Abstract
The interactions of drugs with serum proteins are often stereoselective and can affect the distribution, activity, toxicity and rate of excretion of these drugs in the body. A number of approaches based on affinity chromatography, and particularly high-performance affinity chromatography (HPAC), have been used as tools to study these interactions. This review describes the general principles of affinity chromatography and HPAC as related to their use in drug binding studies. The types of serum agents that have been examined with these methods are also discussed, including human serum albumin, α1-acid glycoprotein, and lipoproteins. This is followed by a description of the various formats based on affinity chromatography and HPAC that have been used to investigate drug interactions with serum proteins and the historical development for each of these formats. Specific techniques that are discussed include zonal elution, frontal analysis, and kinetic methods such as those that make use of band-broadening measurements, peak decay analysis, or ultrafast affinity extraction.
Keywords: High-performance affinity chromatography, Drug-protein binding, Stereoselective interactions, Human serum albumin, α1-Acid glycoprotein, Lipoproteins
1. Introduction
The interactions of drugs with proteins are often stereoselective [1–5]. When these interactions involve agents such as serum proteins, they can play an important role in the distribution, activity, rate of excretion, and toxicity of drugs in the body [6,7]. Information on these interactions can be valuable in the design and optimization of chiral separations [1,3,8–11], as well as in predicting how drugs may behave in the human body, in understanding drug-drug interactions, and in determining the optimum dosages that should be used with such agents for treating patients [6,7,12–17].
The binding of drugs with serum agents is usually a reversible process and may involve several possible proteins or related carriers. Common examples of these binding agents are the proteins human serum albumin (HSA) and α1-acid glycoprotein (AGP), as well as more complex species such as lipoproteins [6,7,12–17]. The extent of these interactions is often significant, with 43% of 1500 most common drugs having 90% or greater binding to serum proteins and carrier agents [18]. As a result, obtaining data on these processes is an important part of the absorption/distribution/metabolism/excretion (ADME) data that are needed prior to the approval of a new drug in the U.S. [7,18].
There are many techniques that have been utilized to study drug interactions with serum proteins. These methods have included equilibrium dialysis, ultrafiltration, absorption spectroscopy, fluorescence spectroscopy, surface plasmon resonance spectroscopy, X-ray crystallography, and nuclear magnetic resonance spectroscopy [19–21]. This review will discuss an alternative group of techniques that are based on affinity chromatography and high-performance affinity chromatography (HPAC) [6–8,22]. The general principles of these methods and the types of serum proteins that these methods have been used to examine will first be considered. This will be followed by an overview of the techniques that have been developed and employed in affinity chromatography and HPAC to study drug-protein interactions, with an emphasis on the historical development of these techniques and their use in the study of stereoselective binding.
2. General principles of affinity chromatography and HPAC
Affinity chromatography can be defined as a type of liquid chromatography which uses a biologically-related agent as a stationary phase for the analysis or separation of samples [23–25]. This type of stationary phase, which is often referred to as the “affinity ligand”, makes use of the reversible and specific interactions that are often present in biological systems, such as the interaction of an enzyme with a substrate or the binding of an antibody with its antigen. This same feature often allows an affinity column to selectively bind a given target compound and makes this a valuable tool for the separation or detection of specific agents in biological samples [23,24].
Affinity chromatography was first used in 1910 for enzyme isolation [26], but it was not until 1968 that this technique became a popular method for compound purification [24,27–29]. Work from the late 1960s through the early 1980s mainly used carbohydrate-based supports such as agarose or cellulose for the immobilization of binding agents and preparation of affinity columns [23,27–30]. This approach is still commonly used for purification purposes and will be referred to in this review as traditional, or low-performance, affinity chromatography [27,29]. Research then began in the early 1980s in combining affinity chromatography with HPLC-grade supports such as silica. The resulting method is now called high-performance affinity chromatography (HPAC), or high-performance liquid affinity chromatography [23,29,31,32]. Other materials besides silica particles that are now used in HPAC include modified polystyrene supports and monolithic supports based on silica or organic polymers [32–36]. The ability to use these supports with HPLC systems and detectors, as well as the speed and precision of the resulting methods, has been important in allowing affinity columns to be used in both analytical applications and in the study of interactions such as drug-protein binding [6,8,11,13,17,23].
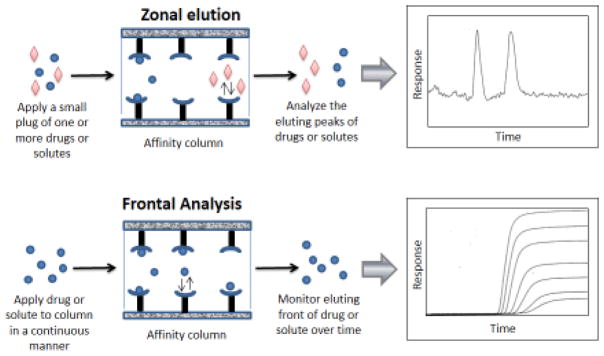
Figure 1 shows a few formats that can be used in affinity chromatography and HPAC for the study of drug-protein binding [6,7,11,13,37,38]. Both of these methods make use of a column that contains an immobilized agent such as a serum protein. A drug or solute is first injected or applied onto this column in the presence of a mobile phase that has the desired pH, ionic strength and solvent composition for the binding study [6,7,11,13,39]. In the method of zonal elution, a small sample containing one or more solutes is applied onto the column and the retention or shape of the resulting peaks is examined. In the technique of frontal analysis, a solution of the solute is continuously applied to the immobilized binding agent as the amount of solute that passes through the column is monitored. These and other approaches (e.g., see Sections 4–6, which also include kinetic methods) can provide information on such things as the binding constants and rate constants for the interaction, as well as the number and types of binding sites involved in this process. After the study has been completed, the bound solute can be eluted and the original buffer applied to regenerate the column for the next experiment. Either traditional affinity chromatography or HPAC can be used in such methods [6,13,39]. However, HPAC offers the advantages of being easier to automate and allowing these measurements to be carried out with greater speed plus better precision and with various types of on-line HPLC detectors [6,13,40].
Figure 1.
General approaches used in affinity chromatography and HPAC for the study of drug-protein binding. The examples shown on the right are described in more detail in Figures 2(b) and 4(a) and are adapted with permission from Refs. [37,38].
3. Serum proteins examined by affinity chromatography
Affinity chromatography and HPAC have been used to study drug interactions with a variety of serum proteins and serum binding agents. Many of these studies have dealt with HSA, or the closely-related protein bovine serum albumin (BSA) [6,7,13,17]. This is due to the importance of these proteins, their use as chiral stationary phases, and the availability of various immobilization methods that can be used to prepare affinity columns that are good models for the behavior of these proteins in their native form [6–11,40–43] (Note: related chromatographic studies with albumin from other species have also been reported) [44–47]. HSA has a concentration in plasma of 35–50 g/L, a molar mass of 66.5 kDa, and an isoelectric point of 4.7. It consists of a single chain of 585 amino acids which is stabilized by 17 internal disulfide bonds [16]. This protein has two major binding sites for drugs, which are often referred to as Sudlow sites I and II [16,48]. Sudlow site I, also known as the warfarin-azapropazone site, is a hydrophobic pocket in subdomain IIA of HSA. Sudlow site II, or the indole-benzodiazepine site, is located in subdomain IIIA [13,16,48]. There are additional binding sites for some other drugs and solutes on this protein (e.g., the digitoxin and tamoxifen sites), as well as sites for fatty acids, metal ions, and endogenous solutes (e.g., bilirubin) [13,16].
HPAC and affinity chromatography have been used to examine the binding by many chiral and achiral drugs and solutes with HSA [6–8,13,17,21,49]. Examples of a few chiral drugs that have been separated and studied in this manner, as is illustrated in Figure 2(a), include barbiturates, benzodiazepines, benzothiadiazeines, coumarins (e.g., warfarin), α-arylpropionic acids (e.g., ibuprofen), tryptophan, and thyronines, among many others [6,8,10,11,13,21,37,43,50–59]. This has included both measurements of the overall binding by such solutes with HSA, as well as more detailed studies of the equilibrium constants and number of binding sites for these interactions [6,8,10,11,13,21,37]. In some cases, the rates of these interactions have also been examined [22], along with the effects of varying parameters such as the temperature, pH, ionic strength, or solvent polarity on these processes [7,8,10,13].
Figure 2.
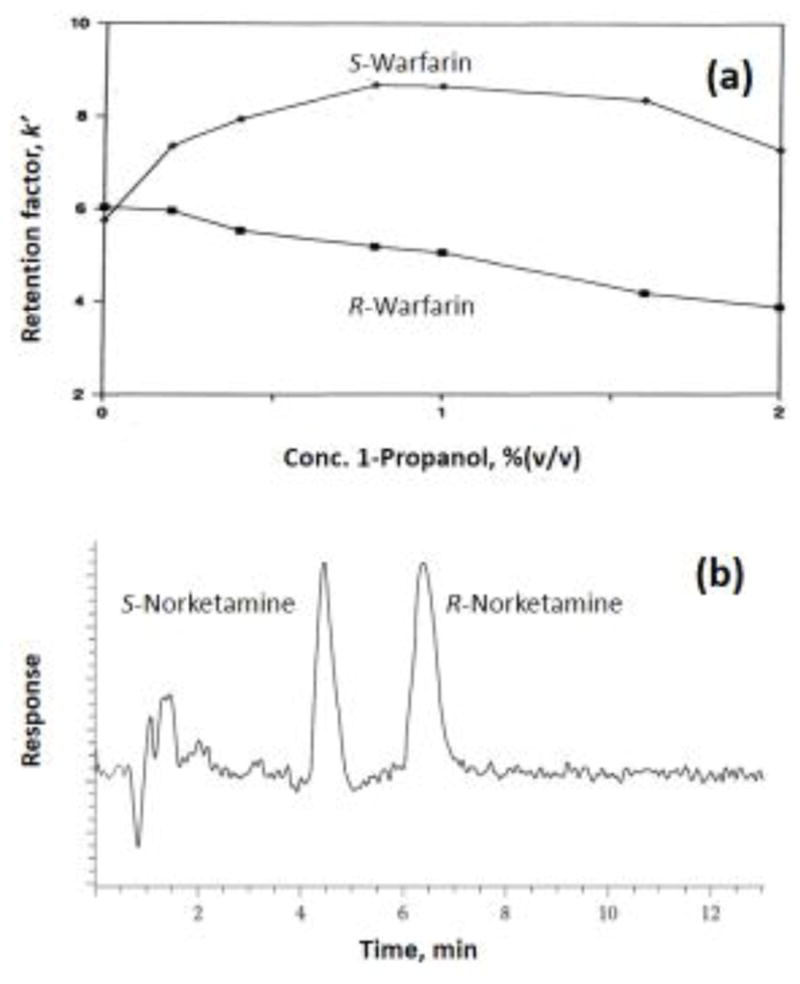
Separation of (a) R- and S-warfarin on a high-performance HSA column and in the presence of various mobile phase concentrations of 1-propranol, and (b) separation and detection of R- and S-norketamine in plasma using a high-performance AGP column with detection based on mass spectrometry. Adapted with permission from Refs. [37,38].
AGP is another serum protein that has been the subject of many studies based on affinity chromatography or HPAC [7,8,13]. This protein has been popular for use as a chiral stationary phase, as shown in Figure 2(b) [8–10,38,60]. However, it has only been over the last decade in which suitably mild immobilization schemes have been developed to place AGP into affinity columns in a form that closely follows the behavior of this protein in its original form [7,60–62]. AGP is a heterogeneous glycoprotein with a molar mass of 41 kDa and a normal concentration in human serum of 0.5–1.0 mg/mL. This protein consists of a single chain of 183 amino acids and has an isoelectric point of 2.7–3.9 [63–66]. AGP also contains five N-linked glycan chains and has a carbohydrate content of 45% (w/w) [67,68]. AGP is believed to have one flexible binding site for many basic and neutral drugs, as well as for some acidic drugs [63–66,69–71].
A number of reports based on HPAC have examined the chiral separation and binding of drugs on AGP columns [7–10]. Some of these studies have looked at the effects of temperature and mobile phase composition on the retention and chiral separation of such drugs as atenolol, warfarin, verapamil, methadone, atropine, disopyramide, ketamine, and various profens (e.g., ketoprofen and ibuprofen) [8–10,72–75]. In addition, detailed thermodynamic studies have been carried out with this protein and such drugs as propranolol, carbamazepine, and warfarin [61,62,76–78]. More recent studies have also employed HPAC and affinity columns to examine the kinetics for some of these interactions [79,80].
Plasma lipoproteins such as high-density lipoprotein (HDL), low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) have also been successfully immobilized in recent years and characterized by HPAC for their drug binding properties [81–84]. Lipoproteins are soluble complexes of lipids and proteins (i.e., apolipoproteins) [85–87]. The general structure of lipoproteins consists of a non-polar core of triacylglycerol and cholesterol esters which is covered with a phospholipid monolayer that also contains apolipoproteins [86,87]. Lipoproteins such as HDL, LDL or VLDL have normal concentrations in human serum of around 280, 410, or 150 mg/dL, respectively [86]. These lipoproteins bind and transport hydrophobic compounds and lipids such as cholesterols and triacylglycerols throughout the body [85,86,88]. However, these carrier agents can also bind and carry various basic or hydrophobic drugs in the bloodstream [7,15,85,86,88].
Recent work with HPAC and lipoprotein columns have shown that drugs can interact with these binding agents through several possible mechanisms, including both partitioning into the hydrophobic core and saturable interactions (e.g., with sites on apolipoproteins) [81–83]. Both of these processes have been noted for propranolol with HDL, LDL or VLDL, as well as for verapamil with HDL [81–84]. The binding parameters for these interactions have been measured by HPAC, and the change in these binding constants with temperature has been investigated. In the case of LDL, stereoselective binding has been observed for R- and S-propranolol, which has been shown by HPAC to be due to the presence of both saturable and non-saturable binding for the R-enantiomer but only non-saturable binding for the S-enantiomer [81].
4. Zonal Elution
The most common method that has been used with affinity columns to study drug-protein interactions is zonal elution (see Figure 3 and top of Figure 1) [6–8,51,54,89,90]. This is also the approach that is typically used for the injection and separation of drugs and other solutes with HPLC columns that contain chiral stationary phases, such as those based on HSA or AGP [8–11,13]. Work starting in the late 1980s and early 1990s used zonal elution to measure retention factors, plate heights, separation factors and peak resolution to aid in the separation of chiral solutes by these columns [8,13,43,53,89,90]. This was accompanied by studies that examined the effects of the mobile phase pH, ionic strength, solvent polarity and temperature on the nature of these separations and the overall degree of stereoselectivity that they provided [89,90]. An example of such a study for R- and S-warfarin is shown in Figure 2(a) [37]. Other reports have examined the stereoselective binding of benzodiazepines or non-steroidal anti-inflammatory drugs with HSA [53,56,90,91], the separation of vinca alkaloids on HSA or AGP columns [92], the stereoselective binding of D/L-tryptophan with HSA [50,93,94], and the interactions of thyronines and thyroxine-related compounds with HSA or BSA [51,52,95].
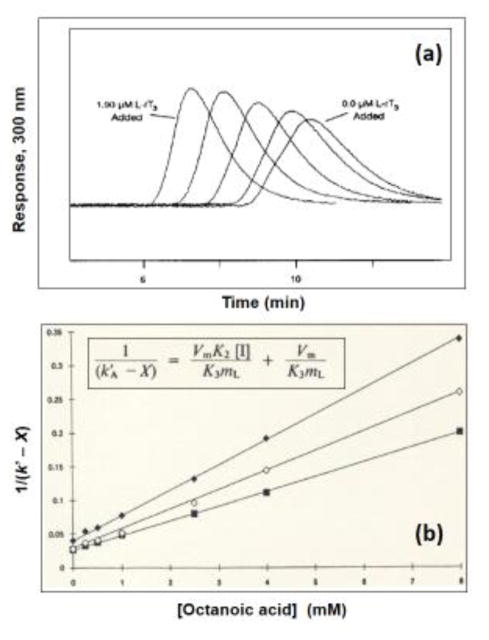
Figure 3.
Examples of competition studies carried out by zonal elution. The chromatograms in (a) were obtained on an HPLC column containing immobilized HSA by using R-warfarin as the injected solute and mobile phases that contained L-reverse triiodothyronine (L-rT3) at concentrations (from right-to-left) of 0.0, 0.24, 0.49, 0.97 or 1.90 μM. The plot in (b) shows the effects of adding octanoic acid to the mobile phase on the observed retention for (◆) R-warfarin, (◇) S-warfarin, and (■) phenylbutazone on an HPLC column containing immobilized HSA. The terms used within the equation in (b) are defined as follows: k′A is the retention factor for the injected solute, X is the retention for this solute due to sites other than those involved in the competitive binding with the mobile phase additive (i.e., octanoic acid), [I] is the mobile phase concentration of the additive, Vm is the void volume, mL is the moles of sites that are involved in the competition process, and K3 or K2 are the association equilibrium constants for the injected solute or mobile phase additive at the site of competition. Adapted with permission from Refs. [51,54].
In the early 1990s, research began in which zonal elution was employed to obtain more quantitative information on the interactions between drugs and proteins that were used as chiral stationary phases [6–8,11,13]. These efforts built on work begun by Dunn and Chaiken in 1974, in which it was shown how zonal elution could be used with low-performance affinity columns to obtain binding constants for enzyme-inhibitor interactions [96]. One way this type of experiment can be carried out is by using a column that has an immobilized protein, onto which is injected a small plug of a solute or drug in the presence of a mobile phase that contains a known concentration of a competing agent. The retention factor for the injected solute is then measured as the concentration of the competing agent is varied. The change in this retention as a function of the competing agent’s concentration is then fit to various models to see how the injected solute is binding to the protein and the extent to which this interaction is affected by the competing agent. It is further possible from such a fit to obtain information on the type of competition and equilibrium constants that are present for these solute-protein interactions [6,7,13].
Figure 3 shows some early experiments that were conducted in this manner with immobilized HSA in HPLC columns [51,54]. The chromatograms in Figure 3(a) show how the retention of an injected solute can shift in the presence of a competing agent on this type of column [51]. Figure 3(b) illustrates how such data may then be plotted and analyzed to determine the types of interactions that are present and to estimate some binding parameters [54]. For instance, the results obtained in Figure 3(b) made it possible to determine that R- or S-warfarin and phenylbutazone (i.e., the injected solutes) had single-site competition with octanoic acid (i.e., the competing agent) on the immobilized HSA column. It was further possible from these plots to determine the association equilibrium constants for octanoic acid at its site of competition with each of these solutes [54]. Similar results and related equations have been used to examine the interactions of many other drugs and compounds with HSA, AGP, lipoproteins, and related binding agents [6,7,13,17,50,55,60,82–84,97–102].
A variation of this approach appeared in 1992, when zonal elution competition studies were carried out by using injected solutes that were probes for particular sites on a protein [52]. This work began with competition studies such as those shown in Figure 3(b), which used R- or S-warfarin as probes for Sudlow site I of HSA [37,50–52,54]. It was then shown that equations like the one provided in Figure 3(b) could be used to obtain binding constants for the competing agent at specific regions on a protein by injecting separate probe compounds for each region [6,51,52]. This led to the identification of other site-selective probes that could be used for either HSA or AGP [50–52,60,76,103–105]. Some applications that followed included the development of affinity maps for drugs on these proteins [7,22,106,107] and reports that examined how modifications of these proteins affected local drug interactions at given sites [22,108–110].
The shape of plots like those in Figure 3(b) also makes it possible to discriminate between direct competition and other types of interactions that may occur between two drugs or solutes on an immobilized protein [6,7,22,54]. For example, if this plot is linear with a positive slope, such a result indicates that direct competition is occurring between the competing agent and injected solute at a single type of binding site. A slope of essentially zero indicates that no competition is present, and a negative slope is a signal that positive allosteric effects are occurring. In addition, negative allosteric effects or multi-site binding can be detected by the presence of a non-linear relationship with a positive slope [6,7,22]. It was shown in 2004 how this same data can be used in a more quantitative manner to not only identify the type of interaction that is taking place but to determine the coupling constants and binding parameters that are present for this interaction [111,112]. This quantitative approach has been used to examine the allosteric effects that occur between tamoxifen and warfarin as these drugs both bind to HSA [113], the allosteric interactions that occur between warfarin and S-propranolol on AGP [114], the interactions between ibuprofen and benzodiazepines on HSA [115], and the effect of tolbutamide on the interactions of R-warfarin with HSA [116].
Another approach that appeared in the early-to-mid 1990s was to use HPAC to study a binding site on an immobilized protein by comparing the retention factors or retention times that were seen for a series of related compounds [22,51]. Early work in this field involved a qualitative evaluation to determine which structural features were the most important contributors to retention [13,53]. This was followed by more detailed studies that compared the changes in binding affinities that were seen for the related compounds or that examined the forces that created such interactions [51,53,91]. This then led to the creation of quantitative structure-retention relationships (QSRRs), or models of how a protein’s binding site was interacting with a given class of solutes [22,91,117–119]. QSRRs have since been employed to study the binding of AGP with antihistamines, beta-adrenolytic drugs, or other pharmaceutical agents [7,13,43,119–121] and the binding of HSA with benzodiazepines or 2,3-substituted and 2-(4-biphenyl)-3-substituted 3-hydroxypropionic acids [118,122,123].
A related application of zonal elution has been to see how the retention of a set of drugs or solutes is changed as the structure of the immobilized protein is altered [7,22]. This method has been utilized to see how the modification of HSA at specific amino acids (e.g., Tyr-411, Trp-214 or Cys-34) will affect its ability to bind to drugs or site-specific probes [124–128]. This same method has been recently used to see how non-enzymatic glycation, as occurs during diabetes, affects the ability of HSA to bind to sulfonylureas and other drugs or solutes [108–110].
5. Frontal Analysis
Another method that is often used in affinity chromatography and HPAC for studying stereoselective drug-protein interactions is frontal analysis, or frontal affinity chromatography [6,7,22]. This approach was illustrated in the bottom portion of Figure 1 [37]. Frontal analysis was first used with low-performance affinity chromatography in 1975 by Kasai and Ishii to examine the interactions of trypsin and related enzymes with immobilized peptides [129]. A similar low-performance approach was used in 1978 and 1979 to study the binding of salicylate with BSA [130,131]. This method was then used with HPAC starting in 1992 through 1994 to examine the binding of HSA with various solutes (see example in Figure 4) [37]. These studies originally involved characterization of the stereoselective interactions of R- or S-warfarin and D- or L-tryptophan with HSA [37,50]. This method has now become a routine tool for characterizing the overall interactions and binding sites of various drugs and solutes with proteins such as HSA, AGP, and lipoproteins [7,11,13,22].
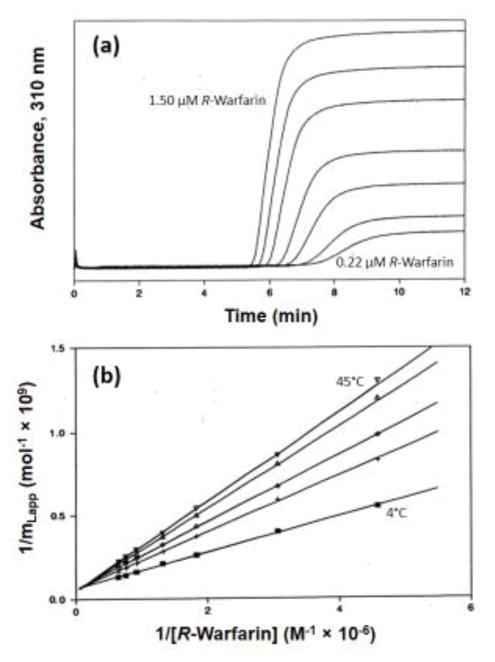
Figure 4.
Frontal analysis (a) chromatograms and (b) plots obtained for R-warfarin by HPAC on an HSA column. The results in (a) were acquired at 4°C and by using solutions that contained (from right-to-left) 0.22, 0.33, 0.55, 0.76, 1.10, 1.30 or 1.50 μM R-warfarin. The results in (b) were generated at temperatures (from bottom-to-top) of 4, 15, 25, 37 or 45°C. The term mLapp represents the moles of applied warfarin that were needed to reach the mean point of the breakthrough curve at a given concentration of this drug. Adapted with permission from Ref. [37].
Figure 4 provides some typical results from these early frontal analysis studies that made use of HPAC [37]. This type of experiment is usually carried out by continuously applying a solution that contains a known concentration of the drug or desired solute to a column that contained an immobilized binding agent, such as HSA or AGP. As the solute binds to the immobilized agent, the column begins to become saturated and a breakthrough curve is formed as more solute elutes from the column [6,22]. The mean position of this curve is then determined at several solute concentrations. If sufficiently fast association and dissociation rates are present between the applied solute and the immobilized binding agent, the resulting data can be used to provide information on the equilibrium constants for the interaction and the number of binding sites that are involved in this interaction [6,22].
Early work in this area focused on the examination of systems that could be described by relatively simple single-site binding models and linear fits to the data [37,50]. However, it is now also common to employ more complex models and equations that may involve non-linear fits [7,22]. Examples of such models include those that involve multi-site binding, non-saturable interactions, and combinations of saturable plus non-saturable interactions, such as have been observed for the interactions of some drugs with modified forms of HSA and with AGP or lipoproteins [6,22,76–78,80–84,108–110,132–134]. This approach has further been employed in the study of other systems, such as drug-receptor interactions [135] and the binding of various solutes with lectins or enzymes [136–139].
An important advantage of this method over zonal elution is that the same experiments can be used in frontal analysis to provide information on both the equilibrium constants and number of binding sites that are involved in a solute-protein interaction [7,22]. In addition, frontal analysis can be used to provide precise estimates of these values, especially when this technique is carried out in the form of HPAC [6]. Like zonal elution, frontal analysis can be used with various supports and detection methods [6,7,22]. One disadvantage of frontal analysis is that it does tend to take longer to perform than zonal elution, and it requires a larger amount of solute because studies must be carried out with many solutions of the applied compound [6,22].
Besides providing information on the overall processes involved in a drug-protein interaction, frontal analysis can be used to study the competition between two compounds for a protein or binding agent in an affinity column [22]. This is usually done by combining frontal analysis with mass spectrometry, giving a hybrid method known as frontal affinity chromatography-mass spectrometry (FAC-MS), as is illustrated in Figure 5 [6,136,138,140]. This method appeared in the late 1990s through early 2000s and typically uses an applied solution that contains both a solute and a competing agent [136,140]. The resulting breakthrough curves are then examined to see how the concentration of the competing agent affects binding by the solute to the column. This data, in turn, makes it possible to see if either direct competition or allosteric effects are present between these applied solutes [6,136,140–144]. This method can also be used to compare the binding of a set of competing agents to the immobilized agent and to determine the equilibrium constants for these interactions [136,138,140–144]. Recent examples of this approach have included studies of the binding of drugs with receptor domains [135] and the binding and displacement properties of flavonoids in the presence of histone deacetylase SIRT6 [141]. FAC-MS has also been employed in several studies to screen mixtures of drug candidates such as oligosaccharides, enzyme inhibitors and peptides for their binding and interactions with targets such as lectins, enzymes, receptors, and antibodies [136,140,143–146].
Figure 5.
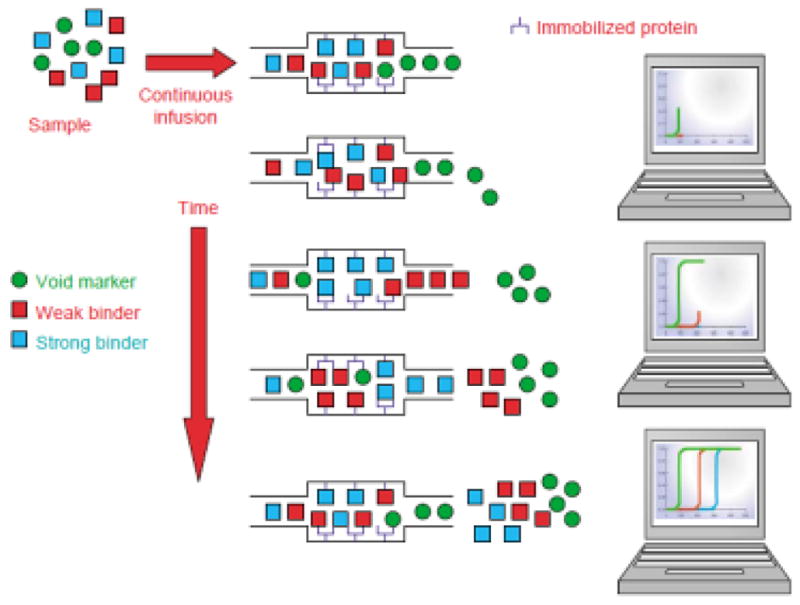
General principles behind frontal affinity chromatography-mass spectrometry (FAC-MS). Reproduced with permission from Ref. [140].
6. Kinetic methods
Zonal elution and frontal analysis experiments usually focus on equilibria that occur between an injected or applied solute and an immobilized binding agent. However, it is further possible to use affinity chromatography and HPAC to examine the rate of this interaction [22,39,147–149]. The earliest approach that was developed for this purpose is one based on band-broadening measurements [39,147–149]. This approach was suggested as early as 1975 [150] and was used with low-performance affinity columns to study the self-association of neurophysin II and its binding to a neuropeptide [151]. This method was then explored for use with HPAC in the mid-1980s for the study of lectin-sugar interactions [39,152,153] and was adapted for use in the study of drug or solute interactions with HSA by the mid-1990s [93,154]. Band-broadening measurements have since been used in various forms to study the binding of drugs and drug metabolites with both HSA and AGP [80,155–157].
A band-broadening experiment is usually carried out by measuring the plate heights for a drug or solute at one or more flow rates on an affinity column and then comparing these results to those that are obtained on an inert control column [147–149]. This comparison makes it possible to find the part of the total plate height for the drug or solute that is a result of stationary phase mass transfer (Hk). The plate height term that is obtained for this process is related to the dissociation rate for the drug/solute as it is released from the immobilized agent and can be used, through various approaches, to obtain the dissociation rate constant [39,147–149]. An example of such a study is provided in Figure 6 [157].
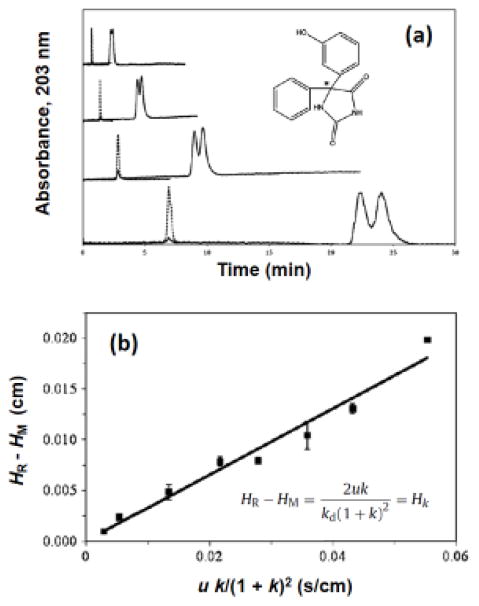
Figure 6.
Use of band-broadening measurements to examine the interaction kinetics for the enantiomers of phenytoin metabolites with HSA immobilized onto an HPLC column. The chromatograms in (a) were obtained at flow rates (from bottom-to-top) of 0.25, 0.5, 1.0 or 2.0 mL/min for injections of racemic 5-(3-hydroxyphenyl)-5-phenylhydantoin (i.e., m-HPPH; see structure given within this figure, were the asterisk shows the chiral center). The solid lines in (a) show the results for m-HPPH and the dashed lines show the results for sodium nitrate (i.e., a non-retained solute). The plot in (b) was obtained for the second eluting enantiomer of 5-(4-hydroxyphenyl)-5-phenylhydantoin (i.e., p-HPPH). In (b), HR is the total plate height measured for an enantiomer on the HSA column, and HM is the plate height determined for the same enantiomer on a control column; u is the linear velocity, k is the retention factor for the enantiomer, Hk is the plate height due to stationary phase mass transfer, and kd is the dissociation rate constant for the enantiomer with HSA. Adapted with permission from Ref. [157].
Several variations on this band-broadening approach have been reported. One form is a technique known as the plate height method [22,39,147–149]. In this method, a plot is made of Hk versus the injection flow rate or linear velocity, or a combined function of the flow rate/linear velocity and the retention factor for the solute. The slope of this plot can then be used to provide the dissociation rate constant for the solute with the immobilized binding agent [22,147–149]. The stereoselective interactions of HSA with D/L-tryptophan and R/S-warfarin have been examined by this approach [93,154]. Another form of the band-broadening approach is peak profiling, which makes use of the difference in plate heights that are measured for a drug or solute on an affinity column and on an inert control column (see Figure 6) [157]. This difference is determined at one or more flow rates and is used to calculate the dissociation rate constant for the solute with the immobilized binding agent [155]. Peak profiling has been used in recent years to estimate the dissociation rate constants for a number of chiral or achiral drugs and solutes with both HSA and AGP [80,155–158].
Another method that can be used with HPAC and affinity columns to obtain rate constants is the peak decay method [22,147–149]. The method makes use of small affinity columns and elution conditions in which the solute is prevented from rebinding to the immobilized binding agent as the retained solute is released from the column [159–161]. This method was first reported in the mid-1980s and used displacing agents to dissociate solutes (e.g., fluorescent sugars) that had moderate-to-strong binding to an immobilized agent in the column (e.g., an immobilized lectin) [39,159]. When this was done at a suitable high flow rate, the elution profile of the solute was found to form a first-order decay curve with a slope that gave the dissociation rate constant for the solute from the column [22,39,159]. An alternative peak decay approach was described in 2009 that could be used for systems with weak-to-moderate strength interactions and that did not require a displacing agent [160]. This second method has been employed to measure dissociation rate constants for various chiral and achiral drugs with both HSA [79,160,161] and AGP [79].
An alternative approach that can be used with affinity columns for either equilibrium or kinetic studies is ultrafast affinity extraction (see Figure 7) [149,162–169]. This method was first used for equilibrium-related measurements in 2001 [166] and for both equilibrium and rate constant measurements in 2014 [162]. This technique can be used to study a drug-protein interaction in solution by using a secondary binding agent in a small affinity column to quickly extract the non-bound form of the solute from an injected sample. This extracted fraction is then measured and used to provide information on both the rate and extent of the drug-protein binding. Both antibodies and more general binding agents such as HSA have been used to extract the non-bound form of drugs in this method [162–169]. The column size and flow are selected to minimize or control the level of dissociation that occurs by the solutes from soluble proteins as the sample passes through the column; these conditions often involve the use of sample residence times in the affinity column that range from a few hundred milliseconds up to a few seconds [149].
Figure 7.
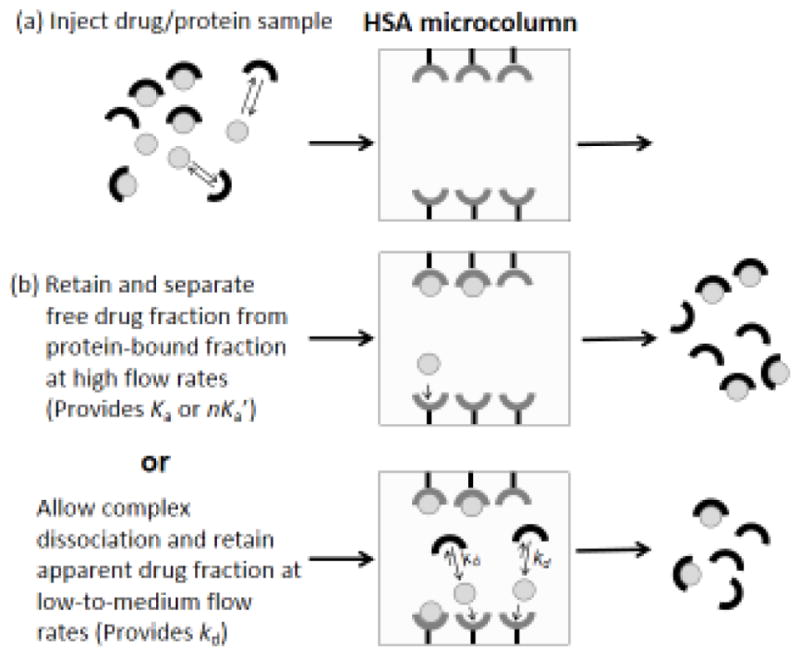
Scheme for (a) the injection of a drug/protein sample onto an HSA microcolumn, and (b) use of ultrafast affinity extraction with this sample and microcolumn to determine the association equilibrium constant (Ka) or global affinity (nKa′) for the drug-protein interaction in solution (middle panel) or the dissociation rate constant (kd) for the drug from the soluble drug-protein complex (bottom panel). Reproduced with permission from Ref. [162].
Systems with a wide range of binding strengths and dissociation rates have been examined by ultrafast affinity extraction [149,165,167]. Initial work with this method used it with small antibody columns to measure the non-bound fraction of warfarin in samples that contained this drug and HSA [166] and the non-bound fractions of phenytoin and thyroxine in serum [165,167]. This method was then adapted for use with small HSA columns to estimate the equilibrium constants for soluble HSA with warfarin, ibuprofen and imipramine [163] and to measure both the non-bound fractions and binding constants for R- and S-warfarin in serum and in mixtures with HSA [169]. This method was then adapted for kinetic studies and used with small HSA columns to study the interactions of several drugs with HSA [162], along with the interactions of testosterone with both HSA and sex hormone binding globulin [168].
7. Conclusions
This review examined the use of affinity chromatography and HPAC for the study of stereoselective drug interactions with serum binding agents, within an emphasis on the history of this field. The general principles of affinity chromatography and HPAC were first discussed, as related to drug-protein binding studies. Various types of serum proteins and binding agents that have been investigated by these methods were also examined. Several formats by which affinity chromatography and HPAC can be used in drug-protein binding studies were then described. These formats included zonal elution and frontal analysis methods, as well as several approaches for kinetic studies. It was shown how this group of methods could be used to examine and measure such things as the binding constants, rate constants, and number of binding sites for drugs with agents such as HSA, AGP, and lipoproteins. The use of these approaches in studying drug-drug competition, in characterizing binding sites, and in examining the mechanism of a drug-protein interaction was also discussed. In addition, several examples of these methods were provided. As HPAC and affinity chromatography continue to be developed for such work, it is expected that even more applications and formats will appear for these techniques in the study of stereoselective interactions by drugs with serum proteins and other binding agents.
Highlights.
The use of affinity chromatography in the study of drug-protein binding is reviewed.
The focus is on high-performance methods and serum proteins or related binding agents.
The use of these methods to examine stereoselective drug interactions is considered.
Important historical developments in this field are discussed.
Acknowledgments
This work was supported, in part, by National Institutes of Health under grants R01 GM044931 and R01 DK069629, and the National Science Foundation (NSF) under grant CHE 1309806.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.He Y. Chiral analysis in drug discovery and development. Innov Pharm Technol. 2010;35:18–23. [Google Scholar]
- 2.Kasprzyk-Hordern B. Pharmacologically active compounds in the environment and their chirality. Chem Soc Rev. 2010;39:4466–4503. doi: 10.1039/c000408c. [DOI] [PubMed] [Google Scholar]
- 3.Nagori BP, Deora MS, Saraswat P. Chiral drug analysis and their application. Int J Pharm Sco Rev Res. 2011;6:106–113. [Google Scholar]
- 4.Nguyer LA, He H, Pham-Huy C. Chiral drugs: an overview. Int J Biomed Sci. 2006;2:85–100. [PMC free article] [PubMed] [Google Scholar]
- 5.Ilisz I, Berkecz R, Peter A. Application of chiral derivatizing agents in the high-performance liquid chromatographic separation of amino acid enantiomers: a review. J Pharm Biomed Anal. 2008;47:1–15. doi: 10.1016/j.jpba.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Hage DS. High-performance affinity chromatography: a powerful tool for studying serum protein binding. J Chromatogr B. 2002;768:3–30. doi: 10.1016/s0378-4347(01)00482-0. [DOI] [PubMed] [Google Scholar]
- 7.Hage DS, Jackson A, Sobansky M, Schiel JE, Yoo MJ, Joseph KS. Characterization of drug-protein interactions in blood using high-performance affinity chromatography. J Sep Sci. 2009;32:835–853. doi: 10.1002/jssc.200800640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel S, Wainer IW, Lough WJ. Affinity based chiral stationary phases. In: Hage DS, editor. Handbook of Affinity Chromatography. 2. Chap. 21 New York: Taylor & Francis; 2006. [Google Scholar]
- 9.Allenmark SG. Chromatographic Enantioseparation Methods: Methods and Applications. Ellis Horwood; New York: 1991. [Google Scholar]
- 10.Wainer IW. Drug Stereochemistry: Analytical Methods and Pharmacology. 2. Marcel Dekker; New York: 1993. [Google Scholar]
- 11.Hage DS. Chromatographic and electrophoretic studies of protein binding to chiral solutes. J Chromatogr A. 2001;906:459–481. doi: 10.1016/s0021-9673(00)00957-2. [DOI] [PubMed] [Google Scholar]
- 12.Lindup WE. Plasma protein binding of drug-some basic and clinical aspects. In: Bridges JW, Chasseaud LF, Gibson GG, editors. Progress in Drug Metabolism. Chap. 4 New York: Taylor & Francis; 1987. [Google Scholar]
- 13.Patel S, Wainer IW, Lough WJ. Chromatographic studies of molecular recognition and solute binding to enzymes and plasma proteins. In: Hage DS, editor. Handbook of Affinity Chromatography. 2. Chap. 24 New York: Taylor & Francis; 2006. [Google Scholar]
- 14.Kratochwil NA, Huber W, Muller F, Kansy M, Gerber PR. Predicting plasma protein binding of drugs: a new approach. Biochem Pharmacol. 2002;64:1355–1374. doi: 10.1016/s0006-2952(02)01074-2. [DOI] [PubMed] [Google Scholar]
- 15.Kwong TC. Free drug measurements: methodology and clinical significance. Clin Chim Acta. 1985;151:193–216. doi: 10.1016/0009-8981(85)90082-8. [DOI] [PubMed] [Google Scholar]
- 16.Peters T., Jr . All About Albumin: Biochemistry, Genetics and Medical Applications. New York: Academic Press; 1996. [Google Scholar]
- 17.Hage DS, Tweed SA. Recent advances in chromatographic and electrophoretic methods for the study of drug-protein interactions. J Chromatogr B. 1997;699:499–525. doi: 10.1016/s0378-4347(97)00178-3. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Beeram SR, Bi C, Suresh D, Zheng X, Hage DS. High-performance affinity chromatography: applications in drug-protein binding studies and personalized medicine. In: Donev Rossen., editor. Advances in Protein Chemistry and Structural Biology. Vol. 102. Chap. 1. 2016. [DOI] [PubMed] [Google Scholar]
- 19.Williams MA, Daviter T, editors. Methods and Applications. New York: Springer; 2013. Protein-Ligand Interactions. [Google Scholar]
- 20.Cantor CR, Schimmel PR. Biophysical Chemistry, Part 2: Techniques for the Study of Biological Structure and Function. San Francisco: Freeman; 1980. [Google Scholar]
- 21.Matsuda R, Bi C, Anguizola J, Sobansky M, Rodriguez E, Badilla JV, Zheng X, Hage B, Hage DS. Studies of metabolite-protein interactions: a review. J Chromatogr B. 2014;966:48–58. doi: 10.1016/j.jchromb.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hage DS, Chen J. Quantitative affinity chromatography: practical aspects. In: Hage DS, editor. Handbook of Affinity Chromatography. 2. Chap. 22 New York: Taylor & Francis; 2006. [Google Scholar]
- 23.Walters RR. Affinity chromatography. Anal Chem. 1985;57:1099A–1101A. doi: 10.1021/ac00288a001. [DOI] [PubMed] [Google Scholar]
- 24.Hage DS. Handbook of Affinity Chromatography. 2. New York: Taylor & Francis; 2006. [Google Scholar]
- 25.Ettre LS. Nomenclature for chromatography. Pure Appl Chem. 1993;65:819–872. [Google Scholar]
- 26.Starkenstein E. Ferment action and the influence upon it of neutral salts. Biochem Z. 1910;24:210–218. [Google Scholar]
- 27.Hage DS, Matsuda R. Affinity chromatography a historical perspective. In: Reichelt S, editor. Affinity Chromatography, Methods in Molecular Biology series. Vol. 1286. Springer; Heidelberg: 2015. pp. 1–19. [DOI] [PubMed] [Google Scholar]
- 28.Cuatrecasas P, Wilchek M, Anfinsen CB. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci USA. 1968;68:636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hage DS, Ruhn PF. An introduction to affinity chromatography. In: Hage DS, editor. Handbook of Affinity Chromatography. 2. Boca Raton: CRC Press; 2005. pp. 3–13. [Google Scholar]
- 30.Turkova J. Affinity Chromatography. Amsterdam: Elsevier; 1978. [Google Scholar]
- 31.Hage DS. Affinity chromatography: a review of clinical applications. Clin Chem. 1999;45:593–615. [PubMed] [Google Scholar]
- 32.Gustavsson PE, Larsson PO. Support materials for affinity chromatography. In: Hage DS, editor. Handbook of Affinity Chromatography. 2. Boca Raton: CRC Press; 2005. pp. 16–32. [Google Scholar]
- 33.Mallik R, Jiang T, Hage DS. High-performance affinity monolith chromatography: development and evaluation of human serum albumin columns. Anal Chem. 2004;76:7013–7022. doi: 10.1021/ac049001q. [DOI] [PubMed] [Google Scholar]
- 34.Mallik R, Hage DS. Development of an affinity silica monolith containing human serum albumin for chiral separations. J Pharm Biomed Anal. 2008;46:820–830. doi: 10.1016/j.jpba.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfaunmiller EL, Paulemond ML, Dupper CM, Hage DS. Affinity monolith chromatography: a review of principles and recent analytical applications. Anal Bioanal Chem. 2013;405:2133–2145. doi: 10.1007/s00216-012-6568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mallik R, Hage DS. Affinity monolith chromatography. J Sep Sci. 2006;29:1686–1704. doi: 10.1002/jssc.200600152. [DOI] [PubMed] [Google Scholar]
- 37.Loun B, Hage DS. Chiral separation mechanism in protein-based HPLC columns. 1. Thermodynamic studies of (R)- and (S)- warfarin binding to immobilized human serum albumin. Anal Chem. 1994;66:3814–3822. doi: 10.1021/ac00093a043. [DOI] [PubMed] [Google Scholar]
- 38.Rosas MER, Patel S, Wainer IW. Determination of enantiomers of ketamine and norketamine in human plasma by enantioselective liquid chromatography-mass spectrometry. J Chromatogr B. 2003;794:99–108. doi: 10.1016/s1570-0232(03)00420-3. [DOI] [PubMed] [Google Scholar]
- 39.Chaiken IM. Analytical Affinity Chromatography. Boca Raton: CRC Press; 1987. [Google Scholar]
- 40.Hage DS, Austin J. High-performance affinity chromatography and immobilized serum albumin as probes for drug- and hormone-protein binding. J Chromatogr B. 2000;739:39–54. doi: 10.1016/s0378-4347(99)00445-4. [DOI] [PubMed] [Google Scholar]
- 41.Haginaka J. Protein-based chiral stationary phases for high-performance liquid chromatography enantioseparations. J Chromatogr A. 2001;906:253–273. doi: 10.1016/s0021-9673(00)00504-5. [DOI] [PubMed] [Google Scholar]
- 42.Millot MC. Separation of drug enantiomers by liquid chromatography and capillary electrophoresis, using immobilized proteins as chiral selectors. J Chromatogr B. 2003;797:131–159. doi: 10.1016/j.jchromb.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 43.Domenici E, Bertucci C, Salvadori P, Motellier S, Wainer IW. Immobilized serum albumin: rapid HPLC probe of stereoselective protein-binding interactions. Chirality. 1990;2:263–268. doi: 10.1002/chir.530020412. [DOI] [PubMed] [Google Scholar]
- 44.Lagercrantz C, Larsson T, Denfors I. Stereoselective binding of the enantiomers of warfarin and tryptophan to serum albumin from some different species studied by affinity chromatography on columns of immobilized serum albumin. Comp Biochem Physiol. 1981;69:375–378. doi: 10.1016/0306-4492(81)90153-2. [DOI] [PubMed] [Google Scholar]
- 45.Massolini G, Aubry AF, McGann A, Wainer IW. Determination of the magnitude and enantioselectivity of ligand binding to rat and rabbit serum albumins using immobilized-protein high performance liquid chromatography stationary phases. Biochem Pharmacol. 1993:1285–1293. doi: 10.1016/0006-2952(93)90478-f. [DOI] [PubMed] [Google Scholar]
- 46.Aubry AF, Markoglou N, McGann A. Comparison of drug binding interactions on human, rat and rabbit serum albumin using high-performance displacement chromatography. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1995;112:257–266. doi: 10.1016/0742-8413(95)02019-5. [DOI] [PubMed] [Google Scholar]
- 47.Pistolozzi M, Fortugno C, Franchini C, Corbo F, Muraglia M, Roy M, Félix G, Bertucci C. Species-dependent binding of tocainide analogues to albumin: affinity chromatography and circular dichroism study. J Chromatogr B. 2014;968:69–78. doi: 10.1016/j.jchromb.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Petitpas I, Bhattacharya AA, Twine S, East M, Curry S. Crystal structure analysis of warfarin binding to human serum albumin-anatomy of drug site I. J Biol Chem. 2011;276:22804–22809. doi: 10.1074/jbc.M100575200. [DOI] [PubMed] [Google Scholar]
- 49.Lammers N, De Bree H, Groen CP, Ruiiten HM, De Jong BJ. Determination of drug protein-binding by high-performance liquid chromatography using a chemically bonded bovine serum albumin stationary phase. J Chromatogr. 1989;496:291–300. doi: 10.1016/s0378-4347(00)82578-5. [DOI] [PubMed] [Google Scholar]
- 50.Yang J, Hage DS. Characterization of the binding and chiral separation of D- and L-tryptophan on a high-performance immobilized human serum albumin column. J Chromatogr A. 1993;645:241–250. doi: 10.1016/0021-9673(93)83383-4. [DOI] [PubMed] [Google Scholar]
- 51.Loun B, Hage DS. Characterization of thyroxine-albumin binding using high-performance affinity chromatography. II. Comparison of the binding of thyroxine, triiodothyronines and related compounds at the warfarin and indole sites of human serum albumin. J Chromatogr B. 1995;665:303–314. doi: 10.1016/0378-4347(94)00547-i. [DOI] [PubMed] [Google Scholar]
- 52.Loun B, Hage DS. Characterization of thyroxine-albumin binding using high-performance affinity chromatography. J Chromatogr. 1992;579:225–235. [PubMed] [Google Scholar]
- 53.Noctor TAG, Pham CD, Kaliszan R, Wainer IW. Stereochemical aspects of benzodiazepine binding to human serum albumin. I: Enantioselective high-performance liquid affinity chromatographic examination of chiral and achiral binding interactions between 1,4-benzodiazepines and human serum albumin. Mol Pharmacol. 1992;42:506–511. [PubMed] [Google Scholar]
- 54.Noctor TAG, Wainer IW, Hage DS. Allosteric and competitive displacement of drugs from human serum albumin by octanoic acid, as revealed by high-performance liquid chromatography, on a human serum albumin-based stationary phase. J Chromatogr B. 1992;577:305–315. doi: 10.1016/0378-4347(92)80252-l. [DOI] [PubMed] [Google Scholar]
- 55.Hage DS, Noctor TAG, Wainer IW. Characterization of the protein-binding of chiral drugs by high-performance affinity chromatography. Interactions of R-ibuprofen and S-ibuprofen with human serum albumin. J Chromatogr A. 1995;693:23–32. doi: 10.1016/0021-9673(94)01009-4. [DOI] [PubMed] [Google Scholar]
- 56.Domenici E, Bertucci C, Salvadori P, Wainer IW. The use of a human serum albumin based HPLC chiral stationary phase for the investigation of protein binding: the detection of the allosteric interaction between warfarin and benzodiazepine binding sites. J Pharm Sci. 1991;80:164–166. doi: 10.1002/jps.2600800216. [DOI] [PubMed] [Google Scholar]
- 57.Noctor TAG, Diaz-Perez MJ, Wainer IW. The use of human serum albumin based stationary phase for high performance liquid chromatography as a tool for the rapid determination of drug-plasma protein binding. J Pharm Sci. 1993;82:675–676. doi: 10.1002/jps.2600820629. [DOI] [PubMed] [Google Scholar]
- 58.Bertucci C, Canepa A, Ascoli GA, Lopes Guimaraes LF, Felix G. Site I on human albumin: differences in the binding of (R)- and (S)-warfarin. Chirality. 1999;11:675–679. doi: 10.1002/(SICI)1520-636X(1999)11:9<675::AID-CHIR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 59.Ascoli G, Bertucci C, Salvadori P. Ligand binding to a human serum albumin stationary phase: use of same-drug competition to discriminate relevant interactions. Biomed Chromatogr. 1998;12:248–254. doi: 10.1002/(SICI)1099-0801(199809/10)12:5<248::AID-BMC742>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 60.Xuan H, Hage DS. Immobilization of α1-acid glycoprotein for chromatographic studies of drug-protein binding. Anal Biochem. 2005;346:300–310. doi: 10.1016/j.ab.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 61.Mallik R, Xuan H, Guiochon G, Hage DS. Immobilization of alpha1-acid glycoprotein for chromatographic studies of drug-protein binding. II. Correction for errors in association constant measurements. Anal Biochem. 2008;376:154–156. doi: 10.1016/j.ab.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mallik R, Xuan H, Hage DS. Development of an affinity silica monolith containing alpha1-acid glycoprotein for chiral separations. J Chromatogr A. 2007;1149:294–304. doi: 10.1016/j.chroma.2007.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schonfeld DL, Ravelli RB, Mueller U, Skerra A. The 1.8-Å crystal structure of alpha1-acid glycoprotein (orosomucoid) solved by UV RIP reveals the broad drug-binding activity of this human plasma lipocalin. J Mol Biol. 2008;384:393–405. doi: 10.1016/j.jmb.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 64.Taguchi K, Nishi K, Chuang VTG, Maruyama T, Otagiri M. Molecular aspects of human alpha-1-acid glycoprotein structure and function. In: Janciauskiene S, editor. Acute Phase Proteins. InTech; Rijeka, Croatia: 2013. pp. 139–162. [Google Scholar]
- 65.Fournier T, Medjoubi-N N, Porquet D. Alpha-1-acid glycoprotein. Biochim Biophys Acta. 2000;1482:157–171. doi: 10.1016/s0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 66.Zsila F, Iwao Y. The drug binding site of human α1-acid glycoprotein: insight from induced circular dichroism and electronic absorption spectra. Biochim Biophys Acta. 2007;1770:797–809. doi: 10.1016/j.bbagen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 67.Ceciliani F, Pocacqua V. The acute phase protein α1-acid glycoprotein: a model for altered glycosylation during diseases. Curr Protein Pept Sci. 2007;8:91–108. doi: 10.2174/138920307779941497. [DOI] [PubMed] [Google Scholar]
- 68.Schmid K, Nimerg RB, Kimura A, Yamaguchi H, Binette JP. The carbohydrate units of human plasma α1-acid glycoprotein. Biochim Biophys Acta. 1977;492:291–302. doi: 10.1016/0005-2795(77)90080-0. [DOI] [PubMed] [Google Scholar]
- 69.Filip Z, Jan K, Vendula S, Jana KZ, Kamil M, Kamil K. Albumin and α1-acid glycoprotein: old acquaintances. Expert Opin Drug Metab Toxicol. 2013;9:943–954. doi: 10.1517/17425255.2013.790364. [DOI] [PubMed] [Google Scholar]
- 70.Israili ZH, Dayton PG. Human alpha-1-glycoprotein and its interactions with drugs. Drug Metab Rev. 2001;33:161–235. doi: 10.1081/dmr-100104402. [DOI] [PubMed] [Google Scholar]
- 71.Burton ME, Shaw LM, Schentag JJ, Evans WE. Applied Pharmacokinetics and Pharmacodynamics: Principles of Therapeutic Drug Monitoring. Lippincott; Baltimore: 2006. [Google Scholar]
- 72.Enquist M, Hermansson J. Separation and quantitation of (R)- and (S)-atenolol in human plasma and urine using an α1-AGP column. Chirality. 1989;1:209–215. doi: 10.1002/chir.530010306. [DOI] [PubMed] [Google Scholar]
- 73.Locatelli I, Kmetec V, Mrhar A, Grabnar I. Determination of warfarin enantiomers and hydroxylated metabolites in human blood plasma by liquid chromatography with achiral and chiral separation. J Chromatogr B. 2005;818:191–198. doi: 10.1016/j.jchromb.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 74.Sandstrom E, Lennernas H, Ohlen K, Karlsson A. Enantiomeric separation of verapamil and norverapamil using Chiral-AGP as the stationary phase. J Pharm Biomed Anal. 1999;21:43–49. doi: 10.1016/s0731-7085(99)00093-x. [DOI] [PubMed] [Google Scholar]
- 75.Etter ML, George S, Graybiel K, Eichhorst J, Lehotay DC. Determination of free and protein-bound methadone and its major metabolite EDDP: enantiomeric separation and quantitation by LC/MS/MS. Clin Biochem. 2005;38:1095–1102. doi: 10.1016/j.clinbiochem.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 76.Xuan H, Joseph KS, Wa C, Hage DS. Biointeraction analysis of carbamazepine binding to alpha 1-acid glycoprotein by high-performance affinity chromatography. J Sep Sci. 2010;33:2294–2301. doi: 10.1002/jssc.201000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anguizola J. PhD dissertation. University of Nebraska; Lincoln: 2013. Affinity Chromatographic Studies of Drug-Protein Binding in Personalized Medicine. [Google Scholar]
- 78.Anguizola J, Bi C, Koke M, Jackson A, Hage DS. On-column entrapment of alpha1-acid glycoprotein for studies of drug-protein binding by high-performance affinity chromatography. Anal Bioanal Chem. 2016;408:5745–5756. doi: 10.1007/s00216-016-9677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoo MJ, Hage DS. High-throughput analysis of drug dissociation from serum proteins using affinity silica monoliths. J Sep Sci. 2011;34:2255–2263. doi: 10.1002/jssc.201100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bi C. PhD dissertation. University of Nebraska; Lincoln: 2016. Analysis of Drug Interactions with Alpha1-Acid Glycoprotein using High-Performance Affinity Chromatography. [Google Scholar]
- 81.Sobansky MR, Hage DS. Identification and analysis of stereoselective drug interactions with low-density lipoprotein by high-performance affinity chromatography. Anal Bioanal Chem. 2012;402:563–571. doi: 10.1007/s00216-012-5816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen S, Sobansky MR, Hage DS. Analysis of drug interactions with high-density lipoprotein by high-performance affinity chromatography. Anal Biochem. 2010;397:107–114. doi: 10.1016/j.ab.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sobansky MR, Hage DS. Analysis of drug interactions with very low density lipoprotein by high-performance affinity chromatography. Anal Biochem Chem. 2014;406:6203–6211. doi: 10.1007/s00216-014-8081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sobansky MR, Hage DS. Analysis of drug interactions with lipoproteins by high-performance affinity chromatography. In: Berhardt LV, editor. Advances in Medicine and Biology. Vol. 53. Nova Science Publishers; 2012. Chapter 9. [PMC free article] [PubMed] [Google Scholar]
- 85.Jonas A. Lipoprotein structure. In: Vance DE, Vance JE, editors. Biochemistry of Lipids, Lipoproteins, and Membranes. Elsevier; Amsterdam: 2002. pp. 483–504. [Google Scholar]
- 86.Barklay M. Lipoprotein class distribution in normal and disease states. In: Nelson GJ, editor. Blood Lipids and Lipoproteins: Quantitation, Composition, and Metabolism. New York: Wiley; 1972. pp. 587–603. [Google Scholar]
- 87.Kostner GM, Laggner P. Human Plasma Lipoproteins. Berlin: Walter de Gruyter; 1989. [Google Scholar]
- 88.Wasan KM, Cassidy SM. Role of plasma lipoproteins in modifying the biological activity of hydrophobic drugs. J Pharm Sci. 1998;87:411–424. doi: 10.1021/js970407a. [DOI] [PubMed] [Google Scholar]
- 89.Domenici E, Bertucci C, Salvadori P, Felix G, Cahagne I, Motellier S, Wainer IW. Synthesis and chromatographic properties of an HPLC chiral stationary phase based upon human serum albumin. Chromatographia. 1990;29:170–176. [Google Scholar]
- 90.Noctor TAG, Felix G, Wainer IW. Stereochemical resolution of enantiomeric 2-aryl propionic acid nonsteroidal anti-inflammatory drugs on a human albumin based high-performance liquid chromatographic chiral stationary phase. Chromatographia. 1991;31:55–59. [Google Scholar]
- 91.Kaliszan R, Noctor TAG, Wainer IW. Stereochemical aspects of benzodiazepine binding to human serum albumin. II: Quantitative relationships between structure and enantioselective retention in high-performance liquid affinity chromatography. Mol Pharmacol. 1992;42:512–517. [PubMed] [Google Scholar]
- 92.Fitos I, Visy J, Simonyi M, Hermansson J. Chiral high-performance liquid chromatographic separations of vinca alkaloid analogs on α1-acid glycoprotein and human serum albumin columns. J Chromatogr. 1992;609:163–171. doi: 10.1016/0021-9673(92)80159-r. [DOI] [PubMed] [Google Scholar]
- 93.Yang J, Hage DS. Effect of mobile phase composition on the binding kinetics of chiral solutes on a protein-based high-performance liquid chromatography column: interactions of D- and L-tryptophan with immobilized human serum albumin. J Chromatogr A. 1997;766:15–25. doi: 10.1016/s0021-9673(96)01040-0. [DOI] [PubMed] [Google Scholar]
- 94.Yang J, Hage DS. Role of binding capacity versus binding strength in the separation of chiral compounds on protein-based high-performance liquid chromatography columns: interactions of D- and L-tryptophan with human serum albumin. J Chromatogr A. 1996;725:273–285. doi: 10.1016/0021-9673(95)01009-2. [DOI] [PubMed] [Google Scholar]
- 95.Dalgaard L, Hansen JJ, Pedersen JL. Resolution and binding site determination of DL-thyronine by high-performance liquid chromatography using immobilized albumin as chiral stationary phase: determination of the optical purity of thyroxine in tablets. J Pharm Biomed Anal. 1989;7:361–368. doi: 10.1016/0731-7085(89)80103-7. [DOI] [PubMed] [Google Scholar]
- 96.Dunn BM, Chaiken IM. Quantitative affinity chromatography: determination of binding constants by elution with competitive inhibitors. Proc Natl Acad Sci USA. 1974;71:2382–2385. doi: 10.1073/pnas.71.6.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim HS, Mallik R, Hage DS. Chromatographic analysis of carbamazepine binding to human serum albunmin: II. Comparison of the Schiff base and N-hydroxysuccinimide immobilization methods. J Chromatogr B. 2006;837:138–146. doi: 10.1016/j.jchromb.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 98.Mallik R, Wa C, Hage DS. Development of sulfhydryl-reactive silica for protein immobilization in high-performance affinity chromatography. Anal Chem. 2007;79:1411–1424. doi: 10.1021/ac061779j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bertucci C. Enantioselective inhibition of the binding of rac-profens to human serum albumin induced by lithocholate. Chirality. 2001;13:372–378. doi: 10.1002/chir.1047. [DOI] [PubMed] [Google Scholar]
- 100.Bertucci C, Andrisano V, Gotti R, Cavrini V. Use of an immobilised human serum albumin HPLC column as a probe of drug-protein interactions: the reversible binding of valproate. J Chromatogr B. 2002;768:147–155. doi: 10.1016/s0378-4347(01)00494-7. [DOI] [PubMed] [Google Scholar]
- 101.Bertucci C, Pistolozzi M, Felix G, Danielson UH. HSA binding of HIV protease inhibitors: a high-performance affinity chromatography study. J Sep Sci. 2009;32:1625–1631. doi: 10.1002/jssc.200900051. [DOI] [PubMed] [Google Scholar]
- 102.Bertucci C, Cimitan S, Riva A, Morazzoni P. Binding studies of taxanes to human serum albumin by bioaffinity chromatography and circular dichroism. J Pharm Biomed Anal. 2006;42:81–87. doi: 10.1016/j.jpba.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 103.Hage DS, Sengupta A. Studies of protein binding to non-polar solutes by using zonal elution and high-performance affinity chromatography: interactions of cis- and trans-clomiphene with human serum albumin in the presence of β-cyclodextrin. Anal Chem. 1998;70:4602–4609. doi: 10.1021/ac980734i. [DOI] [PubMed] [Google Scholar]
- 104.Sengupta A, Hage DS. Characterization of the binding of digitoxin and acetyldigitoxin to human serum albumin by high-performance affinity chromatography. J Chromatogr B. 1999;725:91–100. doi: 10.1016/s0378-4347(98)00589-1. [DOI] [PubMed] [Google Scholar]
- 105.Sengupta A, Hage DS. Characterization of minor site probes for human serum albumin by high-performance affinity chromatography. Anal Chem. 1999;71:3821–3827. doi: 10.1021/ac9903499. [DOI] [PubMed] [Google Scholar]
- 106.Chen J, Ohnmacht C, Hage DS. Studies of phenytoin binding to human serum albumin by high-performance affinity chromatography. J Chromatogr B. 2004;809:137–145. doi: 10.1016/j.jchromb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 107.Kim H, Hage DS. Chromatographic analysis of carbamazepine binding to human serum albumin. J Chromatogr B. 2005;816:57–66. doi: 10.1016/j.jchromb.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 108.Matsuda R, Li Z, Zheng X, Hage DS. Analysis of multi-site drug-protein interactions by high-performance affinity chromatography: binding of glimepiride to normal or glycated human serum albumin. J Chromatogr A. 2015;1408:133–144. doi: 10.1016/j.chroma.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Anguizola J, Joseph KS, Barnaby OS, Matsuda R, Alvarado G, Clarke W, Cerny RL, Hage DS. Development of affinity microcolumns for drug-protein binding studies in personalized medicine: interactions of sulfonylurea drugs with in vivo glycated human serum albumin. Anal Chem. 2013;85:4453–4460. doi: 10.1021/ac303734c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Matsuda R, Anguizola J, Joseph KS, Hage DS. Analysis of drug interactions with modified proteins by high-performance affinity chromatography: binding of glibenclamide to normal and glycated human serum albumin. J Chromatogr A. 2012;1265:114–122. doi: 10.1016/j.chroma.2012.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen J, Hage DS. Quantitative analysis of allosteric drug-protein binding by biointeraction chromatography. Nature Biotechnol. 2004;22:1445–1448. doi: 10.1038/nbt1022. [DOI] [PubMed] [Google Scholar]
- 112.Wainer IW. Finding time for allosteric interactions. Nature Biotechnol. 2004;22:1376–1377. doi: 10.1038/nbt1104-1376. [DOI] [PubMed] [Google Scholar]
- 113.Chen J, Hage DS. Quantitative studies of allosteric effects by biointeraction chromatography: analysis of protein binding to low solubility drugs. Anal Chem. 2006;78:2672–2683. doi: 10.1021/ac052017b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anguizola J, Bi C, Koke M, Jackson A, Hage DS. On-column entrapment of alpha1-acid glycoprotein for studies of drug-protein binding by high-performance affinity chromatography. Anal Bioanal Chem. 2016;408:5745–5756. doi: 10.1007/s00216-016-9677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen J, Fitos I, Hage DS. Chromatographic analysis of allosteric effects between ibuprofen and benzodiazepines on human serum albumin. Chirality. 2006;18:24–36. doi: 10.1002/chir.20216. [DOI] [PubMed] [Google Scholar]
- 116.Joseph KS, Hage DS. Characterization of the binding of sulfonylurea drugs to HSA by high-performance affinity chromatography. J Chromatogr B. 2010;878:1590–1598. doi: 10.1016/j.jchromb.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Turowski M, Kaliszan R. Keratin immobilized on silica as a new stationary phase for chromatographic modelling of skin permeation. J Pharm Biomed Anal. 1997;15:1325–1333. doi: 10.1016/s0731-7085(96)02009-2. [DOI] [PubMed] [Google Scholar]
- 118.Kaliszan R, Kaliszan A, Noctor TAG, Purcell WP, Wainer IW. Mechanism of retention of benzodiazepines in affinity, reversed-phase and adsorption high-performance liquid chromatography in view of quantitative structure retention relationships. J Chromatogr A. 1992;609:69–81. [Google Scholar]
- 119.Kaliszan R, Nasal A, Turowski M. Quantitative structure-retention relationships in the examination of the topography of the binding site of antihistamine drugs on α1-acid glycoprotein. J Chromatogr A. 1996;722:25–32. doi: 10.1016/0021-9673(95)00523-4. [DOI] [PubMed] [Google Scholar]
- 120.Karlsson A, Aspegren A. Enantiomeric separation of amino alcohols on protein phases using statistical experimental design: a comparative study. J Chromatogr A. 2000;866:15–23. doi: 10.1016/s0021-9673(99)01040-7. [DOI] [PubMed] [Google Scholar]
- 121.Gyimesi-Forras K, Szasz G, Gergely A, Szabo M, Kokosi K. Optical resolution of a series of potential cholecystokinin antagonist 4(3H)-quinazolone derivatives by chiral liquid chromatography on alpha(1)-acid glycoprotein stationary phase. J Chromatogr Sci. 2000;38:430–434. doi: 10.1093/chromsci/38.10.430. [DOI] [PubMed] [Google Scholar]
- 122.Andrisano V, Bertucci C, Cavrini V, Recanatini M, Cavalli A, Varoli L, Felix G, Wainer IW. Stereoselective binding of 2,3-substituted-3-hydroxy-propionic acids on an immobilised human serum albumin chiral stationary phase: stereochemical characterisation and QSRR study. J Chromatogr A. 2000;876:75–86. doi: 10.1016/s0021-9673(00)00195-3. [DOI] [PubMed] [Google Scholar]
- 123.Andrisano V, Gotti R, Recanatini M, Cavalli A, Varoli L, Bertucci C. Stereoselective binding of 2-(4-biphenylyl)-3-substituted-3-hydroxypropionic acids on an immobilised human serum albumin chiral stationary phase. J Chromatogr B. 2002;768:137–145. doi: 10.1016/s0378-4347(01)00493-5. [DOI] [PubMed] [Google Scholar]
- 124.Noctor TAG, Wainer IW. The in situ acetylation of an immobilized human serum albumin chiral stationary phase for high-performance liquid chromatography in the examination of drug protein binding phenomena. Pharmaceut Res. 1992;9:480–484. doi: 10.1023/a:1015884112039. [DOI] [PubMed] [Google Scholar]
- 125.Chattopadhyay A, Tian T, Kortum L, Hage DS. Development of tryptophan-modified human serum albumin columns for site-specific studies of drug protein interactions by high-performance affinity chromatography. J Chromatogr B. 1998;715:183–190. doi: 10.1016/s0378-4347(98)00140-6. [DOI] [PubMed] [Google Scholar]
- 126.Bertucci C, Nanni B, Raffaelli A, Salvadori P. Chemical modification of human albumin at Cys34 by ethacrynic acid: structural characterisation and binding properties. J Pharm Biomed Anal. 1998;18:127–136. doi: 10.1016/s0731-7085(98)00163-0. [DOI] [PubMed] [Google Scholar]
- 127.Zheng X, Podariu M, Bi C, Hage DS. Development of enhanced capacity affinity microcolumns by using a hybrid of protein cross-linking/modification and immobilization. J Chromatogr A. 2015;1400:82–90. doi: 10.1016/j.chroma.2015.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bertucci C, Wainer IW. Improved chromatographic performances of a modified human albumin based stationary phase. Chirality. 1997;9:335–340. doi: 10.1002/(SICI)1520-636X(1997)9:4<335::AID-CHIR4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 129.Kasai K, Ishii S. Affinity chromatography of trypsin and related enzymes. I: Preparation and characteristics of an affinity adsorbent containing tryptic peptides from protamine as ligands. J Biochem (Tokyo) 1975;78:653–662. doi: 10.1093/oxfordjournals.jbchem.a130952. [DOI] [PubMed] [Google Scholar]
- 130.Nakano NI, Osio T, Fujimoto Y, Amiya T. Study of drug-protein binding by affinity chromatography: interaction of bovine serum albumin and salicylic acid. J Pharmaceut Sci. 1978;67:1005–1008. doi: 10.1002/jps.2600670737. [DOI] [PubMed] [Google Scholar]
- 131.Lagercrantz C, Larsson T, Karlsson H. Binding of some fatty acids and drugs to immobilized bovine serum albumin studied by column affinity chromatography. Anal Biochem. 1979;99:352–364. doi: 10.1016/s0003-2697(79)80019-6. [DOI] [PubMed] [Google Scholar]
- 132.Jacobson SC, Andersson S, Allenmark SG, Guiochon G. Estimation of the number of enantioselective sites of bovine serum-albumin using frontal chromatography. Chirality. 1993;5:513–515. doi: 10.1002/chir.530050707. [DOI] [PubMed] [Google Scholar]
- 133.Tweed SA, Loun B, Hage DS. Effects of ligand heterogeneity in the characterization of affinity columns by frontal analysis. Anal Chem. 1997;69:4790–4798. doi: 10.1021/ac970565m. [DOI] [PubMed] [Google Scholar]
- 134.Tong Z, Hage DS. Detection of heterogeneous drug-protein binding by frontal analysis and high-performance affinity chromatography. J Chromatogr A. 2011;1218:8915–8924. doi: 10.1016/j.chroma.2011.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Temporini C, Pochetti C, Frachhiolla G, Piemontese L, Montanari R, Moaddel R, Lazhezza A, Altieri F, Cervoni L, Ubiali D, Prada E, Loidoice F, Massolini G, Calleri E. Open tubular columns containing the immobilized ligand binding domain of peroxisome proliferator-activated receptors and for dual agonists characterization by frontal affinity chromatography with mass spectrometry detection. J Chromatogr A. 2013;1284:36–43. doi: 10.1016/j.chroma.2013.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schriemer DS. Biosensor alternative: frontal affinity chromatography. Anal Chem. 2004;76:440A–448A. doi: 10.1021/ac041684m. [DOI] [PubMed] [Google Scholar]
- 137.Tetala KKK, Chen B, Visser GM, van Beek TA. Single step synthesis of carbohydrate monolithic capillary columns for affinity chromatography of lectins. J Sep Sci. 2007;30:2828–2835. doi: 10.1002/jssc.200700356. [DOI] [PubMed] [Google Scholar]
- 138.Kovarik P, Hodgson RJ, Covey T, Brook MA, Brennan JD. Capillary-scale frontal affinity chromatography/MALDI tandem mass spectrometry using protein-doped monolithic silica columns. Anal Chem. 2005;77:3340–3350. doi: 10.1021/ac048263p. [DOI] [PubMed] [Google Scholar]
- 139.Hodgson RJ, Chen Y, Zhang Z, Tleugabulova E, Long H, Zhao X, Organ M, Brook MA, Brennan JD. Protein-doped monolithic silica columns for capillary liquid chromatography prepared by the sol gel method: applications to frontal affinity chromatography. Anal Chem. 2004;76:2780–2790. doi: 10.1021/ac0352124. [DOI] [PubMed] [Google Scholar]
- 140.Slon-Usakiewicz JJ, Ng W, Dai JR, Pasternak A, Redden RR. Frontal affinity chromatography with MS detection (FAC-MS) in drug discovery. Drug Discov Today. 2005;10:409–416. doi: 10.1016/S1359-6446(04)03360-4. [DOI] [PubMed] [Google Scholar]
- 141.Singh N, Ravichandran S, Norton DD, Fugmann SD, Moaddel R. Synthesis and characterization of a SIRT6 open tubular column: predicting deacetylation activity using frontal chromatography. Anal Biochem. 2013;436:78–83. doi: 10.1016/j.ab.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moaddel R, Wainer IW. The preparation and development of cellular membrane affinity chromatography columns. Nature Protocols. 2009;4:197–205. doi: 10.1038/nprot.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schriemer DC, Bundle DR, Li L, Hindsgaul O. Micro-scale frontal affinity chromatography with mass spectrometric detection: a new method for the screening of compound libraries. Angew Chem Int Ed. 1998;37:3383–3397. doi: 10.1002/(SICI)1521-3773(19981231)37:24<3383::AID-ANIE3383>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 144.Zhang B, Palcic MM, Mo H, Goldstein IJ, Hindsgaul O. Rapid determination of the binding affinity and specificity of the mushroom Polyporus squamosus lectin using frontal affinity chromatography coupled to electrospray mass spectrometry. Glycobiology. 2001;11:141–147. doi: 10.1093/glycob/11.2.141. [DOI] [PubMed] [Google Scholar]
- 145.Calleri E, Fracchiolla G, Montanari R, Pochetti G, Lavecchia A, Loiodice F, Laghezza A, Piemontese L, Massolini G, Temporini C. Frontal affinity chromatography with MS detection of the ligand binding domain of PPARγ receptor: ligand affinity screening and stereoselective ligand macromolecule interaction. J Chromatogr A. 2012;1232:84–92. doi: 10.1016/j.chroma.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 146.Zhang B, Palcic MM, Schriemer DC, Alarez-Manilla G, Pierce M, Hindsgaul O. Frontal affinity chromatography coupled to mass spectrometry for screening mixtures of enzyme inhibitors. Anal Biochem. 2001;299:173–182. doi: 10.1006/abio.2001.5417. [DOI] [PubMed] [Google Scholar]
- 147.Bi C, Beeram S, Li Z, Zheng X, Hage DS. Kinetic analysis of drug-protein interactions by affinity chromatography. Drug Discov Today Technol. 2015;17:16–21. doi: 10.1016/j.ddtec.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schiel JE, Hage DS. Kinetic studies of biological interactions by affinity chromatography. J Sep Sci. 2009;32:1507–1522. doi: 10.1002/jssc.200800685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zheng X, Bi C, Li Z, Podariu M, Hage DS. Analytical methods for kinetic studies of biological interactions: a review. J Pharm Biomed Anal. 2005;113:163–180. doi: 10.1016/j.jpba.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Denziot FC, Delaage MA. Statistical theory of chromatography: new outlooks for affinity chromatography. Proc Natl Acad Sci USA. 1975;72:4840–4843. doi: 10.1073/pnas.72.12.4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Swaisgood HE, Chaiken IM. Analytical high-performance affinity chromatography: evaluation by studies of neurophysin self-association and neurophysin-peptide hormone interaction using glass matrices. Biochemistry. 1986;25:4148–4155. doi: 10.1021/bi00362a024. [DOI] [PubMed] [Google Scholar]
- 152.Anderson DJ, Walters RR. Equilibrium and rate constants of immobilized concanavalin A determined by high-performance affinity chromatography. J Chromatogr. 1986;376:69–85. [Google Scholar]
- 153.Muller AJ, Carr PW. Chromatographic study of the thermodynamic and kinetic characteristics of silica-bound concanavalin A. J Chromatogr. 1984;284:33–51. [Google Scholar]
- 154.Loun B, Hage DS. Chiral separation mechanism in protein-based HPLC columns. 2. Kinetic studies of (R)- and (S)- warfarin binding to immobilized human serum albumin. Anal Chem. 1996;68:1218–1225. doi: 10.1021/ac950827p. [DOI] [PubMed] [Google Scholar]
- 155.Schiel JE, Ohnmacht CM, Hage DS. Measurement of drug-protein dissociation rates by high-performance affinity chromatography and peak profiling. Anal Chem. 2009;81:4320–4333. doi: 10.1021/ac9000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tong Z, Schiel JE, Papastavros E, Ohnmacht CM, Smith QR, Hage DS. Kinetic studies of drug-protein interactions by using peak profiling and high-performance affinity chromatography: examination of multi-site interactions of drugs with human serum albumin columns. J Chromatogr A. 2011;1218:2065–2071. doi: 10.1016/j.chroma.2010.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tong Z, Hage DS. Characterization of interaction kinetics between chiral solutes and human serum albumin by using high-performance affinity chromatography and peak profiling. J Chromatogr A. 2011;1218:6892–6897. doi: 10.1016/j.chroma.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Talbert AM, Tranter GE, Holmes E, Frances PW. Determination of drug-plasma protein binding kinetics and equilibria by chromatographic profiling: exemplification of the method using L-tryptophan and albumin. Anal Chem. 2002;74:446–452. doi: 10.1021/ac010643c. [DOI] [PubMed] [Google Scholar]
- 159.Moore RM, Walters RR. Peak-decay method for the measurement of dissociation rate constants by high-performance affinity chromatography. J Chromatogr. 1987;384:91–103. [Google Scholar]
- 160.Chen J, Schiel JE, Hage DS. Noncompetitive peak decay analysis of drug-protein dissociation by high-performance affinity chromatography. J Sep Sci. 2009;32:1632–1641. doi: 10.1002/jssc.200900074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yoo MJ, Hage DS. Use of peak decay analysis and affinity microcolumns containing silica monoliths for rapid determination of drug-protein dissociation rates. J Chromatogr A. 2011;1218:2072–2078. doi: 10.1016/j.chroma.2010.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zheng X, Li Z, Podariu DS, Hage M. Determination of rate constants and equilibrium constants for solution-phase drug-protein interactions by ultrafast affinity extraction. Anal Chem. 2014;86:6454–6460. doi: 10.1021/ac501031y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bi C, Zheng X, Hage DS. Analysis of free drug fractions in serum by ultrafast affinity extraction and two-dimensional affinity chromatography using α1-acid glycoprotein microcolumns. J Chromatogr A. 2016;1432:49–57. doi: 10.1016/j.chroma.2015.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mallik R, Yoo MJ, Briscoe CJ, Hage DS. Analysis of drug-protein binding by ultrafast affinity chromatography using immobilized human serum albumin. J Chromatogr A. 2010;1217:2796–2803. doi: 10.1016/j.chroma.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ohnmacht CM, Schiel JE, Hage DS. Analysis of free drug fractions using near infrared fluorescent labels and an ultrafast immunoextraction/displacement assay. Anal Chem. 2006;78:7547–7556. doi: 10.1021/ac061215f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Clarke WC, Choudhuri AR, Hage DS. Analysis of free drug fractions by ultra-fast immunoaffinity chromatography. Anal Chem. 2001;73:2157–2164. doi: 10.1021/ac0009752. [DOI] [PubMed] [Google Scholar]
- 167.Clarke W, Schiel JE, Moser A, Hage DS. Analysis of free hormone fractions by an ultrafast immunoextraction/displacement immunoassay: studies using free thyroxine as a model system. Anal Chem. 2005;77:1859–1866. doi: 10.1021/ac040127x. [DOI] [PubMed] [Google Scholar]
- 168.Zheng X, Brooks M, Hage DS. Analysis of hormone-protein binding in solution by ultrafast affinity extraction: interactions of testosterone with human serum albumin and sex hormone binding globulin. Anal Chem. 2015;87:11187–11194. doi: 10.1021/acs.analchem.5b03007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zheng X, Yoo MJ, Hage DS. Analysis of free fractions for chiral drugs using ultrafast extraction and multi-dimensional high-performance affinity chromatography. Analyst. 2013;138:6262–6265. doi: 10.1039/c3an01315d. [DOI] [PMC free article] [PubMed] [Google Scholar]