Abstract
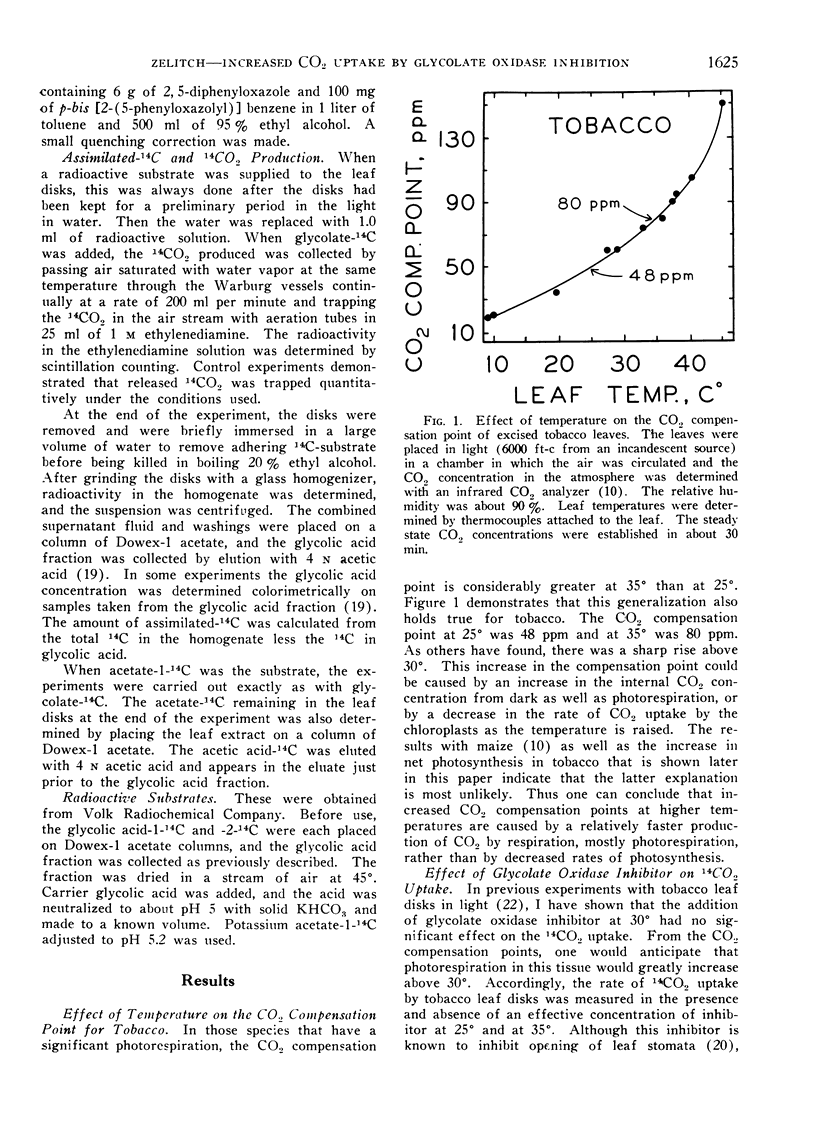
There is considerable variation among species in their rate of photorespiration, and photorespiration increases greatly at higher temperatures. The addition of an inhibitor of glycolate oxidase, α-hydroxy-2-pyridinemethanesulfonic acid, to tobacco leaf disks at 35° stimulated photosynthetic 14CO2 uptake at least 3-fold, but 14CO2 uptake was not changed by the inhibitor at 25°. The inhibitor did not increase photosynthesis in maize leaf disks at either temperature.
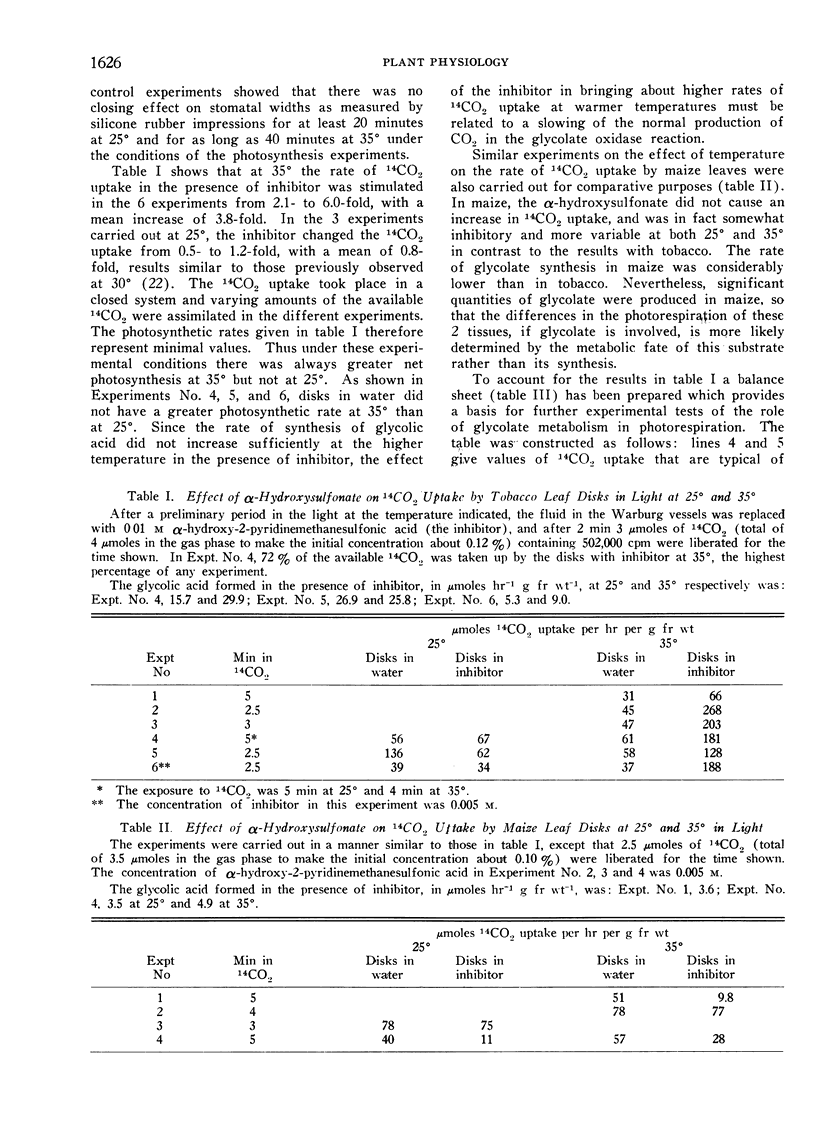
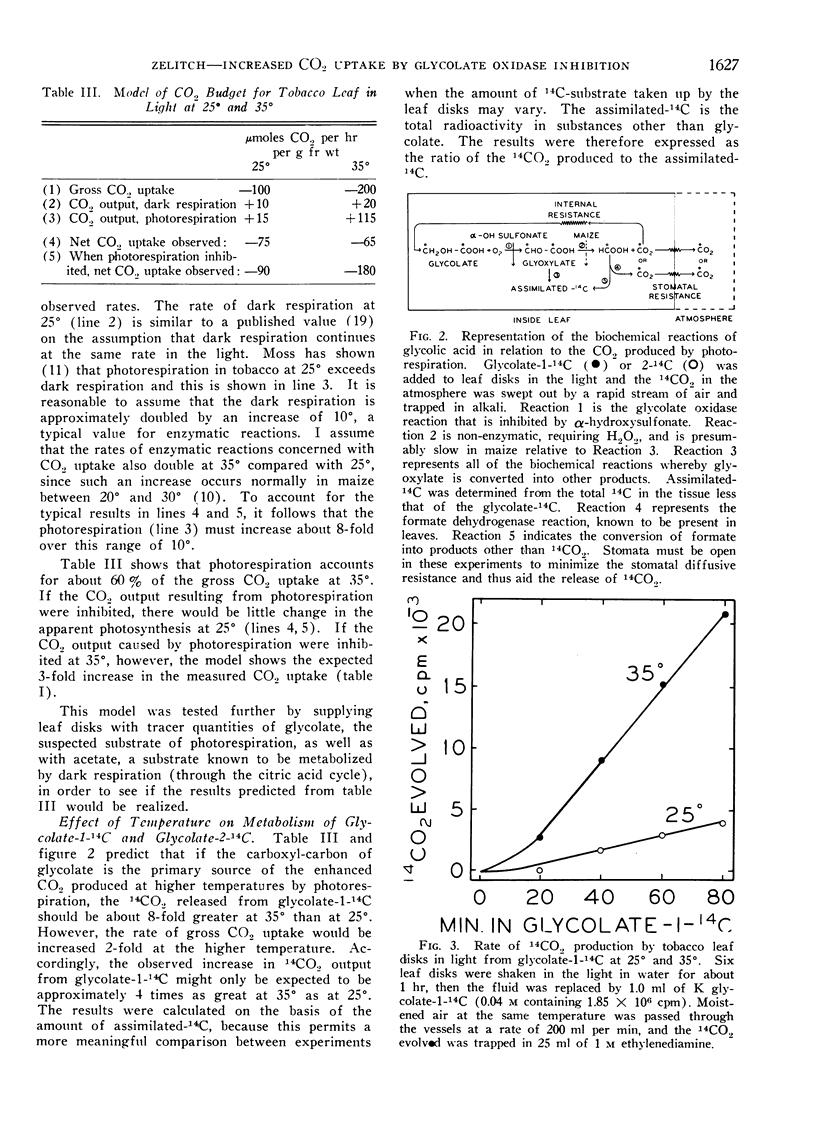
The evolution of CO2 from glycolate was greatly enhanced in tobacco at 35° compared with 25°. Labeling of the glycolate of tobacco with glycolate-1-14C and -2-14C showed that the increased CO2 evolved in the light (photorespiration) arose specifically from the carboxyl-carbon atom of glycolate. Maize, a species known to have a negligible photorespiration, produced 14CO2 poorly from glycolate-1-14C in comparison to tobacco.
Acetate-1-14C, a substrate metabolized by dark respiration, produced similar amounts of 14CO2 in the light in both tobacco and maize. This respiration was changed little relative to photosynthesis by increasing temperature.
Most plants, such as tobacco, have a high photorespiration. The loss of fixed carbon causes an increase in the internal concentration of CO2 especially at higher temperatures, and results in a lower CO2 concentration gradient and therefore a lower net photosynthetic CO2 uptake. Some species, like maize, have a negligible photorespiration and are thus more efficient photosynthetically. The use of an inhibitor of the oxidation of glycolate, the substrate for photorespiration, changed tobacco so that it behaved photosynthetically like maize. Thus high rates of photorespiration may limit the net CO2 uptake in many plant species.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Forrester M. L., Krotkov G., Nelson C. D. Effect of oxygen on photosynthesis, photorespiration and respiration in detached leaves. I. Soybean. Plant Physiol. 1966 Mar;41(3):422–427. doi: 10.1104/pp.41.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSS D. N. The limiting carbon dioxide concentration for photosynthesis. Nature. 1962 Feb 10;193:587–587. doi: 10.1038/193587a0. [DOI] [PubMed] [Google Scholar]
- Ozbun J. L., Volk R. J., Jackson W. A. Effects of Light and Darkness on Gaseous Exchange of Bean Leaves. Plant Physiol. 1964 Jul;39(4):523–527. doi: 10.1104/pp.39.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. D., Hendricks R. H., Hill G. R. APPARENT EQUILIBRIUM BETWEEN PHOTOSYNTHESIS AND RESPIRATION IN AN UNRENEWED ATMOSPHERE. Plant Physiol. 1944 Apr;19(2):370–376. doi: 10.1104/pp.19.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregunna B. Flavin mononucleotide control of glycolic Acid oxidase and photorespiration in corn leaves. Science. 1966 Mar 11;151(3715):1239–1241. doi: 10.1126/science.151.3715.1239. [DOI] [PubMed] [Google Scholar]
- Walker D. A., Zelitch I. Some Effects of Metabolic Inhibitors, Temperature, & Anaerobic Conditions on Stomatal Movement. Plant Physiol. 1963 Jul;38(4):390–396. doi: 10.1104/pp.38.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELITCH I. THE RELATION OF GLYCOLIC ACID SYNTHESIS TO THE PRIMARY PHOTOSYNTHETIC CARBOXYLATION REACTION IN LEAVES. J Biol Chem. 1965 May;240:1869–1876. [PubMed] [Google Scholar]
- ZELITCH I. The relationship of glycolic acid to respiration and photosynthesis in tobacco leaves. J Biol Chem. 1959 Dec;234:3077–3081. [PubMed] [Google Scholar]
- Zelitch I. BIOCHEMICAL CONTROL OF STOMATAL OPENING IN LEAVES. Proc Natl Acad Sci U S A. 1961 Sep;47(9):1423–1433. doi: 10.1073/pnas.47.9.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]



