drug-naïve DT correlated with DT after 28 days (r2=0.38, p=0.006) of abstinence and had a trend to correlate after 7 days of abstinence (r2=0.23, p=0.083).
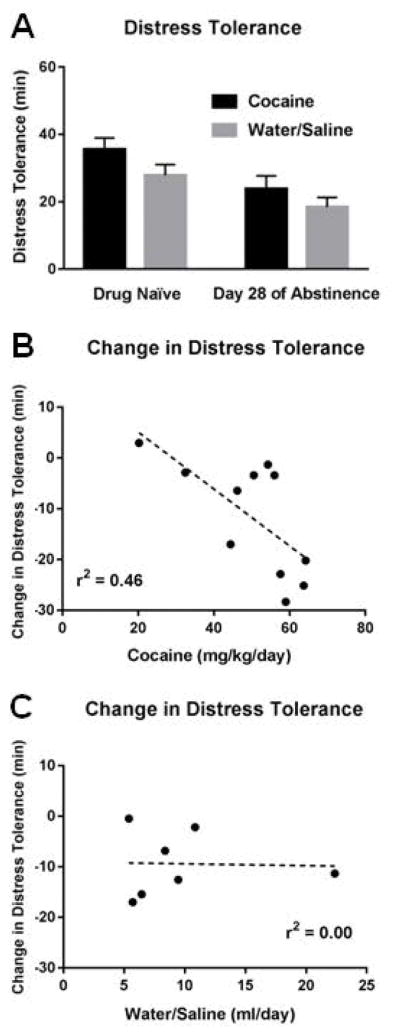
One objective of the study was to determine if cocaine SA altered subsequent DT. Using a 2x2 mixed design ANOVA with time (drug-naïve, 28 d of abstinence) and drug (cocaine, water/saline) as the factors, we found a main effect of time (F(1,16)=20.32, p<0.001), indicating that all rats decreased DT across test days (drug naïve DT: 30.97 ± 2.07 min, day 28 DT: 21.52 ± 2.88 min), independent of drug condition (Fig. 4A). However, there was no main effect of drug (F(1,16)=2.10, p=0.167) and no time x drug interaction (F(1,16)=0.23, p=0.639). The latter suggests that a history of cocaine SA had no unique effect on subsequent DT on day 28. Next, we examined if the change in DT in each group (i.e., Day 28 DT - drug naïve DT) correlated with how much cocaine or water was consumed. Here, we found that within cocaine rats, greater cocaine consumption during SA (average of days 1–14) significantly correlated with a greater decrease in DT on day 28 (r2=0.46, p=0.022; Fig. 4B), suggesting that the amount of cocaine consumed had a negative effect on DT. This correlation was not significant for water rats (r2=0.00, p=0.944; Fig. 4C).
Figure 4.
Relationship between cocaine self-administration and subsequent distress tolerance. A) All rats decreased distress tolerance across test days, independent of whether they had a history of water or cocaine self-administration. B) A history of greater cocaine consumption significantly correlated with a greater decrease in distress tolerance. C) A history of water consumption did not correlate with a decrease in distress tolerance.
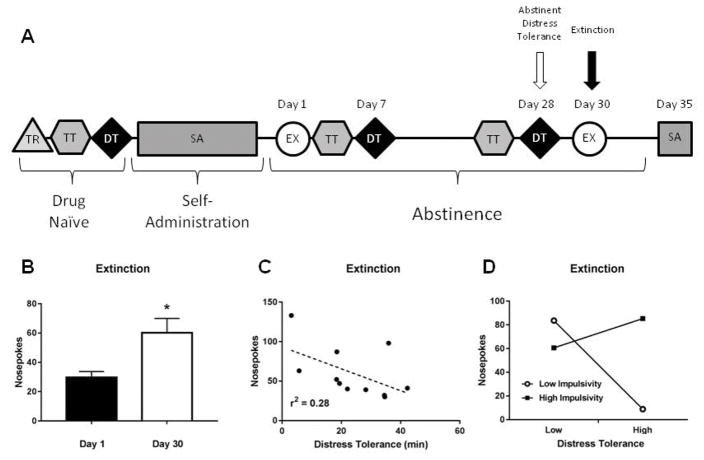
Extinction (Day 30)
A second 2 hr extinction session was administered on the 30th day of abstinence (Fig. 5A). Here, rats with a history of cocaine SA showed an ‘incubation of craving’ effect, exhibiting a significant increase in the number of nosepokes compared to extinction after 1 day of abstinence (t(10)=2.95, p=0.014, see Fig. 5B). Next, we determined if DT (drug naïve, day 7, or day 28) would predict the heightened drug-seeking behavior during extinction seen after 30 days of abstinence. We found that neither drug naïve nor day 7 DT predicted number of nosepokes or latency during extinction (all p’s > 0.05). However, low DT after 28 days of abstinence did have a trend to predict the high number of nosepokes during extinction (r2=0.28, p=0.092, see Fig. 5C).
Figure 5.
Relationship between distress tolerance following abstinence and subsequent drug seeking behavior during extinction. A) Experimental timeline highlighting analysis time points. B) Rats exhibit ‘incubation of craving’ (i.e., increased drug seeking) following 30 days of drug abstinence, C) Distress tolerance after abstinence had a trend to predict number of responses under extinction. D) There was a significant interaction between distress tolerance and impulsivity: In animals with low impulsivity, low distress tolerance significantly predicted more responses under extinction. No such relationship was seen in animals with high impulsivity.
Interestingly, there was an interaction between DT (day 28) and impulsivity (days 25–27) that significantly predicted the number of extinction nosepokes (day 30). Specifically, the interactive effect of impulsivity and DT on extinction responses was estimated with an OLS regression model, with impulsivity as the moderator. The interaction term was significant (β =15.150, SE=3.611, 95% CI: 6.606–23.694, p=0.004). To understand the nature of this moderation, conditional effects (“simple slopes”) of DT on extinction nosepokes was estimated for low and high impulsivity (±1 SD). As illustrated in Figure 5D, high DT was associated with fewer extinction nosepokes among rats with low (β =−2.979, SE=0.582, 95% CI: −4.357 – −1.602, p=0.001), but not high (β =0.983, SE=0.717, 95% CI: −0.715 – 2.680, p=0.213), impulsivity. These data suggest that, following extended abstinence, animals with high DT were protected against elevated drug-seeking behavior, but only if they also displayed low impulsivity. Importantly, impulsivity alone did not predict the number of nosepokes during extinction (r2 =0.00, p=0.869). We also found a significant interactive effect of impulsivity (average of days 4–6) and DT (day 7) on the latency to first press for extinction (β =0.374, SE=0.116, 95% CI: 0.996 – 0.648, p=0.015). Upon examination of conditional effects of DT on extinction latency, we found that low DT predicted short latency to press in animals with high (β =0.075, SE=0.024, 95% CI: 0.017 – 0.132, p=0.018), but not low (β =−0.040, SE=0.026, 95% CI: −0.100 – 0.021, p=0.165), impulsivity. This suggests that rats with low DT and high impulsivity in early abstinence may more quickly initiate drug-seeking behavior after extended abstinence.
Self-Administration (Day 35)
A final 2 hr self-administration session occurred on the 35th day of abstinence. Here, rats self-administered 10.59 ± 1.83 mg/kg of cocaine during the session (ITI=6.76 ± 0.81 min). As illustrated in Figure 6A, we determined if following extended abstinence, DT (drug naïve, day 7, day 28) would predict cocaine SA (Day 35). Neither drug naïve nor day 7 DT predicted amount of cocaine administered, loading or latency to first press (all p’s > 0.05). However, DT after 28 days of abstinence significantly predicted loading during the task (r2=0.54, p=0.038, Fig. 6B) and had a strong trend to predict the amount of cocaine self-administered (r2=0.49, p=0.052, Fig. 6C). Impulsivity (drug naïve, days 4–6, days 25–27) did not predict SA, loading or latency on day 35 (all p’s > 0.05). These data suggest that, following extended abstinence, animals with lower DT rapidly load up more drug and have greater drug consumption overall.
Figure 6.
Relationship between distress tolerance following abstinence and subsequent drug taking behavior during self-administration. A) Experimental timeline highlighting analysis time points. Distress tolerance after abstinence B) significantly predicted loading behavior and C) had a trend to predict amount of cocaine self-administered.
Discussion
We developed a novel rodent model of DT based upon a human model, the Computerized Paced Auditory Serial Attention Task (PASAT-C, Lejeuz et al., 2003). Similar to human laboratory paradigms, DT was defined in our rat model as the amount of time it took the animal to continue to seek reward, while experiencing ‘psychological’ distress, before quitting the task. Animals exhibited a wide range of DT, with some rats quitting quickly and others nearly reaching the one hour time limit. We assessed the relationship of DT with cocaine seeking and taking both before and after one month of abstinence from SA. To build on existing human work with current substance users, we were particularly interested in examining the unique effects of drug naïve DT and post cocaine self-administration DT on cocaine seeking and taking. We found that drug naïve DT did not predict cocaine seeking and taking. However, low DT measured after abstinence from cocaine SA significantly predicted high levels of early session cocaine taking. Additionally, high DT measured after abstinence protected against high cocaine seeking, but this protective effect was blocked in rats with high impulsivity.
Our primary finding of interest was that animals with low DT following cocaine SA exhibited heightened cocaine seeking and taking after a period of extended drug abstinence (30–35 days). This result is consistent with several human studies showing that substance users with low DT have a high rate of treatment dropout and relapse (Brown et al., 2002; Brandon et al., 2003; Daughters et al., 2005a,b; Brown et al., 2009; Strong et al., 2012). However, our findings also demonstrate the important role that extended drug abstinence plays in the relationship between DT and substance use. Previous reports showed extended abstinence from cocaine leads to an ‘incubation of craving’ which results in greater cocaine seeking (Grimm et al., 2001; also replicated here) as well as a host of molecular and neural adaptations in corticolimbic circuitry (Grimm et al., 2003; Lu et al., 2003; Lu et al., 2004). It is possible that our measurement of DT after extended abstinence is capturing individual differences in these effects, with rats demonstrating low DT experiencing higher incubation of craving. As such, it may be fruitful to investigate the differences in neural adaptation between high and low DT animals after incubation of craving.
DT’s ability to predict relapse and treatment dropout is thought to reflect how much a given individual can tolerate the distress associated with drug abstinence (Magidson et al., 2013). This is in line with negative reinforcement theories of addiction (Baker et al., 2004; Koob, 2013), which suggest that the heightened distress associated with drug withdrawal/abstinence drives an individual to seek and consume drug to alleviate that distress. These theories suggest that chronic drug use alters the hedonic set point of the brain by upregulating “antireward” brain systems, thereby requiring a greater amount of reward (natural or drug) to elicit the same response (Koob, 2013). It may therefore be valuable to investigate the effects of downregulating these systems (e.g. by blocking kappa opioid receptor function in reward circuitry, or corticotrophin-releasing factor receptor function in the extended amygdala) on DT.
We also found that motor impulsivity significantly influenced the role of DT in cocaine seeking (but not taking). Specifically, high DT after extended abstinence protected against elevated drug seeking, but high impulsivity was able to overcome this protective effect. Furthermore, low DT measured during early abstinence predicted faster latency to initiate cocaine seeking, but only in highly impulsive rats. Importantly, impulsivity alone did not predict drug seeking and taking, nor were DT and impulsivity related, collectively providing further evidence for the specificity of these constructs (Daughters et al., 2005a; Kiselica et al., 2015; however note that motor impulsivity in a 5-choice task does predict drug seeking and taking; Belin et al., 2008; Dalley et al., 2007). The results suggest that under psychologically distressing conditions, one’s ability to persist in goal-directed behavior is influenced by levels of disinhibition, with a combined low DT and high impulsivity presenting heightened risk for drug seeking.
In addition to determining DT’s predictive capacity, we investigated if a history of cocaine SA altered DT. Previous research has shown that cocaine history can disrupt goal-directed behaviors, and neural mechanisms underlying associative learning and decision making (Stalnaker et al., 2007; Mitchell et al., 2014; Saddoris & Carelli, 2014; Saddoris et al., 2016). A history of cocaine SA had no unique effect on DT when compared to a history of water/saline self-administration. However, within cocaine rats, greater amounts of cocaine SA did correlate with a steeper decrease in DT, while among water rats there was no relationship with water/saline self-administration (although the latter had a relatively small sample size). Nevertheless, these findings are in line with previous research reporting a relationship between DT and degree of substance use (Daughters et al., 2016; Quinn et al., 1996).
In summary, using a translational approach, we developed a rodent model of DT to examine the relationships between aspects of DT and cocaine self-administration. Our results corroborate features of DT reported in human addicts and support our paradigm as a model for DT and drug relapse in rodents. It is important to note however that our primary task measure, DT, is not easily dissociated from other measures such as attentional ability or cognitive effort (e.g. Cocker et al., 2012), and future studies should investigate the relationships between these constructs. Nevertheless, our work sets the foundation for additional studies that can be designed to investigate the neural underpinnings of DT.
Acknowledgments
The work was supported by National Institute on Drug Abuse Grant DA014339 to RMC. We thank Dominic Stone and Xuefei Wang for technical assistance.
Footnotes
Authors’ contribution: TM and RMC were responsible for the study concept and design. TM and DT contributed to the acquisition of animal data. TM, SD and RMC assisted with data analysis and interpretation of findings. TM and DT drafted the manuscript. TM, SD and RMC provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved the final version for publication.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Gratz KL, Daughters SB, Hunt ED, Lejuez CW. Initial RCT of a distress tolerance treatment for individuals with substance use disorders. Drug Alcohol Depend. 2012;122:70–76. doi: 10.1016/j.drugalcdep.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Herzog TA, Juliano LM, Irvin JE, Lazev AB, Simmons NV. Pretreatment task persistence predicts smoking cessation outcome. J Abnorm Psychol. 2003;112:448–456. doi: 10.1037/0021-843x.112.3.448. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong D. Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol. 2002;111:180–185. [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, Niaura R, Price LH. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine Tob Res. 2009;11:493–502. doi: 10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Palm KM, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, Hayes SC, Wilson KG, Gifford EV. Distress tolerance treatment for early-lapse smokers rationale, program description, and preliminary findings. Behav Modif. 2008;32:302–332. doi: 10.1177/0145445507309024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Deadwyler SA. Dose-dependent transitions in nucleus accumbens cell firing and behavioral responding during cocaine self-administration sessions in rats. J Pharmacol Exp Ther. 1996;277:385–393. [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JR, Bornovalova MA, Ojanen T, Hunt E, MacPherson L, Lejuez C. Time doesn’t change everything: The longitudinal course of distress tolerance and its relationship with externalizing and internalizing symptoms during early adolescence. J Abnorm Child Psychol. 2013;41:735–748. doi: 10.1007/s10802-012-9704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocker PJ, Hosking JG, Benoit J, Winstanley CA. Sensitivity to cognitive effort mediates psychostimulant effects on a novel rodent cost/benefit decision-making task. Neuropsychopharmacol. 2012;37:1825–1837. doi: 10.1038/npp.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters SB, Lejuez CW, Bornovalova MA, Kahler CW, Strong DR, Brown RA. Distress tolerance as a predictor of early treatment dropout in a residential substance abuse treatment facility. J Abnorm Psychol. 2005a;114:729–734. doi: 10.1037/0021-843X.114.4.729. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Lejuez CW, Kahler CW, Strong DR, Brown RA. Psychological distress tolerance and duration of most recent abstinence attempt among residential treatment-seeking substance abusers. Psychol Addict Behav. 2005b;19:208–211. doi: 10.1037/0893-164X.19.2.208. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Reynolds EK, MacPherson L, Kahler CW, Danielson CK, Zvolensky M, Lejuez CW. Distress tolerance and early adolescent externalizing and internalizing symptoms: The moderating role of gender and ethnicity. Behav Res Ther. 2009;47:198–205. doi: 10.1016/j.brat.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters SB, Ross TJ, Bell RP, Yi JY, Ryan J, Stein EA. Distress tolerance among substance users is associated with functional connectivity between prefrontal regions during a distress tolerance task. Addict Biol. 2016 doi: 10.1111/adb.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation: incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. An introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; New York: 2013. [Google Scholar]
- Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry. 2015;72:584–592. doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Kahler CW, Brown RA. A modified computer version of the Paced Auditory Serial Addition Task (PASAT) as a laboratory-based stressor. Behav Ther. 2003;26:290–293. [Google Scholar]
- Kiselica AM, Rojas E, Bornovalova MA, Dube C. The nomological network of self-reported distress tolerance. Assessment. 2015;22:715–729. doi: 10.1177/1073191114559407. [DOI] [PubMed] [Google Scholar]
- Koob GF. Negative reinforcement in drug addiction: the darkness within. Curr Opin Neurobiol. 2013;23:559–563. doi: 10.1016/j.conb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47:214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Shaham Y, Hope BT. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochem. 2003;85:1604–1613. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- Magidson JF, Ali B, Listhaus A, Daughters SB. Distress tolerance. In: MacKillop A, de Wit H, editors. The Wiley-Blackwell Handbook of Addiction Psychopharmacology. John Wiley & Sons; 2013. pp. 233–256. [Google Scholar]
- Mitchell MR, Weiss VG, Ouimet DJ, Fuchs RA, Morgan D, Setlow B. Intake-dependent effects of cocaine self-administration on impulsive choice in a delay discounting task. Behav Neurosci. 2014;128:419–429. doi: 10.1037/a0036742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn EP, Brandon TH, Copeland AL. Is task persistence related to smoking and substance abuse? The application of learned industriousness theory to addictive behaviors. Exp Clin Psychopharm. 1996;4:186–190. [Google Scholar]
- Saddoris MP, Carelli RM. Cocaine self-administration abolishes associative neural encoding in the nucleus accumbens necessary for higher-order learning. Biol Psychiatry. 2014;75:156–164. doi: 10.1016/j.biopsych.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Wang X, Sugam JA, Carelli RM. Cocaine Self-Administration Experience Induces Pathological Phasic Accumbens Dopamine Signals and Abnormal Incentive Behaviors in Drug-Abstinent Rats. J Neurosci. 2016;36:235–250. doi: 10.1523/JNEUROSCI.3468-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Roesch MR, Franz TM, Calu DJ, Singh T, Schoenbaum G. Cocaine-induced decision-making deficits are mediated by miscoding in basolateral amygdala. Nature Neurosci. 2007;10:949–951. doi: 10.1038/nn1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev Psychol. 2008;44:1764–1768. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Strong DR, Brown RA, Sims M, Herman DS, Anderson BJ, Stein MD. Persistence on a stress-challenge task before initiating buprenorphine treatment was associated with successful transition from opioid use to early abstinence. J Addiction Med. 2012;6:219–225. doi: 10.1097/ADM.0b013e31825d927f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57:774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]