Abstract
Background:
We previously showed that intradermal injection of statins is a successful treatment for hypertrophic scarring. Topical application has many advantages over intradermal injection. In this study, we demonstrate the efficacy of topical statin treatment in reducing scar in our validated rabbit ear scar model.
Methods:
Twenty New Zealand White rabbits were divided into 2 study groups, with 6 rabbits receiving 10 μm pravastatin intradermally at postoperative days 15, 18, and 21, and 14 rabbits receiving 0.4%, 2%, and 10% simvastatin topical application at postoperative days 14–25. Four or 6 full-thickness circular dermal punches 7 mm in diameter were made on the ventral surface of the ear down to but not including the perichondrium. Specimens were collected at 28 days to evaluate the effects of statins on hypertrophic scarring.
Results:
Treatment with pravastatin intradermal administration significantly reduced scarring in terms of scar elevation index. Topical treatment with both medium- and high-dose simvastatin also significantly reduced scarring. High-dose simvastatin topical treatment showed a major effect in scar reduction but induced side effects of scaling, erythema, and epidermal hyperplasia, which were improved with coapplication of cholesterol. There is a dose response in scar reduction with low-, medium- and high-dose simvastatin topical treatment. High-dose simvastatin treatment significantly reduced the messenger ribonucleic acid (mRNA) expression of connective tissue growth factor, consistent with our previously published work on intradermally injected statins. More directly, high-dose simvastatin treatment also significantly reduced the mRNA expression of collagen 1A1.
Conclusions:
Topical simvastatin significantly reduces scar formation. The mechanism of efficacy for statin treatment through interference with connective tissue growth factor mRNA expression was confirmed.
BACKGROUND
Hypertrophic scarring is a common sequela of burns and other traumatic injuries with significant cosmetic and functional consequences, especially when occurring in the face and across joints. There are limited therapeutic options for the reduction of scarring following these injuries beyond promotion of reepithelialization and control of inflammation. Available chemotherapeutic options with solid evidence of clinical efficacy are limited to topical silicone gel and intralesional steroid injections, each having major limitations.1,2 Mechanical manipulation to reduce tension in surgical incisions can improve scar outcome3 but is not practical for burn scars or large posttraumatic injuries. It is unlikely that any single therapy will be sufficient to produce optimal scar outcomes. Therefore, there is great need for new, efficacious therapeutic options that can be delivered alone or in conjunction with current therapies through a local route with minimal toxicity or impairment of healing. The etiology of scarring is multifactorial, so optimal treatment may involve a combination of modalities such as a topical agent mixed with silicone gel, and so on.
We previously utilized intradermally injected statins as a treatment for hypertrophic scarring and found a reduction of scarring in a validated rabbit ear model of hypertrophic scarring.4 The study also showed that this reduction in hypertrophic scarring might be mediated through inhibition of connective tissue growth factor (CTGF) expression, a major regulator of fibrosis.4
Administration of pharmaceutical agents by intradermal injection is a challenging treatment modality, which is painful, and requires the expertise of a health care professional. Compared with intradermal injection, topical application has many advantages such as ease of handling, the localized delivery of product, and the reduced effect of first pass metabolism.5 Furthermore, a suitable concentration of reagent(s) can be applied topically without the risk of systemic effects.6 However, a major difficulty in topical administration is adequate transdermal delivery, which requires both high solubility and penetration capability of topical agents. Through extensive search, we found a suitable vehicle for statin topical application in this study.
In this study, we demonstrate the efficacy of topical statin treatment in reducing scar in our validated rabbit ear scar model. The presumed mechanism of action by interference with CTGF signaling is confirmed.
MATERIALS AND METHODS
Statin Preparation and Administration
Statins can be subgrouped according to their hydrophobicity or hydrophilicity. Pravastatin [molecular weight (MW), 446 Da] and rosuvastatin (MW, 500 Da) are hydrophilic, whereas atorvastatin (MW, 604 Da), cerivastatin (MW, 481 Da), fluvastatin (MW, 433 Da), lovastatin (MW, 404 Da), and simvastatin (MW, 418 Da) are hydrophobic. Hydrophobic statins easily diffuse through the cell membrane, whereas hydrophilic statins have poor permeability7 and require active transport into cells via transporters.8–10 As such, hydrophilic statins were administered by intradermal injection and hydrophobic statins by topical application in this study.
For intradermal administration, a 100 μL dose of 10 μm pravastatin in phosphate buffered saline (PBS) per healed wound was chosen based on in vitro and in vivo studies,4,11–13 and 3 doses were given at postoperative days (POD) 15, 18, and 21 after epithelialization was complete (Table 1). Six rabbits were used for pravastatin intradermal injection, with 4 or 6 wounds per ear. Wounds in 1 ear received pravastatin intradermal injection and that in the other ear received PBS injection and served as controls.
Table 1.
Treatment Methods
Transdermal penetration is the critical limitation to topical delivery of medications. A suitable statin for topical application should have both high solubility and high permeability or penetration14–16 with a molecular weight less than 500 Da.17 The stratum corneum is hydrophobic and a barrier for any topically applied reagent, and so hydrophobic statins should have increased penetration.18 Of the hydrophobic statins, lovastatin has limited solubility in the vehicle that we tested; atorvastatin has a relatively heavier molecular weight (604 Da)17,19 and so its penetration is limited. Simvastatin is a hydrophobic statin with a moderate molecular weight (418 Da) and potentially increased penetration capability and most importantly high solubility in the self-microemulsifying drug delivery system.20 A modified Capmul medium chain mono- and diglycerides European Pharmacopoeia (MCM EP)-based microemulsion formulation with Transcutol as cosurfactant was developed for topical delivery of simvastatin with 1:1 (v/v) Capmul MCM EP/Transcutol21. Dosing schedules were chosen based upon in vitro dosing necessary to interfere with CTGF expression11–13,22,23 and extrapolated to animal models based upon our experience with dose response curves for growth factors in our rabbit ear model.24 To assess whether simvastatin demonstrates a dose response, effects of low- (0.4%), medium- (2%), and high-dose (10%) simvastatin on scar reduction were explored. Topical statin inhibits synthesis of cholesterol in the epidermis, which interferes with the stratum corneum. In previous reports, treated skin developed scale, erythema, and epidermal hyperplasia when statin was used topically.25–27 Topical cholesterol application has been reported to help to restore skin barrier function,11,25,27–29 so 2% cholesterol was coapplied with high-dose simvastatin to see if it would mitigate those side effects.
In summary, low- (0.4%), medium- (2%), and high-dose (10%) simvastatin with or without 2% cholesterol were prepared in Transcutol/Capmul MCM EP 1:1 (v:v)21,30–32 for topical application. Fourteen rabbits were used for simvastatin topical treatment, with 2, 6, 3, and 3 rabbits for 0.4%, 2%, 10% simvastatin, and 10% simvastatin with 2% cholesterol treatments, respectively, totaling 84 treated and 84 control wounds (6 wounds per ear). Wounds in 1 ear received topical simvastatin treatment, and those on the other ear received vehicle alone and served as controls. Daily topical application (10 μL per wound) of these agents or their corresponding vehicles was performed between PODs 14 and 25 (Table 1). Transcutol (Diethylene glycol monoethyl ether) was purchased from Sigma-Aldrich (St. Louis, Mo.), and Capmul MCM EP [glycerol monocaprylocaprate (type I)] was kindly donated by Abitec Corporation (Janesville, Wis.).
Animal Models
The Northwestern University Animal Care and Use Committee approved the use of animals in this study. Twenty New Zealand White rabbits (3–6 months, ~3 kg; Covance Research Products, Inc, Cumberland, Va.) were divided into 2 study groups, with 6 rabbits for intradermal injection, and 14 rabbits for topical application.
The rabbit ear hypertrophic scar model was used as previously described.33 Briefly, 4 or 6 full-thickness dermal punches 7 mm in diameter were made on the ventral surface of the ear down to but not including the perichondrium. The cartilage was scored around the circumference of the wound to allow for histomorphometric analysis. The wounds were covered with a semiocclusive dressing Tegaderm (3M Health Care, St. Paul, Minn.), which was replaced as needed.
As previously reported, each wound was considered a separate sample because of independent healing and response to treatments.
Tissue Harvest and Histological Analysis
Animals were euthanized at POD 28. Rectangular samples including scar tissue and about 3.5 mm normal skin at each side were harvested.
One half of a rectangular biopsy was taken for histological analysis. Tissues underwent routine processing, paraffin embedding, and sectioning. A 4-μm cross section through the center of each rectangular biopsy was taken to approximate the diameter of the scar section to the initial 7 mm diameter. The tissues were stained with hematoxylin and eosin (H&E) and examined under light microscopy. Several histomorphometric measurements were determined using a digital image analysis system (NIS-Elements Basic Research, Nikon Corporation, Kanagawa, Japan) at 2× and 10× magnification as previously described.33 Each parameter was measured in a blind manner. Scar elevation index (SEI) was calculated to quantify the extent of hypertrophic scarring in the scarring model. Scored nicks in the cartilage served as references of the original wound diameter. Relative scar reduction rate (RSRR) was calculated from the difference of SEI values between control and treatment divided by control SEI minus 1.
Inflammatory cells with dark and/or round nuclei were counted in randomly chosen high-power microscopic fields (HPFs, 400× magnification) in H&E stained slides. Fibroblasts with spindle nuclei were excluded from inflammatory cell counting. This initial analysis does not allow differentiation between fibroblasts with round nuclei and macrophages that can look very similar but is a useful initial analysis when combined with specific immunohistochemical information.
For immunohistochemical studies, the Avidin-Biotin Complex method (Vector Laboratories, Burlingame, Calif.) was used. After deparaffinization and rehydration, 4-μm-thick sections were treated with 0.05% trypsin (pH, 7.8), either mouse antimacrophage (Abcam, Cambridge, Mass.) or antineutrophil (Santa Cruz Biotechnology, Dallas, Tex.) was used as a primary antibody. Signal was detected using the Vectastain kit (Vector Laboratories) and visualized using 3,3′-diaminobenzidine. Cells exhibiting positive stain were counted in randomly chosen HPFs (200× magnification).
Quantification of CTGF, transforming growth factor beta 1, and Collagen 1A1 mRNA Expression
Total ribonucleic acid (RNA) was extracted from dermal scar tissue of high-dose simvastatin topical treatment and control scars using TRI reagent (Sigma-Aldrich, St. Louis, Mo.), and contaminated DNA was removed with Turbo DNA-free kit (Ambion, Austin, Tex.). The complementary DNA was synthesized from 1 μg of total RNA by Superscript II reverse transcriptase (Invitrogen, Grand Island, N.Y.) with 100 ng of random primers in 20 μl of volume. Synthesized cDNA was quantified in a sequence detection system (ABI StepOnePlus Real Time PCR System; Applied Biosystems, Foster City, Calif.) using SYBR green master mixes and specific primers for CTGF and collagen 1A1; glyceraldehyde 3-phosphate dehydrogenase was set as endogenous control. The sequence of primers is as follows: CTGF (5′-CTT CTG TCG GCT GGA GAA AC-3′ & 5′-TTA GCC CGG TAC GTC TTC AC-3′); transforming growth factor beta 1 (5′-AAA GTC GGC ACA GCG TCT AT-3′ & 5′-TGC TGC ATT TCT GGT ACA GC-3′); collagen 1A1: (5′-TAA GAG CTC CAA GGC CAA GA-3′ & 5′-TGT TCT GAG AGG CGT GAT TG-3′); GAPDH (5′-AGG TCA TCC ACG ACC ACT TC-3′ & 5′-GTG AGT TTC CCG TTC AGC TC-3′).
Statistical Analysis
Some samples were excluded from the study because their cartilage was heavily damaged and/or missing or because their epidermis was lost during tissue processing and/or sectioning for any reason; their counterparts in the opposite ears were also omitted to do paired t test analysis. Twenty-four samples for both the treatment and control groups were included in the pravastatin intradermal injection study. Nine, 31, 17, and 17 samples for both treatment and control groups were included for each of 0.4%, 2%, and 10% simvastatin alone as well as 10% simvastatin with 2% cholesterol (Table 1).
For statistical analysis of histological and molecular analysis results, Student’s paired t test was applied to assess differences between treatment and control groups, P < 0.05 was used as significant. All values were represented as mean ± standard error of the mean.
RESULTS
Pravastatin Intradermal Administration Reduces Scar Formation
We previously reported that local administration of statins by intradermal injection reduced scarring with a concomitant reduction in CTGF expression.4 There are multiple statins in clinical use including hydrophobic and hydrophilic statins. We confirm the previous report4 that the hydrophilic statin pravastatin is effective in reducing scar formation. Treatment with pravastatin intradermal injection significantly reduced scarring when compared with saline control in terms of SEI (pravastatin 1.6 ± 0.1 versus saline 1.7 ± 0.1; n = 24; P = 0.0009).
Simvastatin Topically Reduces Scarring with the Additional Cholesterol
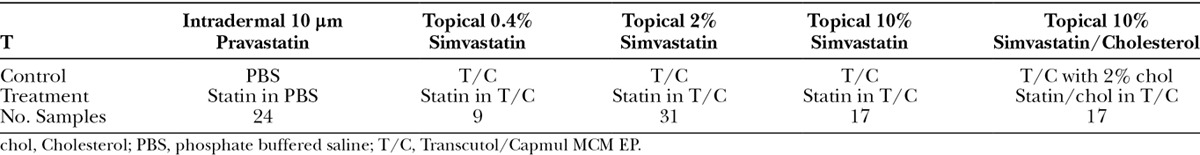
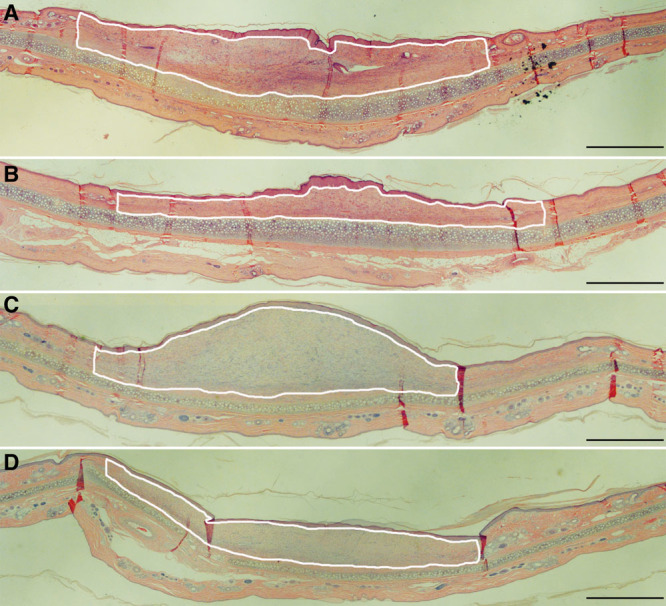
Topical treatment with low-dose simvastatin showed no effect on scarring when compared to vehicle control in terms of SEI (low simvastatin 2.1 ± 0.2 versus vehicle 2.1 ± 0.1; n = 9; P = 0.6). Topical treatment with both medium- and high-dose simvastatin, however, significantly reduced scarring shown in histological pictures (Fig. 1) and SEI (medium-dose 1.6 ± 0.1 versus vehicle 1.9 ± 0.1; n = 31; P = 0.001; high-dose 1.3 ± 0.1 versus vehicle 1.6 ± 0.1; n = 17; P = 0.004; Fig. 2). With coapplication of cholesterol, high-dose simvastatin treatment also significantly reduced scarring in histological pictures and SEI (coapplication 1.1 ± 0.1 versus vehicle 1.4 ± 0.1; n = 17; P = 0.005; Fig. 2), which is similar to that by high-dose simvastatin treatment only. When the high-dose simvastatin groups with and without cholesterol are combined, the significance is even greater (high-dose simvastatin 1.2 ± 0.0 versus vehicle 1.5 ± 0.1; n = 34; P = 0.00005). In summary, there is a dose response in scar reduction with low-, medium- and high-dose simvastatin topical treatment.
Fig. 1.
Medium- (B) and high-dose simvastatin topical application (D) significantly reduced scarring when compared with vehicle alone (A and C). Representative histological pictures are shown. Bar is 1,000 μm in length. Scar tissue is shown within the white line.
Fig. 2.
. Effects of simvastatin topical application on hypertrophic scar formation. There is a dose response with low-, medium-, and high-dose simvastatin on scar reduction. Coapplication of high-dose simvastatin with cholesterol also significantly reduced hypertrophic scarring compared with controls as shown by improvements in SEI. hsim/c, High-dose simvastatin with cholesterol; lsim, msim, hsim, low-, medium-, and high-dose simvastatin; N.S., not significant; veh, vehicle; ***P < 0.005.
Addition of Cholesterol Reduces Epidermal Hyperplasia
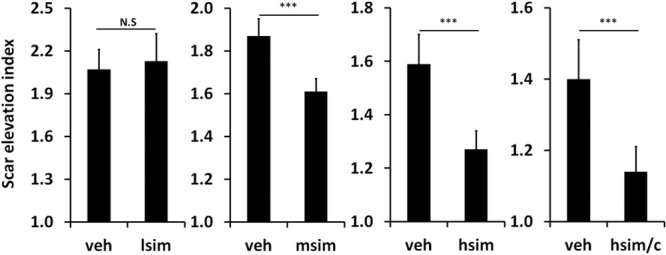
High-dose simvastatin showed a major effect in scar reduction but induced the previously reported side effects of visible scaling, erythema, and epidermal hyperplasia (Figs. 3, 4) presumably due to interference with synthesis of cholesterol, which is an important component of the stratum corneum. Vehicle controls did not show these abnormalities. The addition of cholesterol improved the scaling, erythema, and epidermal hyperplasia caused by high-dose simvastatin treatment (Figs. 3, 4). Immunohistochemistry showed only rare macrophages at day 28, as well as scant neutrophils (data not shown). Inflammatory cell counts in H&E–stained scar samples showed no significant difference in inflammatory cells infiltration among high-dose simvastatin with or without cholesterol and their respective control groups (Fig. 5). In summary, the addition of cholesterol to high-dose simvastatin reduced epidermal hyperplasia, possibly through the restoration of epidermal barrier function, which we have previously demonstrated.34
Fig. 3.
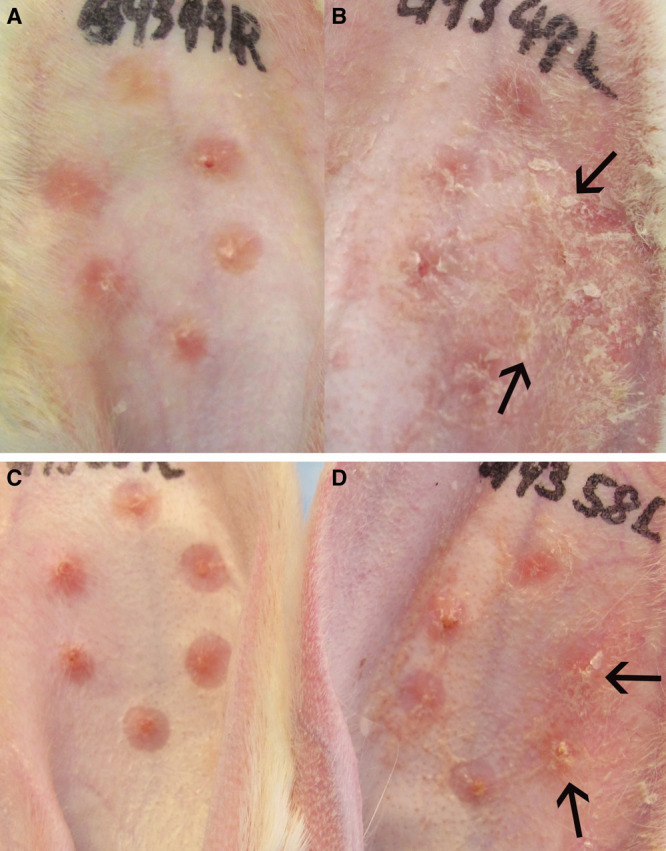
Some scars with high-dose simvastatin treatment developed scaling [arrows; (B)], erythema, and epidermal hyperplasia, when compared with vehicle control (A). Scars treated with coapplication of high-dose simvastatin and cholesterol developed less scaling [arrows; (D)], erythema, and epidermal hyperplasia when compared with high-dose simvastatin treatment alone (B). Vehicle alone both with and without cholesterol did not induce scaling (C and A). Representative gross pictures are shown.
Fig. 4.
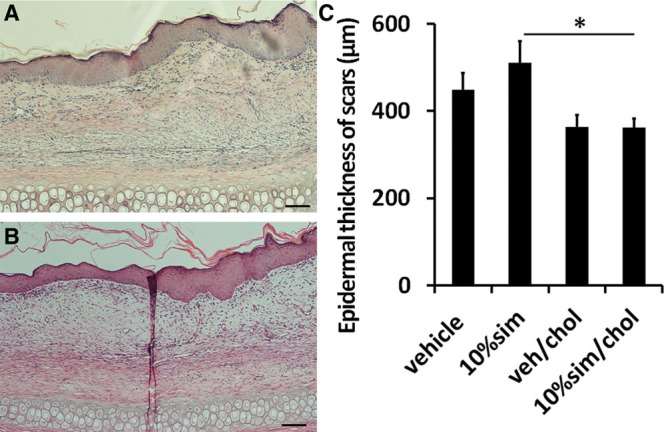
Some scars with high-dose simvastatin treatment developed scaling, erythema, and epidermal hyperplasia (A), which was significantly decreased with coapplication of cholesterol (B). There was significant difference in epidermal thickness [(C); P = 0.012] between statin treatment alone and coapplication of statin and cholesterol. Representative histological pictures are shown. Scale bar is 500 μm in length, *P < 0.05. 10% sim, 10% sim/chol, 10% simvastatin without or with cholesterol; veh/chol, vehicle with cholesterol.
Fig. 5.
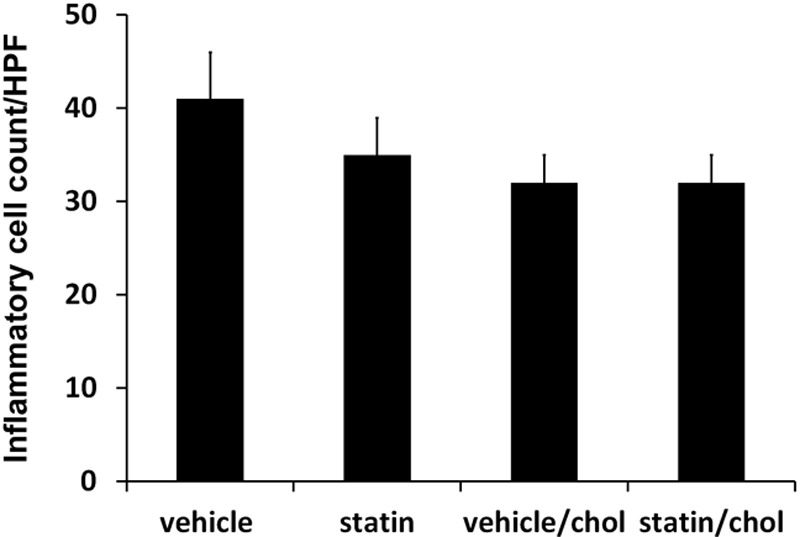
There is no significant difference in inflammatory cell counts among high-dose simvastatin with or without cholesterol and their respective control groups. chol, Cholesterol; HPF, high-power field.
Simvastatin Topical Application Decreases CTGF and Collagen 1A1 mRNA Expression in Hypertrophic Scar
Collagen synthesis is regulated by CTGF, which is a downstream effector of TGF-β; elevated expression of CTGF may maintain a fibrotic phenotype.35 Our previous work demonstrated that statins downregulate CTGF mRNA expression4 confirming previous in vitro observations regarding statins.11–13,22,23 In this study, high-dose simvastatin treatment significantly reduced the expression of CTGF to 53.8 ± 8.0% (P < 0.01; n = 12) of vehicle control (Fig. 6). More directly, high-dose simvastatin treatment also significantly reduced the expression of collagen 1A1 to 48.5 ± 14% (P < 0.01; n = 12). Although it did not reach statistical significance, the expression of TGFβ1 was reduced to 75.0 ± 6.0% (P = 0.13; n = 12). Data are presented as mean ± the standard error of the mean. Our mRNA expression analysis confirmed the mechanism of efficacy for statin treatment through interference with CTGF mRNA expression.
Fig. 6.
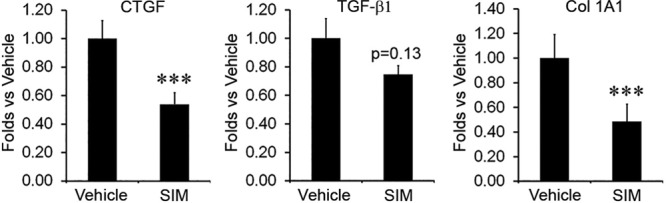
High-dose simvastatin topical application significantly decreased both CTGF and collagen gene expression but did not significantly decrease TGFβ1 expression. SIM 10, 10% simvastatin.
DISCUSSION
Our laboratory has previously reported that intradermal administration of statins can significantly reduce hypertrophic scarring.4 In addition to further confirming this finding, we report that topical application of a hydrophobic statin reduces scarring. The critical limitation of topical application is absorption through the stratum corneum barrier. We employed a microemulsion system as the vehicle for simvastatin topical application with a combination of Transcutol as a surfactant and Capmul MCM EP as an oil base.
As our results showed, both medium- and high-dose simvastatin treatment significantly reduced scarring with the latter having a more dramatic effect. However, the higher dose also caused apparent side effects such as scale, erythema, and epidermal hyperplasia in some cases (Figs. 3, 4). Although surfactant had been reported to cause scaling,36 this was unlikely to be the case in this study because vehicle controls with surfactant did not show those side effects, so they were likely local side effects from topical high-dose statin treatment.37 Coapplied cholesterol was shown to mitigate somewhat the scaling effect caused by simvastatin25 (Figs. 3, 4). It is unclear if cholesterol plays a direct role by restoring deficient cholesterol as a critical component of the stratum corneum or by an indirect role in this finding.29 We have previously reported in multiple publications that restoring epidermal barrier function reduces scarring, by a mechanism of reducing Na flux, and inflammatory pathways mediated by Na channels.38,39 The existence of the dose response among low-, medium-, and high-dose simvastatin treatments further validated the effect of simvastatin topical treatment on scar reduction.
CTGF is an important downstream mediator of TGF-β, which regulates collagen synthesis without major effects on inflammatory cells, and has been demonstrated to specifically modulate scarring.35 CTGF overexpression is reported in some profibrotic conditions such as scleroderma.40 After injury, it continues to rise steadily through day 40, and blockade of CTGF mRNA by antisense oligonucleotides is associated with reduction of collagen types I and III and scarring.35 Our results indicate that the scar reduction effect of statins is at least partly due to the decrease of CTGF expression, and consequently the decrease of collagen expression, which is consistent with Mun et al.’s41 in vitro study and Watts and Spiteri’s12 in vivo study. Whether statin affected the expression of TGF-βRII is unclear in this study.42 Furthermore, biphasic effects of simvastatin on host cells have been noted in in vitro experiments where high doses of statins induced cell apoptosis and inversely inhibited angiogenesis.43–45
As a modern drug carrier system, microemulsions are defined as single optically isotropic and thermodynamically stable solutions with droplet sizes in the submicron range. In general, they consist of an oil phase, a surfactant, a cosurfactant, and an aqueous phase. The core benefits offered by microemulsions include improvement in drug solubility and release, enhanced penetration, and bioavailability.46 Additional benefits have been reported such as ease of manufacturing, less inter- and intraindividual variability in drug pharmacokinetics, and a long shelf life.21,46–48 Transcutol (transcutolylene glycol monoethyl ether, 2-(2-ethoxyethoxy)ethanol) is a widely used nonionic surfactant with powerful solubilizing ability.46 It is reported to increase the permeability of the drugs48,49 and is listed in the Food and Drug Administration Inactive Ingredients Database for topical use.50 Capmul MCM EP (mono/diglycerides of capric acid) is an emulsifier and natural lipophilic surfactant enhancer and helps to dissolve hydrophobic substances with good solubilizing ability.46 In addition, it improves permeation14 and is also listed in the Food and Drug Administration Inactive Ingredients Database.51 A mixture of Transcutol and Capmul MCM EP was chosen for topical statin treatment in this study.52–57
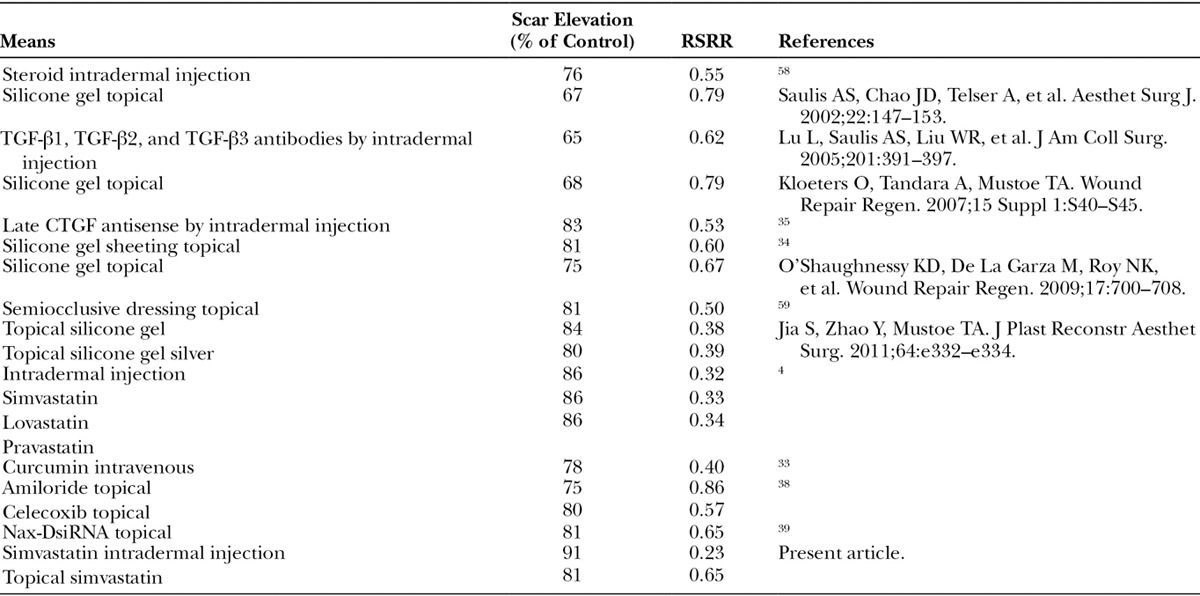
We have used our rabbit ear hypertrophic scar model in the past to evaluate the effects of age and various treatments including steroids, TGF-β antibody, CTGF antisense RNA, silicone gel, and other methods of occlusion over 20 years.58 The scar-reductive therapeutic effect of statin is comparable to results with other treatments that have required injections (steroids) or have other mechanisms of action (Table 2). Interfering with CTGF signaling by topical statin treatment will have minimal regulatory issues and is a promising therapy for clinical use that could be in conjunction with other topical treatment like silicone gel.
Table 2.
Scar Elevation and RSRR with Various Means
The rabbit ear scar model, as with any animal model, has some limitations. However, previous publications from our laboratory have documented similar behavior to humans in terms of response to accepted treatment modalities,58 the role of the epithelium in scarring,39,59 and the role of aging in scarring.60 Compared with rodent models, scarring in rabbit ear wounds does not require special methods to induce significant scarring, and compared with pig models the ease of working with animals, the time course to develop scarring, and the reproducibility and elevated visual appearance are substantial advantages.58
ACKNOWLEDGMENTS
The authors would like to thank Dr. Hari Iyer in the Laboratory for Tissue Repair and Regenerative Surgery for the critical review of this article.
Footnotes
Presented in part at the 25th and 26th Annual Meetings of the Wound Healing Society, in San Antonio, Tex., April 29 through May 3, 2015, and in Atlanta, Ga., April 13 through 17, 2016, respectively.
Drs. Jia and Xie contributed equally to this work.
Disclosure: Supported by the Armed Forces Institute of Regenerative Medicine (AFIRM), contract number W81XWH-13-2-0054 from the U.S. Army Medical Research and Material Command (USAMRMC). The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Mustoe TA, Cooter RD, Gold MH, et al. International Advisory Panel on Scar Management. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560–571. [DOI] [PubMed] [Google Scholar]
- 2.Sidgwick GP, McGeorge D, Bayat A.A comprehensive evidence-based review on the role of topicals and dressings in the management of skin scarring. Arch Dermatol Res. 2015;307:461–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurtner GC, Dauskardt RH, Wong VW, et al. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg. 2011;254:217–225. [DOI] [PubMed] [Google Scholar]
- 4.Ko JH, Kim PS, Zhao Y, et al. HMG-CoA reductase inhibitors (statins) reduce hypertrophic scar formation in a rabbit ear wounding model. Plast Reconstr Surg. 2012;129:252e–261e. [DOI] [PubMed] [Google Scholar]
- 5.Subedi RK, Oh SY, Chun MK, et al. Recent advances in transdermal drug delivery. Arch Pharm Res. 2010;33:339–351. [DOI] [PubMed] [Google Scholar]
- 6.Asai J, Takenaka H, Hirakawa S, et al. Topical simvastatin accelerates wound healing in diabetes by enhancing angiogenesis and lymphangiogenesis. Am J Pathol. 2012;181:2217–2224. [DOI] [PubMed] [Google Scholar]
- 7.Germershausen JI, Hunt VM, Bostedor RG, et al. Tissue selectivity of the cholesterol-lowering agents lovastatin, simvastatin and pravastatin in rats in vivo. Biochem Biophys Res Commun. 1989;158:667–675. [DOI] [PubMed] [Google Scholar]
- 8.Hughes A, Rogers MJ, Idris AI, et al. A comparison between the effects of hydrophobic and hydrophilic statins on osteoclast function in vitro and ovariectomy-induced bone loss in vivo. Calcif Tissue Int. 2007;81:403–413. [DOI] [PubMed] [Google Scholar]
- 9.Ichihara K, Satoh K.Disparity between angiographic regression and clinical event rates with hydrophobic statins. Lancet. 2002;359:2195–2198. [DOI] [PubMed] [Google Scholar]
- 10.Fukami M, Maeda N, Fukushige J, et al. Effects of HMG-CoA reductase inhibitors on skeletal muscles of rabbits. Res Exp Med (Berl). 1993;193:263–273. [DOI] [PubMed] [Google Scholar]
- 11.Watts KL, Cottrell E, Hoban PR, et al. RhoA signaling modulates cyclin D1 expression in human lung fibroblasts; implications for idiopathic pulmonary fibrosis. Respir Res. 2006;7:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watts KL, Spiteri MA.Connective tissue growth factor expression and induction by transforming growth factor-beta is abrogated by simvastatin via a Rho signaling mechanism. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1323–L1332. [DOI] [PubMed] [Google Scholar]
- 13.Watts KL, Sampson EM, Schultz GS, et al. Simvastatin inhibits growth factor expression and modulates profibrogenic markers in lung fibroblasts. Am J Respir Cell Mol Biol. 2005;32:290–300. [DOI] [PubMed] [Google Scholar]
- 14.Shah SM, Jain AS, Kaushik R, et al. Preclinical formulations: insight, strategies, and practical considerations. AAPS PharmSciTech. 2014;15:1307–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahan A, Miller JM.The solubility-permeability interplay and its implications in formulation design and development for poorly soluble drugs. AAPS J. 2012;14:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petyaev IM.Improvement of hepatic bioavailability as a new step for the future of statin. Arch Med Sci. 2015;11:406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finnin BC, Morgan TM.Transdermal penetration enhancers: applications, limitations, and potential. J Pharm Sci. 1999;88:955–958. [DOI] [PubMed] [Google Scholar]
- 18.Schmalfuss U, Neubert R, Wohlrab W.Modification of drug penetration into human skin using microemulsions. J Control Release 1997;46:279–85. [Google Scholar]
- 19.Elnaggar YS, El-Refaie WM, El-Massik MA, et al. Lecithin-based nanostructured gels for skin delivery: an update on state of art and recent applications. J Control Release. 2014;180:10–24. [DOI] [PubMed] [Google Scholar]
- 20.Murtaza G.Solubility enhancement of simvastatin: a review. Acta Pol Pharm. 2012;69:581–590. [PubMed] [Google Scholar]
- 21.Solanki SS, Sarkar B, Dhanwani RK.Microemulsion drug delivery system: for bioavailability enhancement of ampelopsin. ISRN Pharm. 2012;2012:108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer-Ter-Vehn T, Katzenberger B, Han H, et al. Lovastatin inhibits TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci. 2008;49:3955–3960. [DOI] [PubMed] [Google Scholar]
- 23.Eberlein M, Heusinger-Ribeiro J, Goppelt-Struebe M.Rho-dependent inhibition of the induction of connective tissue growth factor (CTGF) by HMG CoA reductase inhibitors (statins). Br J Pharmacol. 2001;133:1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mustoe TA, Pierce GF, Morishima C, et al. Growth factor-induced acceleration of tissue repair through direct and inductive activities in a rabbit dermal ulcer model. J Clin Invest. 1991;87:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feingold KR, Man MQ, Menon GK, et al. Cholesterol synthesis is required for cutaneous barrier function in mice. J Clin Invest. 1990;86:1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feingold KR, Man MQ, Proksch E, et al. The lovastatin-treated rodent: a new model of barrier disruption and epidermal hyperplasia. J Invest Dermatol. 1991;96:201–209. [DOI] [PubMed] [Google Scholar]
- 27.Menon GK, Feingold KR, Mao-Qiang M, et al. Structural basis for the barrier abnormality following inhibition of HMG CoA reductase in murine epidermis. J Invest Dermatol. 1992;98:209–219. [DOI] [PubMed] [Google Scholar]
- 28.Paller AS, van Steensel MA, Rodriguez-Martín M, et al. Pathogenesis-based therapy reverses cutaneous abnormalities in an inherited disorder of distal cholesterol metabolism. J Invest Dermatol. 2011;131:2242–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murota H, Itoi S, Terao M, et al. Topical cholesterol treatment ameliorates hapten-evoked cutaneous hypersensitivity by sustaining expression of 11β-HSD1 in epidermis. Exp Dermatol. 2014;23:68–70. [DOI] [PubMed] [Google Scholar]
- 30.Inugala S, Eedara BB, Sunkavalli S, et al. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) of darunavir for improved dissolution and oral bioavailability: in vitro and in vivo evaluation. Eur J Pharm Sci. 2015;74:1–10. [DOI] [PubMed] [Google Scholar]
- 31.Yadav PS, Yadav E, Verma A, et al. Development, characterization, and pharmacodynamic evaluation of hydrochlorothiazide loaded self-nanoemulsifying drug delivery systems. Scientific World J. 2014;2014:274823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alinaghi A, Tan A, Rao S, et al. Impact of solidification on the performance of lipid-based colloidal carriers: oil-based versus self-emulsifying systems. Curr Drug Deliv. 2015;12:16–25. [DOI] [PubMed] [Google Scholar]
- 33.Jia S, Xie P, Hong SJ, et al. Intravenous curcumin efficacy on healing and scar formation in rabbit ear wounds under nonischemic, ischemic, and ischemia-reperfusion conditions. Wound Repair Regen. 2014;22:730–739. [DOI] [PubMed] [Google Scholar]
- 34.Tandara AA, Mustoe TA.The role of the epidermis in the control of scarring: evidence for mechanism of action for silicone gel. J Plast Reconstr Aesthet Surg. 2008;61:1219–1225. [DOI] [PubMed] [Google Scholar]
- 35.Sisco M, Kryger ZB, O’Shaughnessy KD, et al. Antisense inhibition of connective tissue growth factor (CTGF/CCN2) mRNA limits hypertrophic scarring without affecting wound healing in vivo. Wound Repair Regen. 2008;16:661–673. [DOI] [PubMed] [Google Scholar]
- 36.Fulmer AW, Kramer GJ.Stratum corneum lipid abnormalities in surfactant-induced dry scaly skin. J Invest Dermatol. 1986;86:598–602. [DOI] [PubMed] [Google Scholar]
- 37.Brazzelli V, Distante F, Perani G, et al. Effects of systemic treatment with statins on skin barrier function and stratum corneum water-holding capacity. Dermatology. 1996;192:214–216. [DOI] [PubMed] [Google Scholar]
- 38.Xu W, Hong SJ, Zeitchek M, et al. Hydration status regulates sodium flux and inflammatory pathways through epithelial sodium channel (ENaC) in the skin. J Invest Dermatol. 2015;135:796–806. [DOI] [PubMed] [Google Scholar]
- 39.Xu W, Hong SJ, Zhong A, et al. Sodium channel Nax is a regulator in epithelial sodium homeostasis. Sci Transl Med. 2015;7:312ra177. [DOI] [PubMed] [Google Scholar]
- 40.Leask A, Denton CP, Abraham DJ.Insights into the molecular mechanism of chronic fibrosis: the role of connective tissue growth factor in scleroderma. J Invest Dermatol. 2004;122:1–6. [DOI] [PubMed] [Google Scholar]
- 41.Mun JH, Kim YM, Kim BS, et al. Simvastatin inhibits transforming growth factor-β1-induced expression of type I collagen, CTGF, and α-SMA in keloid fibroblasts. Wound Repair Regen. 2014;22:125–133. [DOI] [PubMed] [Google Scholar]
- 42.Shang L, Jia SS, Jiang HM, et al. Simvastatin downregulates expression of TGF-βRII and inhibits proliferation of A549 cells via ERK. Tumour Biol. 2015;36:4819–4824. [DOI] [PubMed] [Google Scholar]
- 43.Zhu XY, Daghini E, Chade AR, et al. Disparate effects of simvastatin on angiogenesis during hypoxia and inflammation. Life Sci. 2008;83:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urbich C, Dernbach E, Zeiher AM, et al. Double-edged role of statins in angiogenesis signaling. Circ Res. 2002;90:737–744. [DOI] [PubMed] [Google Scholar]
- 45.Weis M, Heeschen C, Glassford AJ, et al. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. [DOI] [PubMed] [Google Scholar]
- 46.Pathak R, Prasad Dash R, Misra M, et al. Role of mucoadhesive polymers in enhancing delivery of nimodipine microemulsion to brain via intranasal route. Acta Pharm Sin B. 2014;4:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kogan A, Garti N.Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interface Sci. 2006;123-126:369–385. [DOI] [PubMed] [Google Scholar]
- 48.Lawrence MJ, Rees GD.Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45:89–121. [DOI] [PubMed] [Google Scholar]
- 49.Lee SG, Kang JB, Kim SR, et al. Enhanced topical delivery of tacrolimus by a carbomer hydrogel formulation with transcutol P. Drug Dev Ind Pharm. 2016:1–7. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan DW, Jr, Gad SC, Julien M.A review of the nonclinical safety of Transcutol®, a highly purified form of diethylene glycol monoethyl ether (DEGEE) used as a pharmaceutical excipient. Food Chem Toxicol. 2014;72:40–50. [DOI] [PubMed] [Google Scholar]
- 51.Available at http://www.abiteccorp.com/wp-content/files_mf/1380908873ABITECLipidExcipients.PharmaRegulatoryChart2.pdf. Accessed January 28, 2016.
- 52.Mostafa DM, Ammar NM, Basha M, et al. Transdermal microemulsions of Boswellia carterii Bird: formulation, characterization and in vivo evaluation of anti-inflammatory activity. Drug Deliv. 2015;22:748–756. [DOI] [PubMed] [Google Scholar]
- 53.Duangjit S, Chairat W, Opanasopit P, Rojanarata T, Ngawhirunpat T.Application of Design Expert for the investigation of capsaicin-loaded microemulsions for transdermal delivery. Pharm Dev Technol 2016;21:698–705. [DOI] [PubMed] [Google Scholar]
- 54.Goyal U, Arora R, Aggarwal G.Formulation design and evaluation of a self-microemulsifying drug delivery system of lovastatin. Acta Pharm. 2012;62:357–370. [DOI] [PubMed] [Google Scholar]
- 55.Rajpoot P, Bali V, Pathak K.Anticancer efficacy, tissue distribution and blood pharmacokinetics of surface modified nanocarrier containing melphalan. Int J Pharm. 2012;426:219–230. [DOI] [PubMed] [Google Scholar]
- 56.Pund S, Shete Y, Jagadale S.Multivariate analysis of physicochemical characteristics of lipid based nanoemulsifying cilostazol—quality by design. Colloids Surf B Biointerfaces. 2014;115:29–36. [DOI] [PubMed] [Google Scholar]
- 57.Cho HJ, Ku WS, Termsarasab U, et al. Development of udenafil-loaded microemulsions for intranasal delivery: in vitro and in vivo evaluations. Int J Pharm. 2012;423:153–160. [DOI] [PubMed] [Google Scholar]
- 58.Morris DE, Wu L, Zhao LL, et al. Acute and chronic animal models for excessive dermal scarring: quantitative studies. Plast Reconstr Surg. 1997;100:674–681. [DOI] [PubMed] [Google Scholar]
- 59.Gallant-Behm CL, Mustoe TA.Occlusion regulates epidermal cytokine production and inhibits scar formation. Wound Repair Regen. 2010;18:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marcus JR, Tyrone JW, Bonomo S, et al. Cellular mechanisms for diminished scarring with aging. Plast Reconstr Surg. 2000;105:1591–1599. [DOI] [PubMed] [Google Scholar]


