Abstract
Background:
Approximately 250,000 new cases of breast cancer are diagnosed yearly in the U.S. resulting in more postmastectomy breast reconstructions (PMBRs). The acellular dermal matrix (ADM) expander-implant method became popular in the mid-2000s, but newer techniques such as the inferior deepithelialized flap (IDF) has more recently been described. We hypothesize that ADMs and IDFs provide comparable aesthetic outcomes, with no difference in complication rates and operative characteristics.
Methods:
A retrospective, single-institution study was performed between July 1, 2012, and June 30, 2014, examining all PMBR’s (ADM and IDF). Outcomes were categorized as clinical (e.g., complications requiring surgical intervention) or aesthetic.
Results:
A total of 65 patients (41 ADM; 24 IDF; mean age, 53.4 ± 10.7 years) were included, with 101 PMBR’s evaluated (63 ADM and 38 IDF). Patients who underwent IDFs had higher body mass index (32 versus 25; P < 0.01) and higher grades of breast ptosis. Major complication rates were similar between ADM and IDF groups (22% versus 31.5%; P = 0.34). There were no differences in aesthetic outcomes between groups (rater intraclass correlation, 0.92). The average IDF breast reconstruction took nearly 30 minutes longer per reconstructed side (192 minutes versus 166 minutes; P = 0.02), but operative costs were more expensive for the ADM breast reconstruction.
Conclusions:
The IDF procedure took 30 minutes longer for each reconstructed side, without significant differences in complications or aesthetic outcomes between the 2 PMBRs. IDF reconstructions may be more suitable for patients with grade 3 breast ptosis and higher body mass index. Further studies should focus on long-term outcomes and value-based approaches to PMBR.
INTRODUCTION
Breast cancer continues to have a global impact as a major public health issue.1–3 According to the National Breast Cancer Foundation, 1 in 8 women will develop breast cancer during their lifetime.4 Despite equivalent survival outcomes between mastectomy and breast conservation therapy for breast cancer, there has been an increasing trend for mastectomies.5–7
As the number for mastectomies increased, so did the demand for breast reconstruction (BR) operations, including the use of expanders or implants,8 pedicled flaps,9,10 and free flap options.8,11 Implant-based management continues to dominate, accounting for approximately 70% of BRs.12
Acellular dermal matrix (ADM) became popular in the mid-2000s, especially for the coverage of the inferior pole of the implant.13,14 This allows for faster tissue expansion filling time, greater immediate tissue expansion,13,15 and thus facilitates immediate implant-based postmastectomy BR (PMBR).16,17 A more recent technique has been described using an inferior deepithelialized flap (IDF) to cover the lower pole of the implant.18,19 Advantages of such a technique include autologous blood supply, thicker layer of tissue with a more natural consistency, reduced cost, and better tolerance to post-BR radiation.20,21
Common complications for ADM reconstructions include infections, seromas, and mastectomy skin flap necrosis.13,22,23 Among those, infections are most prominent; however, ADM alone is not an independent risk factor for infection.24 Literature regarding complications for dermal autografts remains sparse, with more data needed to better compare the ADM-assisted versus the IDF-based techniques. Aesthetic outcomes are among the most important metrics in BR. Despite this, data comparing aesthetic outcomes between ADM and IDF-assisted BR are lacking. We hypothesized that ADM and IDF provide comparable aesthetic outcomes, with no difference in complication rates and operative characteristics.
METHODS
A retrospective, single-institution study was performed between July 1, 2012, and June 30, 2014, examining all immediate expander BRs using ADM or IDF. Included were all patients who underwent an acellular dermis or IDF immediate PMBR, with or without radiotherapy treatment. Patients who were younger than 18 years of age were excluded. All the patients underwent immediate PMBR with tissue expanders and none with immediate implant-based reconstruction.
Abstracted data included demographics [age, body mass index (BMI), grade of ptosis), potential risk factors for postoperative morbidity (history of tobacco use, diabetes, pre-BR radiation therapy), and operative characteristics (type of operative procedure, laterality, duration, cost).
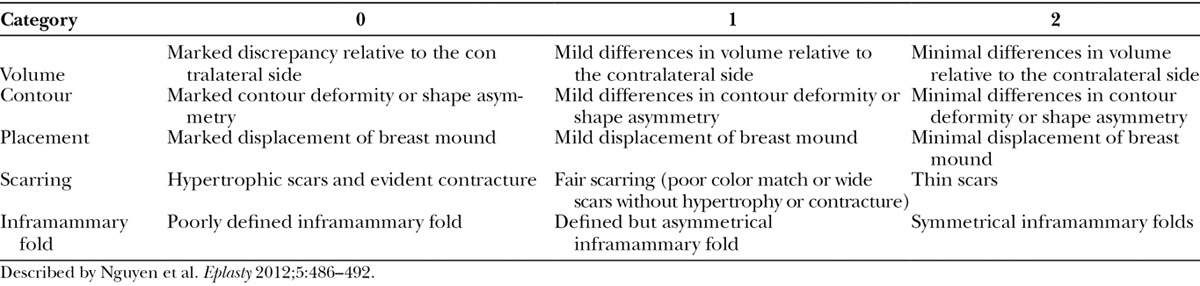
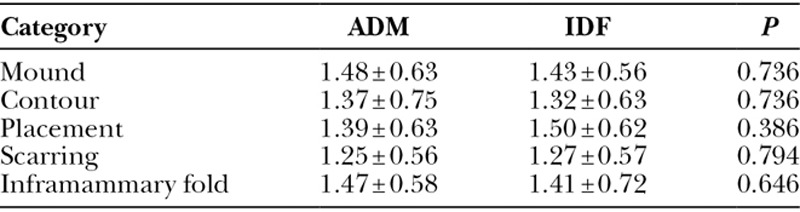
Primary outcomes were categorized into 2 groups: clinical and aesthetic. Clinical complications requiring surgical intervention postoperatively included hematoma, seroma, partial flap necrosis, tissue expander leakage/exposure, and inframammary fold displacement. Aesthetic outcomes were measured by 5 different variables that included BR volume, contour, placement, scarring, and inframammary fold appearance as described by Nguyen et al.20 The scale described was a 3-point scale with ratings from 0 to 2 with higher scores being more favorable (Table 1). Postoperative photographic data were scored by 2 blinded senior-level general surgery residents after being given explicit examples and instructions using the 3-point scale. None of the reviewers were involved with patient care.
Table 1.
Aesthetic 3-Point Outcome Scale
Statistical analysis was performed using the student’s t test for normally distributed continuous variables, Mann-Whitney U test for nonnormally distributed continuous data, and Fisher’s exact test for categorical data. Statistical significance level was set at α = 0.05. SPSS 18 for Windows (IBM, Armonk, N.Y.) was used to perform analyses. Institutional review board approval was granted.
OVERVIEW OF SURGICAL TECHNIQUES
ADM
Our surgeon uses AlloMaxTM (Bard, Davol Inc., Warwick, R.I.) as the surgical biological graft. AlloMaxTM is a sterile, human-derived acellular dermal collagen with elastin fibers (Fig. 1). The ADM procedure consists of using the dermal matrix as an inferior sling for the tissue expander or implant.13,16,25 The inferolateral origin (margin) of the pectoralis major muscles is released and a piece of ADM is sutured into place between the inframammary fold and inferior border of the pectoralis major muscle to create an inferior sling for the tissue expander (Fig. 1A). The suture line extends the length of the ADM and follows the lateral contour of the pectoralis major muscle which re-creates a new inframammary fold and a pocket for the tissue expander or implant. A central pocket is left open where the tissue expander or implant will be inserted through. The tissue expander or implant is then securely placed below the pectoralis muscle superiorly and ADM sling inferiorly before the interface between the pectoralis and leading edge of the acellular dermis is completely closed over the expander (Fig. 1B–2C). Two drains were routinely placed, 1 in the subpectoral space and the other in the suprapectoral space.
Fig. 1.
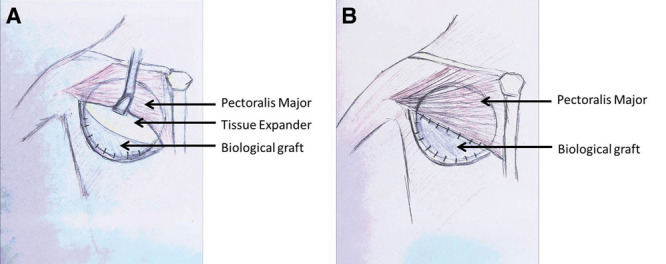
Schematic drawing of an ADM procedure for a right BR. A, Represents the tissue expander exposed under the pectoralis major muscle, with the dermal sling created by the biological graft where the implant will sit. B, The tissue expander is covered by the pectoralis major and biological graft once they are sutured together.
Fig. 2.
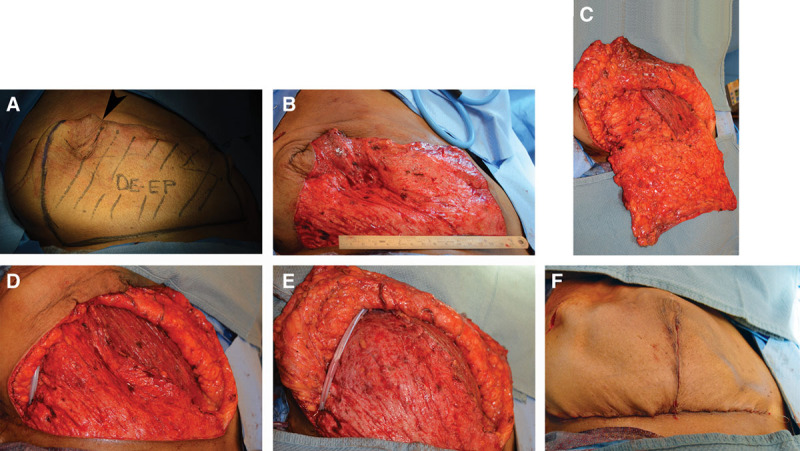
IDF procedure for a right BR. A, Represents the area that inferio-lateral portion before the deepithelialization process. The mastectomy incision is indicated where the arrow is pointing. B, Demonstrates a completed deepithelialized flap. C, The inferio-lateral sling is mobilized and lateral attachments are removed keeping the inframammary fold intact. D, The IDF is created ready for the tissue expander. E, The tissue expander has been placed with the IDF and pectoralis major sutured together. F, Completed reconstruction. Photos courtesy of Dr. Michael Morrissey.
IDF
The IDF procedure evolved later than the ADM procedure (Fig. 2). The concept behind this technique is the same as using the ADM; however, instead of using a biological graft, autologous tissue is used to make an inferior sling where the tissue expander or implant will stay. This newer technique utilizes a deepithelialized portion of the infero-lateral dermis as an infero-lateral sling.5,12,21 The first step to the procedure is deepithelializing the inferior portion of the mastectomy flap (Fig. 2B). Once the inferiorly based dermal flap is deepithelialized, the lateral attachments are taken down (Fig. 2C), to allow for a recreation of the inferior dermal sling where the lateral edge is sutured to the lateral mammary fold (Fig. 2D). As a result, the inframammary fold is already intact and a new one does not need to be recreated unlike the ADM procedure. The general dimensions of the inferior sling was patient dependent but ranged from 20 to 38 cm by 6 to 15 cm in this study. The tissue expander is then securely placed below the pectoralis muscle superiorly and autologous inferior dermal sling inferiorly before the interface between the pectoralis, and leading edge of the acellular dermis was completely closed over the expanders (Fig. 2E). SPY angiography was used at the discretion of the operating surgeon when there was concern for ischemic changes but not routinely used for all cases. Drain placement was the same as the ADM procedure.
RESULTS
A total of 65 patients underwent PMBR during the study period. The overall mean age was 53.4 ± 10.7 years. Of these, 41 patients underwent ADM-based procedure and 24 patients underwent IDF-based BR. Within the patient sample, there were 101 individual BRs (1.6 per patient). Looking at the 2 study subsets, there were 63 ADM-based BRs and 38 IDF-based reconstructions.
Except for BMI and grades of ptosis, the 2 groups were similar in all other aspects of general descriptive characteristics (Table 2). Patients who underwent IDFs had significantly higher BMI (32.2 versus 25.6; P < 0.01) and the majority of these patients had grade 3 breast ptosis (68%; Fig. 3). On the other hand, the majority of the patients who underwent the ADM procedure had grade 1 breast ptosis (50.8%). There were no significant differences between the groups in terms of tobacco use, diabetes, and pre-BR radiation therapy (Table 2). Majority of operative characteristics examined were similar between the 2 groups, including laterality. Self pay costs for the procedures differed significantly (Table 3). Average costs for a 192 minute and 166 minute procedures at our main campus are $8,105 and $6,745 respectively, which includes facility, anesthesia, and surgeon fees. However, the cost for an 8 × 16 cm piece of AlloMax is $3,411, resulting in the ADM BR procedure cost to be approximately $10,156. Although, the IDF cost was less than the ADM procedure, the average IDF procedure took nearly 30 minutes longer per reconstructed side (192 ± 45 minutes) than an average ADM procedure (166 ± 36 minutes; P = 0.02). Procedure times reflect the total operation time from start of the mastectomy procedures to the completion of the reconstruction.
Table 2.
Demographic Characteristics
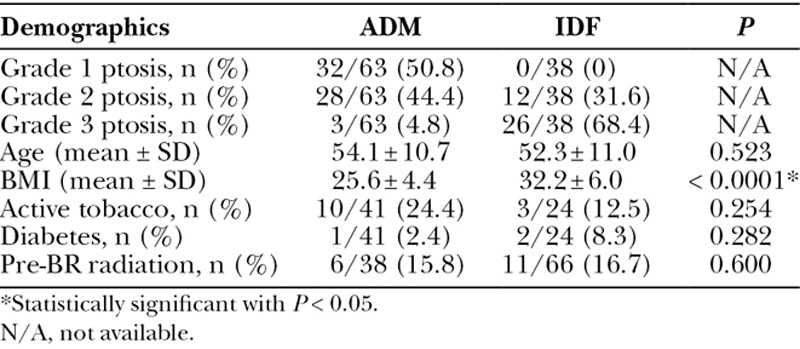
Fig. 3.
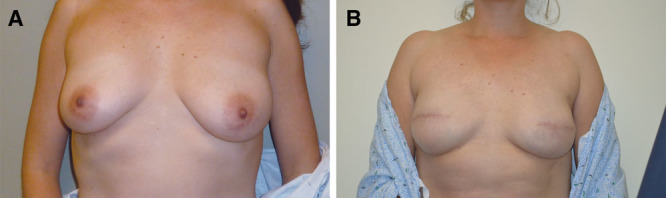
Grades of ptosis. A, Grade 1 breast ptosis. B, Grade 2 breast ptosis. C, Grade 3 breast ptosis. Photos courtesy of Dr. Michael Morrissey.
Table 3.
Self Pay Costs

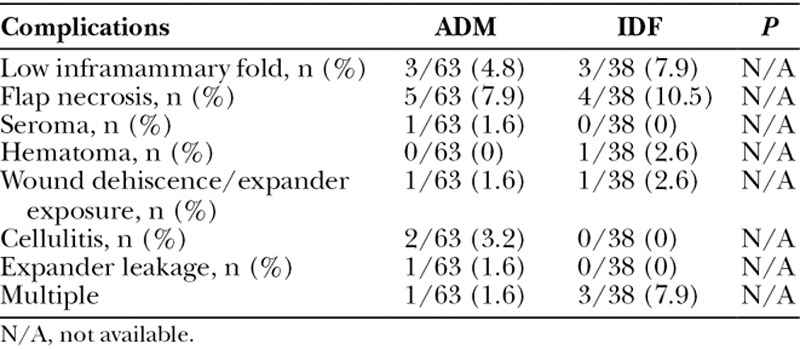
Overall, major complication rates were similar between ADM and IDF groups (22% versus 31%; P = 0.34). Clinical complications requiring subsequent surgical intervention included hematoma, seroma, partial flap necrosis, tissue expander leakage/exposure, and inframammary fold. Outcomes for these categories are shown in Table 4. In addition, there were no cases of poor adherence of ADM or tissue necrosis of the IDF flap appreciated at the time of implant exchanges.
Table 4.
Major Complications
Finally, there were no significant differences in the aesthetic outcomes between the groups rated among our 2 reviewers (Figs. 4, 5). The 5 different aesthetic outcomes measured included BR volume, contour, placement, scarring, and inframammary fold appearance. Details regarding aesthetic outcomes are presented in Table 5. The intraclass correlation was calculated to be 0.92 (95% confidence interval, 0.89–0.94), indicating good rater agreement.
Fig. 4.
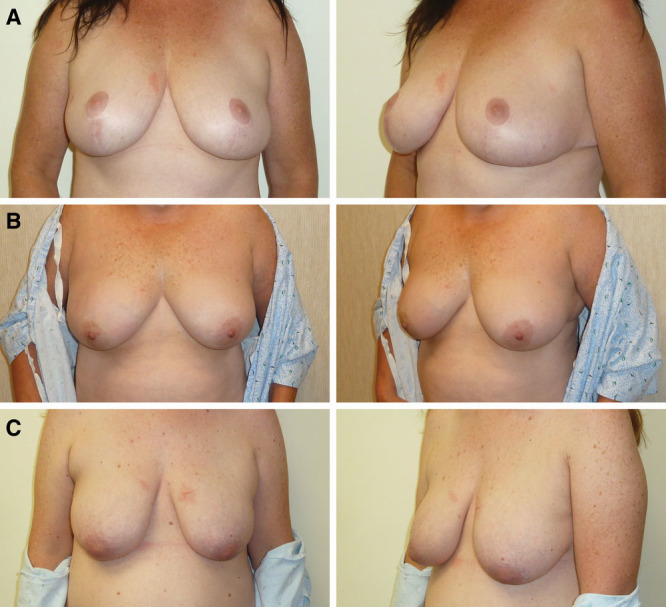
IDF reconstruction, 13-month follow-up. A, Breasts prior to IDF reconstruction. B, Breasts post reconstruction at 13-month follow-up. Photos courtesy of Dr. Michael Morrissey.
Fig. 5.
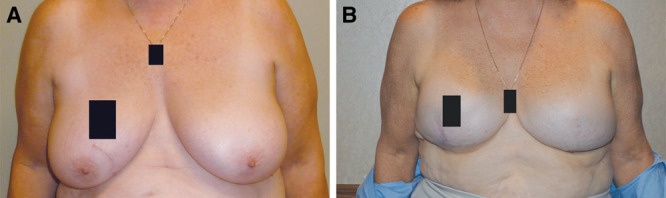
ADM reconstruction, 15-month follow-up. A, Breasts prior to ADM reconstruction. B, Breasts post reconstruction at 15-month follow-up. Photos courtesy of Dr. Michael Morrissey.
Table 5.
Aesthetic Outcomes
DISCUSSION
The demand for PMBR continues to increase for a number of reasons, including the large number of newly diagnosed cases of breast cancer and patient preference for mastectomy over breast conservation.1–3,5–7 BR options vary based on several factors, including breast size, BMI, and type of skin sparing mastectomies (e.g., categories I–IV).26
Of interest, the only parameter that reached statistical significance between the ADM and IDF reconstructed groups was BMI (32 versus 25, respectively). In addition, of importance, patients who underwent IDF reconstructed procedures were more likely to have grade 3 breast ptosis. Although the above finding is not surprising, it is important to note that BMI may play a role in breast cancer risk profile (both primary and recurrent).27,28 In smaller, less ptotic breasts and patients were lower BMIs, the IDF may not be feasible as there would not be sufficient tissue to create a dermal sling used to cover the tissue expander or implant.20,21
Operative characteristics and laterality were similar for the 2 groups. More specifically, the average IDF procedure took nearly 30 minutes longer per each BR than an average ADM procedure. Based on our operative experience, we attribute this increase in operative time to the deepithelialization process of the dermal flap as an extra step is necessary to create the inferior sling to harbor the tissue expander or implant. On the other hand, with ADM, depending on the product being used, cadaveric versus acellular human-derived tissue is readily available to create the inferior pocket. In terms of self pay costs, the ADM BR procedure was more expensive because of the use of AllomaxTM, despite the IDF procedure being a longer procedure. Granted, most of these procedures will be covered by insurance companies, but self pay costs give us a basis without the hyperinflation of charges that results when insurance companies are involved.
A recent meta-analysis showed that infections, hematomas, and seromas may occur more frequently in ADM-based BRs.29 However, our study suggests that there is no difference in complications between the 2 groups; however, our sample size is relatively small, and definitive conclusions cannot be made. In theory, autologous tissue should be less immunogenic and more compatible, suggesting perhaps that there should be a lower complication especially with regard to infection rates.30 However, we speculated that the increased technical skill and longer operative time required for the deepithelialization process of the inferior flap in the IDF procedure mitigated the differences in complication rates between the 2 procedures.
In addition to complication rates, aesthetics also have a major impact on BR outcomes.31 The optimal result in breast cancer is predicated on elimination of the malignancy and the achievement of satisfactory aesthetic outcome, including the recreation of a natural breast appearance.32 Aesthetic outcomes are difficult to measure objectively due to their inherent subjectivity. Past studies have used overall outcome of reconstruction as an aesthetic endpoint for ADM-assisted closures but lacked quantitative measures.33,34 In our study, the appearance of PMBR was subjectively measured for both groups by third-party reviewers and not the patients’ themselves. Any definitive conclusion about aesthetic outcomes cannot be made, as our scale lacks objective measures and patient satisfaction measures. Overall findings of this study suggest that both options provide similar and adequate aesthetic outcomes. However, this may differ over longer term follow-up, and a more complete study with the subjective opinion of the patient’s overall satisfaction may provide more insight in this matter. One study used patient-reported satisfaction measures and examined aesthetic outcomes between prosthetic and autogenous reconstruction.35 This study studied short-term (< 5 years), intermediate (6–8 years) and long-term (> 8 years) patient-reported aesthetic outcomes and interestingly found that satisfaction was similar between prosthetic and autogenous aesthetic outcomes during short-term follow-up.35 However, in the long-term follow-up group, autogenous tissue reconstruction patients were significantly more satisfied with their aesthetic outcomes.35
Limitations to this study include the inherent retrospective nature of the study. In addition, it is a relatively small study conducted in a single institution by a single surgeon, and an increase in sample size plus a multicenter study may demonstrate a statistical difference in the complication rates between the 2 groups. A power calculation was performed that showed that our sample size would need to be approximately 364 patients to potentially determine a difference between the 2 groups, reiterating a larger study may be of benefit. Aesthetic outcome postoperative grading measures among the patients were performed over a period of 3–32 months. Granted the patients who required additional operations secondary to complication rates had longer follow ups, a more standardized methodology for aesthetic outcome measurements at set intervals should be considered for future research. In addition, the aesthetic outcomes scale used in this study was a subjective one, and physical, photographic, 3-dimensional imaging scales, and patient satisfaction scales should be considered. As well, a long-term follow-up study greater than 5 years after the reconstructive procedures with regard to aesthetic outcomes may be of benefit including patient satisfaction. Future directions should also evaluate capsular contracture and whether or not the use of ADM decreased the rate of capsular contracture, when compared with IDF.
CONCLUSIONS
Our study suggests that there are no significant differences in major complications or aesthetic outcomes between the ADM and the IDF approach to immediate expander BR, but no definitive conclusions can be made, given that our sample size was relatively small. Although these preliminary results are favorable, data from additional prospective, randomized multicenter trials would be beneficial. However, our data suggest that IDF may be more suitable for patients with higher grades of breast ptosis (2 or 3) and higher BMIs. On the other hand, ADM may be more appropriate for patients with less available autologous tissue for BR. On average, the ADM procedure took significantly less time for each reconstructed side, but costs were more expensive secondary to the use of the biological graft. As a result, the duration in the operating room must be balanced against the cost of biological graft. The results from this study are preliminary, and further studies are warranted in this area, focusing on complication rates, patient satisfication, long-term outcomes and value-based approaches to BR.
ACKNOWLEDGMENTS
We would like to thank Crystal Corredera for facilitation of patient information.
Footnotes
Presented at the 11th Annual Academic Surgical Congress, February 2–4, 2016, Jacksonville, Fla.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Anderson BO, Yip CH, Ramsey SD, et al. Global Summit Health Care Systems and Public Policy Panel. Breast cancer in limited-resource countries: health care systems and public policy. Breast J. 2006;12:S54–S69. [DOI] [PubMed] [Google Scholar]
- 2.Bouchardy C, Rapiti E, Fioretta G, et al. Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol. 2003;21:3580–3587. [DOI] [PubMed] [Google Scholar]
- 3.Mettlin C.Global breast cancer mortality statistics. CA Cancer J Clin. 1999;49:138–144. [DOI] [PubMed] [Google Scholar]
- 4.breastcancer.org. U.S. breast cancer statistics. 2016. Available at http://www.breastcancer.org/symptoms/understand_bc/statistics. Accessed January 21, 2016.
- 5.Dragun AE, Huang B, Tucker TC, et al. Increasing mastectomy rates among all age groups for early stage breast cancer: a 10-year study of surgical choice. Breast J. 2012;18:318–325. [DOI] [PubMed] [Google Scholar]
- 6.Dragun AE, Pan J, Riley EC, et al. Increasing use of elective mastectomy and contralateral prophylactic surgery among breast conservation candidates: a 14-year report from a comprehensive cancer center. Am J Clin Oncol. 2013;36:375–380. [DOI] [PubMed] [Google Scholar]
- 7.McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. 2009;16:2682–2690. [DOI] [PubMed] [Google Scholar]
- 8.Djohan R, Gage E, Bernard S.Breast reconstruction options following mastectomy. Cleve Clin J Med. 2008;75:S17–S23. [DOI] [PubMed] [Google Scholar]
- 9.Hamdi M, Khuthaila DK, Van Landuyt K, et al. Double-pedicle abdominal perforator free flaps for unilateral breast reconstruction: new horizons in microsurgical tissue transfer to the breast. J Plast Reconstr Aesthet Surg. 2007;60:904–12; discussion 913. [DOI] [PubMed] [Google Scholar]
- 10.Janiga TA, Atisha DM, Lytle IF, et al. Ipsilateral pedicle TRAM flaps for breast reconstruction: are they as safe as contralateral techniques? J Plast Reconstr Aesthet Surg. 2010;63:322–326. [DOI] [PubMed] [Google Scholar]
- 11.Emam A, Khan K, Prousskaia E.Point of technique: protecting the pedicle in free flap breast reconstruction from the drain. Plast Reconstr Surg Glob Open. 2015;3:e419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weichman KE, Wilson SC, Saadeh PB, et al. Sterile “ready-to-use” AlloDerm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2013;132:725–736. [DOI] [PubMed] [Google Scholar]
- 13.Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;125:429–436. [DOI] [PubMed] [Google Scholar]
- 14.Ganske I, Verma K, Rosen H, et al. Minimizing complications with the use of acellular dermal matrix for immediate implant-based breast reconstruction. Ann Plast Surg. 2013;71:464–470. [DOI] [PubMed] [Google Scholar]
- 15.Collis GN, TerKonda SP, Waldorf JC, et al. Acellular dermal matrix slings in tissue expander breast reconstruction: are there substantial benefits? Ann Plast Surg. 2012;68:425–428. [DOI] [PubMed] [Google Scholar]
- 16.Breuing KH, Warren SM.Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg. 2005;55:232–239. [DOI] [PubMed] [Google Scholar]
- 17.Salzberg CA.Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg. 2006;57:1–5. [DOI] [PubMed] [Google Scholar]
- 18.King IC, Harvey JR, Bhaskar P.One-stage breast reconstruction using the inferior dermal flap, implant, and free nipple graft. Aesthetic Plast Surg. 2014;38:358–364. [DOI] [PubMed] [Google Scholar]
- 19.Mestak J, Sukop A, Mestak O.Use of deepithelialized flap in mammoplasties: simple method with excellent results. Aesthetic Plast Surg. 2011;35:1106–1111. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen KT, Mioton LM, Smetona JT, et al. Esthetic outcomes of ADM-assisted expander-implant breast reconstruction. Eplasty. 2012;12:e58. [PMC free article] [PubMed] [Google Scholar]
- 21.Torstenson T, Boughey JC, Saint-Cyr M.Inferior dermal flap in immediate breast reconstruction. Ann Surg Oncol. 2013;20:3349. [DOI] [PubMed] [Google Scholar]
- 22.Sbitany H, Sandeen SN, Amalfi AN, et al. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg. 2009;124:1735–1740. [DOI] [PubMed] [Google Scholar]
- 23.Parks JW, Hammond SE, Walsh WA, et al. Human acellular dermis versus no acellular dermis in tissue expansion breast reconstruction. Plast Reconstr Surg. 2012;130:739–746. [DOI] [PubMed] [Google Scholar]
- 24.Peker F, Yuksel F, Karagoz H, et al. Breast reconstruction using de-epithelialized dermal flap after vertical-pattern skin-sparing mastectomy in macromastia. ANZ J Surg. 2015;85:64–68. [DOI] [PubMed] [Google Scholar]
- 25.Sbitany H, Sandeen SN, Amalfi AN, et al. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg. 2009;124:1735–1740. [DOI] [PubMed] [Google Scholar]
- 26.Carlson GW.Technical advances in skin sparing mastectomy. Int J Surg Oncol. 2011;2011:396901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fedele P, Orlando L, Schiavone P, et al. BMI variation increases recurrence risk in women with early-stage breast cancer. Future Oncol. 2014;10:2459–2468. [DOI] [PubMed] [Google Scholar]
- 28.Gathirua-Mwangi WG, Zollinger TW, Murage MJ, et al. Adult BMI change and risk of breast cancer: National Health and Nutrition Examination Survey (NHANES) 2005-2010. Breast Cancer. 2015;22:648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao X, Wu X, Dong J, et al. A meta-analysis of postoperative complications of tissue expander/implant breast reconstruction using acellular dermal matrix. Aesthetic Plast Surg. 2015;39:892–901. [DOI] [PubMed] [Google Scholar]
- 30.Lynch MP, Chung MT, Rinker BD.Dermal autografts as a substitute for acellular dermal matrices (ADM) in tissue expander breast reconstruction: a prospective comparative study. J Plast Reconstr Aesthet Surg. 2013;66:1534–1542. [DOI] [PubMed] [Google Scholar]
- 31.Alderman AK, Wilkins EG, Lowery JC, et al. Determinants of patient satisfaction in postmastectomy breast reconstruction. Plast Reconstr Surg. 2000;106:769–776. [DOI] [PubMed] [Google Scholar]
- 32.Kim MS, Sbalchiero JC, Reece GP, et al. Assessment of breast aesthetics. Plast Reconstr Surg. 2008;121:186e–194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg. 2008;32:418–425. [DOI] [PubMed] [Google Scholar]
- 34.Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg. 2011;128:403e–410e. [DOI] [PubMed] [Google Scholar]
- 35.Hu ES, Pusic AL, Waljee JF, et al. Patient-reported aesthetic satisfaction with breast reconstruction during the long-term survivorship period. Plast Reconstr Surg. 2009;124:1–8. [DOI] [PubMed] [Google Scholar]


