Abstract
Background:
Lymphoscintigraphy has often been used for evaluating arm lymphatic dysfunction, but no optimal approach for quantification has so far emerged. We propose a quantifiable measure of lymphatic function that we applied in patients treated for breast cancer.
Methods:
Eleven patients, aged 34–68 years, with unilateral arm lymphedema following breast cancer treatment underwent bilateral lymphoscintigraphy using intradermal injection in both hands of technetium-99m–labeled human serum albumin and sequential 5 min imaging for 5 hours. The mean transit time (MTT) in the arms was calculated based on time activity curves generated from injection site and arm regions. Visual lymphedema scoring was performed based on dermal backflow and lymph node presence. Excess arm volume was calculated from circumference measurements.
Results:
The MTT (mean ± SD) was significantly longer in the lymphedema arm than in the normal arm: 60.1 ± 27.7 versus 5.4 ± 2.5 minutes (mean difference, 54.7 minutes; 95% confidence interval, 36.5–72.9 minutes; P < 0.0001). Patients with previous erysipelas infection had significantly longer MTT than other patients (mean difference, 43.7 minutes; 95% confidence interval, 18.6–68.7 minutes; P < 0.001). There was a positive correlation between MTT and excess arm volume (r = 0.64; P = 0.04) and number of lymph nodes removed (r = 0.65; P = 0.03) but no correlation between visual score and MTT.
Conclusion:
Measurements of MTT were able to discriminate lymphedema from healthy arm and MTT correlated with relevant markers for lymphedema severity. We encourage further research using the MTT approach for monitoring lymphedema and evaluation of treatment response.
INTRODUCTION
Upper limb lymphedema is one of the most debilitating complications to breast cancer treatment. Lymphedema is associated with decreased quality of life1 and increased risk of serious skin infections.2 About 30% of breast cancer patients with axillary lymph node involvement develop lymphedema, and standard management is life-long compression therapy.3,4
Several reports have dealt with lymphoscintigraphy as a means of quantifying lymphedema and lymphatic dysfunction. Many approaches have been made including simple ratios between regions of interest (ROIs),5,6 time to axillary lymph node presentation,7 and clearance rate from injection depot.8,9 However, results are conflicting, and there is no consensus on the optimal way of quantifying lymphedema, allowing for comparison before and after treatment, or between different therapeutic approaches.5,6,8,9
Emerging microsurgical procedures have shown promise for alleviating lymphedema, but recent reviews have identified that only half of published studies present a functional evaluation of the lymphatic system. Lymphoscintigraphy is one of the most commonly applied techniques.10,11 However, for many studies including functional evaluation, the presentation is either not clear or mostly qualitative in nature, making it difficult to compare results across studies. Thus, there is a need of standardized quantitative techniques that can reliably reflect the lymphatic dysfunction to allow proper evaluation of emerging surgical techniques.
The aim of the present study was to apply a newly described quantification method based on lymphoscintigraphy and examine its correlation to lymphedema patient characteristics.
MATERIALS AND METHODS
Patient Characteristics
We examined 11 female patients with unilateral arm lymphedema following breast cancer treatment including patients treated with axillary lymphadenectomy. All patients had previously been diagnosed with lymphedema by physiotherapists. They had received standard of care treatment and were in a stable phase of lymphedema.
Excess arm volume was calculated based on multiple circumference measurements.12 We measured arm circumference at wrist, middle of the forearm, elbow, and middle of and proximally on the upper arm. Based on these 5 measurements, the arm was divided into 4 segments, and the volume of each segment was calculated using the truncated cone formula given in equation (1)
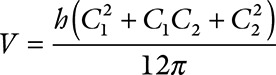 |
(1) |
where V is the segment volume, h is the length of the segment, and C1 and C2 are circumferences at the ends of the segment. The ratio between the 2 arm volumes was used to define excess arm volume percentage.
Body mass index (BMI) was calculated from body weight and height measured at the time of circumference measurements. Hand dominance, location of lymphedema (hand involvement—yes/no), previous erysipelas infection, duration of lymphedema, and number of lymph nodes removed during surgery were registered.
Lymphoscintigraphy Protocol
Lymphoscintigraphy was performed according to a procedure described in detail elsewhere.13 In short, 20 MBq technetium-99m–labeled human serum albumin (Tc-99m-HSA (Vasculocis; CIS Bio International, Paris, France) in 0.1 mL was injected in the finger web between the second and third fingers bilaterally and was followed immediately by dynamic imaging for 30 minutes. Sequential 300-second scans were performed every 30–45 minutes for 5 hours using a dual head Philips Skylight gamma camera equipped with low-energy, high-resolution collimators.
Visual Evaluation
As a semiquantitative visual evaluation, we used the scoring system described previously by Szuba et al.5 This system evaluates 2 parameters, dermal backflow and visualization of axillary lymph nodes giving a total score between 0 and 8, where 0 is normal and 8 is the highest severity of lymphedema. Two physicians scored the 2 parameters; disagreements were solved by consensus.
Quantitative Evaluation
The quantitative evaluation was performed as described in detail elsewhere.13 Briefly, for estimation of mean transit time (MTT) of the tracer, ROIs were manually drawn on each time frame (Fig. 1). Time activity curves from each ROI were background and decay corrected. The time activity curve of the injection depot was described by a 2-phase clearance function.14 The activity input into the arm from the injection depot was calculated as the (minus) time derivative of the modeled injection time activity function. The measured arm time activity curve was mathematically described by a convolution of the input function and a retention function. The retention function reflects the sojourn time of activity entering the arm from the injection depot remaining in the arm compartment until it exited the arm compartment proximally. The best fit of the convolution function and the measured arm activity curve were obtained using the least square fitting method “NonlinearModelFit” from the software Wolfram Mathematica v10.0 (Wolfram Research Inc, Champaign, Ill.). MTT was calculated as the time integral of the retention function.
Fig. 1.
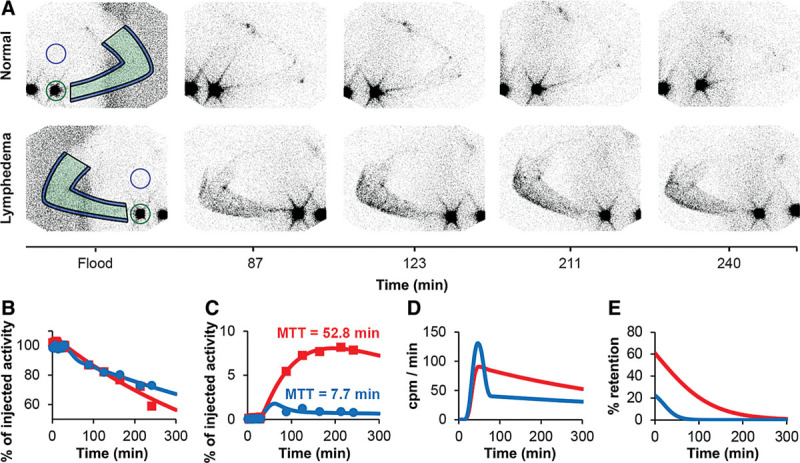
A, Representative scintigrams from both arms of a patient with the normal arm on top and the lymphedema arm on the bottom line. Imaging was performed up to 5 hours after depot injection. On each image, the arm was marked (green), and a background correction in the surrounding arm was drawn (blue). The injection depot was marked (green circle), and a corresponding background correction was made (blue circle). The resulting graphs from the scintigraphy are depicted in B–E. On all graphs, blue is the normal arm and red is the lymphedema arm. B, Time activity curve of injection depots. C, Time activity curve of both arms. In all cases, the MTT was longer in the lymphedema arm compared with the contralateral healthy arm. D, Modeled input function based on the minus derivative of the time activity curve of the injection depots. The y-axis is counts per minute entering the arm per minute. E, The retention function was modeled based on the measured time activity arm curve and the input function. The area under the retention function curve corresponds to the MTT.
Statistical Analysis
MTT is given as mean ± SD. Statistical analysis was performed using STATA/IC14 (StataCorp, LP, College Station, Tex.). Comparison between arms was made with the paired samples t test, and between other groups the independent t test was used. Bivariate correlation was calculated using Spearman’s rank correlation as associations were assumed to be monotonic and nonlinear. A 2-tailed P value < 0.05 was considered statistically significant.
Ethics
This study was part of a clinical trial (NCT02592213) registered with the Danish Data Protection Agency (2008-58-0035) and approved by the Regional Ethics Committee (S-20150109).
RESULTS
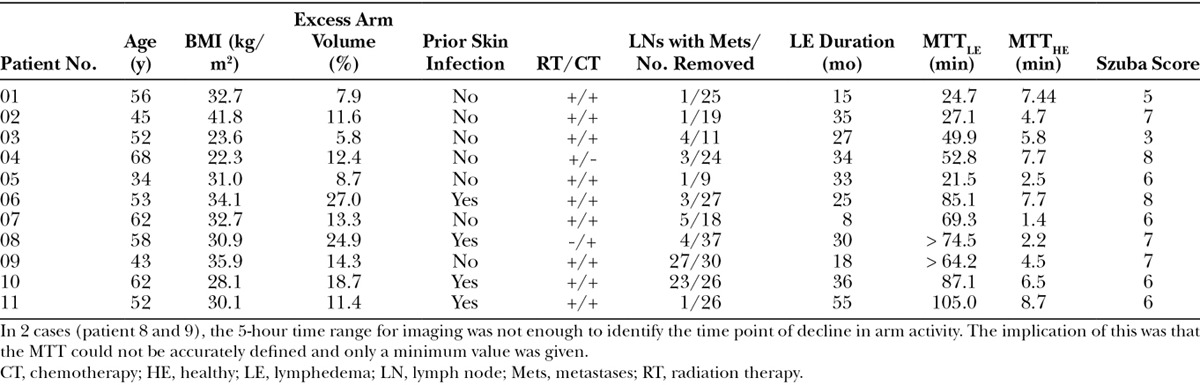
Included patients had a median age of 52 years (range, 34–68) and a median BMI of 31 kg/m2 (range, 22.3–41.8). The duration of lymphedema ranged from 8 to 55 months. Five patients had lymphedema of the dominant arm, and 7 had lymphedema involving the hand. The median excess arm volume was 12.4% (range, 5.8–27.0%). The median Szuba score was 6 (range, 3–8). See Table 1 for characteristics of included patients.
Table 1.
Overview of the Characteristics of Included Patients
There was a clear and highly significant difference in mean MTT between the 2 arms; mean MTT in lymphedema arms (MTTlymph) was 60.1 ± 27.7 minutes versus 5.4 ± 2.5 minutes in the normal, unaffected arms (MTTnorm; mean difference, 54.7 minutes; 95% confidence interval [CI], 36.5–72.9 minutes; P < 0.0001). This was in contrast to the result when looking exclusively on the removal rate from the injection site, where there was no significant difference between arms (0.127 ± 0.053%/min versus 0.132 ± 0.040%/min; P = 0.76).
Four of the included patients had previously been treated for erysipelas in their lymphedema arm. In these, MTTlymph was significantly longer, that is, mean MTTlymph 87.0 ± 11.4 minutes versus 41.7 ± 17.0 minutes in patients without prior erysipelas (mean difference, 43.7 minutes; 95% CI, 18.6–68.7 minutes; P < 0.001). In the bivariate correlation analysis, this naturally lead to a strong correlation between MTTlymph and previous erysipelas (r = 0.84; P < 0.01). In addition, there was a significant positive correlation between MTTlymph and excess arm volume (r = 0.64; P = 0.04) and between MTTlymph and number of lymph nodes removed (r = 0.65; P = 0.03).There was no correlation between MTTlymph and duration of lymphedema, hand involvement or dominance, age, BMI, or Szuba score. The Szuba score was significantly correlated exclusively with excess arm volume (Spearman’s rho, r = 0.64; P = 0.03; Fig. 2).
Fig. 2.
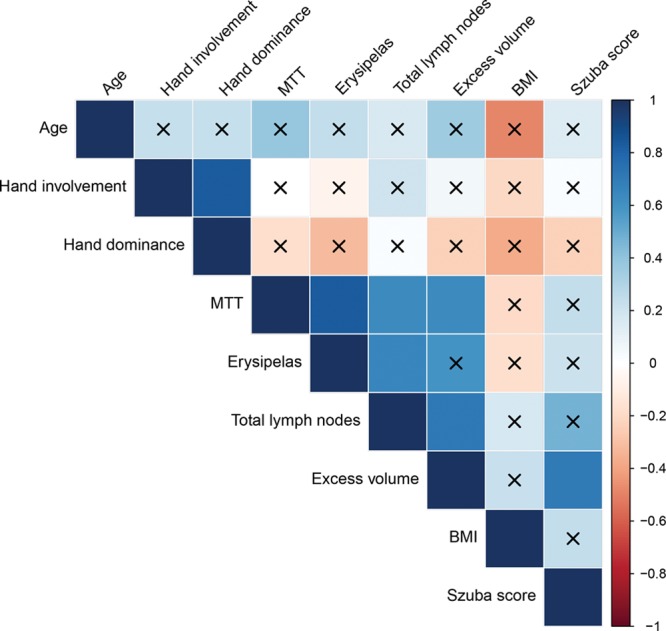
Correlation matrix. Overview of correlations visualized in a correlation matrix. P value was set at 0.05 and x marks all bivariate correlations that were not significant. Spearman Rank correlation was used in all analyses.
Interestingly, a positive correlation was found between number of lymph nodes removed and prior erysipelas (r = 0.66; P = 0.03) as well as excess arm volume (r = 0.72; P = 0.01), suggesting that the more lymph nodes removed, the greater the severity of lymphedema with a higher risk of later infection.
DISCUSSION
We used a newly developed lymphoscintigraphy-based quantitative measure, the MTT, to describe lymphatic function. In our small sample, MTT was strongly related to prior erysipelas infection and correlated with excess arm volume and number of axillary lymph nodes removed during breast cancer surgery, all of which are considered relevant markers of disease severity.3,15,16
Other studies have used simple ratios of ROIs between the healthy and lymphedema arm in the axillary regions, injection depots, or area of dermal backflow.5,6 The approach of using simple ratios between the 2 arms is dependent on the contralateral side being healthy. In contrast, the MTT approach gives an independent value for each arm, which enables it to be used for unilateral as well as bilateral lymphedema. Despite our effort to inject an equal amount of activity on both sides, we observed up to a factor of 3 in difference. A strength of our procedure is that this source of error is eliminated as we calculate the MTT solely based on the dynamics of the injection depot and the arm time activity curves; thus, the absolute amount of injected activity only has influence on the counts statistics.13 An additional challenge with the axillary region is that it will have naturally reduced tracer accumulation following lymphadenectomy. This hampers the use of time to axillary lymph node visualization as a quantitative or semiquantitative measure.7
Removal rate from the injection depot has previously been proposed as an evaluation method. A study by Pain et al.17 showed that this rate was significantly different in affected and healthy arm. In contrast, we found no difference in removal rate between arms, which is in agreement with findings of Lane et al.9 who showed nearly identical removal rates from the injection depot in lymphedema patients and healthy control subjects, suggesting that this measure cannot stand alone as a measure of lymphatic dysfunction.
The MTT methodology distinguished itself by being able to discriminate between healthy and lymphedema arms with no overlap. A main strength of our proposed method is the use of the input and the retention function for calculation of the MTT through the arm. In theory, this makes the method independent of the injection method, whether intradermal, subdermal, or epifascial. Obviously, the same approach can also be applied for measuring lower extremity lymphedema.
Lymphatics are visualized faster with noncolloidal tracers, including human serum albumin and by intradermal rather than subcutaneous injection.18–20 Despite the use of a fast tracer and intradermal injection technique, a decline in arm activity during our 5-hour scan time was not observed in 2 patients, meaning that sometimes the MTT cannot be calculated and only a minimum value can be obtained. In addition, in case of very limited tracer migration from injection depot, the MTT through the arm is determined with great uncertainty; however, this was not the case in any of the examined patients. Other limitations include the small number of patients in our sample, which increased the risk of overestimation bias. The reproducibility of the procedure is unknown due to lack of retesting and this will need to be confirmed before implementation. Results from a previous study repeating lymphoscintigraphy within a week concluded that lymphoscintigraphy is reproducible, at least with regard to the quantitative and qualitative markers used in that study.21 ROIs were manually drawn on each time frame, which may have introduced some error compared with an automated approach with reuse of the same ROIs, which, however, was not available to us.
In conclusion, preliminary clinical results with this novel quantitative lymphoscintigraphic approach seem to indicate that MTT measurements can reliably discriminate between presence and absence of lymphedema. If and when the reproducibility of the method has been confirmed, it may, thus, be suitable also for evaluation of therapeutic interventions. In addition, it may allow for comparison between institutions and is now open for further validation in larger groups of patients being treated for breast cancer–related lymphedema.
ACKNOWLEDGMENTS
We are grateful for the technical assistance of Camilla Bergholdt. The study was conducted as part of a PhD project funded by participating departments, Odense University Hospital, University of Southern Denmark, and Axel Muusfeldt fonden.
Footnotes
Preliminary results presented at the annual meeting of the Danish Society of Nuclear Medicine and Clinical Physiology as a poster, September 2016, Kolding, Denmark.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The study was conducted as part of a PhD project funded by participating departments, Odense University Hospital, University of Southern Denmark, and Axel Muusfeldt fonden. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.McWayne J, Heiney SP.Psychologic and social sequelae of secondary lymphedema: a review. Cancer. 2005;104:457–466. [DOI] [PubMed] [Google Scholar]
- 2.Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg (cellulitis): case-control study. BMJ. 1999;318:1591–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–515. [DOI] [PubMed] [Google Scholar]
- 4.Toyserkani NM, Jørgensen MG, Haugaard K, et al. Seroma indicates increased risk of lymphedema following breast cancer treatment: a retrospective cohort study. Breast. 2017;32:102–104. [DOI] [PubMed] [Google Scholar]
- 5.Szuba A, Strauss W, Sirsikar SP, et al. Quantitative radionuclide lymphoscintigraphy predicts outcome of manual lymphatic therapy in breast cancer-related lymphedema of the upper extremity. Nucl Med Commun. 2002;23:1171–1175. [DOI] [PubMed] [Google Scholar]
- 6.Yoo JN, Cheong YS, Min YS, et al. Validity of quantitative lymphoscintigraphy as a lymphedema assessment tool for patients with breast cancer. Ann Rehabil Med. 2015;39:931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi M, Grassi R, Costa R, et al. Evaluation of the upper limb lymphatic system: a prospective lymphoscintigraphic study in melanoma patients and healthy controls. Plast Reconstr Surg. 2016;138:1321–1331. [DOI] [PubMed] [Google Scholar]
- 8.Pain SJ, Barber RW, Ballinger JR, et al. Side-to-side symmetry of radioprotein transfer from tissue space to systemic vasculature following subcutaneous injection in normal subjects and patients with breast cancer. Eur J Nucl Med Mol Imaging. 2003;30:657–661. [DOI] [PubMed] [Google Scholar]
- 9.Lane KN, Dolan LB, Worsley D, et al. Upper extremity lymphatic function at rest and during exercise in breast cancer survivors with and without lymphedema compared with healthy controls. J Appl Physiol (1985). 2007;103:917–925. [DOI] [PubMed] [Google Scholar]
- 10.Ozturk CN, Ozturk C, Glasgow M, et al. Free vascularized lymph node transfer for treatment of lymphedema: a systematic evidence based review. J Plast Reconstr Aesthet Surg. 2016;69:1234–1247. [DOI] [PubMed] [Google Scholar]
- 11.Penha TR, Ijsbrandy C, Hendrix NA, et al. Microsurgical techniques for the treatment of breast cancer-related lymphedema: a systematic review. J Reconstr Microsurg. 2013;29:99–106. [DOI] [PubMed] [Google Scholar]
- 12.Brorson H, Höijer P.Standardised measurements used to order compression garments can be used to calculate arm volumes to evaluate lymphoedema treatment. J Plast Surg Hand Surg. 2012;46:410–415. [DOI] [PubMed] [Google Scholar]
- 13.Hvidsten S, Toyserkani NM, Sørensen JA, et al. A scintigraphic method for quantitatively measuring lymphatic function in upper extremity lymphedema in humans. EJNMMI Res. [Manuscript in preparation]. [Google Scholar]
- 14.Modi S, Stanton AW, Mortimer PS, et al. Clinical assessment of human lymph flow using removal rate constants of interstitial macromolecules: a critical review of lymphoscintigraphy. Lymphat Res Biol. 2007;5:183–202. [DOI] [PubMed] [Google Scholar]
- 15.Ramos SM, O’Donnell LS, Knight G.Edema volume, not timing, is the key to success in lymphedema treatment. Am J Surg. 1999;178:311–315. [DOI] [PubMed] [Google Scholar]
- 16.Asdourian MS, Skolny MN, Brunelle C, et al. Precautions for breast cancer-related lymphoedema: risk from air travel, ipsilateral arm blood pressure measurements, skin puncture, extreme temperatures, and cellulitis. Lancet Oncol. 2016;17:e392–e405. [DOI] [PubMed] [Google Scholar]
- 17.Pain SJ, Purushotham AD, Barber RW, et al. Variation in lymphatic function may predispose to development of breast cancer-related lymphoedema. Eur J Surg Oncol. 2004;30:508–514. [DOI] [PubMed] [Google Scholar]
- 18.O’Mahony S, Solanki CK, Barber RW, et al. Imaging of lymphatic vessels in breast cancer-related lymphedema: intradermal versus subcutaneous injection of 99mTc-immunoglobulin. AJR Am J Roentgenol. 2006;186:1349–1355. [DOI] [PubMed] [Google Scholar]
- 19.O’Mahony S, Rose SL, Chilvers AJ, et al. Finding an optimal method for imaging lymphatic vessels of the upper limb. Eur J Nucl Med Mol Imaging. 2004;31:555–563. [DOI] [PubMed] [Google Scholar]
- 20.Mortimer PS.Evaluation of lymphatic function: abnormal lymph drainage in venous disease. Int Angiol. 1995;14:32–35. [PubMed] [Google Scholar]
- 21.Devoogdt N, Van den Wyngaert T, Bourgeois P, et al. Reproducibility of lymphoscintigraphic evaluation of the upper limb. Lymphat Res Biol. 2014;12:175–184. [DOI] [PubMed] [Google Scholar]


