Abstract
Objective
Emerging research demonstrates race differences in diurnal cortisol slope, an indicator of HPA-axis functioning associated with morbidity and mortality, with African Americans showing flatter diurnal slopes than their White counterparts. Sleep characteristics are associated with both race and with HPA-axis functioning. The present report examines whether sleep duration may account for race differences in cortisol dynamics.
Methods
Participants were 424 employed African American and White adults (mean age = 42.8 years, 84.2% White, 53.6% female) with no cardiovascular disease (AHAB-II cohort, University of Pittsburgh). Cortisol slope was calculated using 4 salivary cortisol readings, averaged over each of 4 days. Demographic (age, sex), psychosocial (socioeconomic status, affect, discrimination), and health behaviors (smoking, alcohol use, physical activity) variables were used as covariates, and sleep (self-report and accelerometry) was also assessed.
Results
African Americans had flatter slopes than Whites (F(1, 411) = 10.45, B = .02, p = .001) in models adjusting for demographic, psychosocial, and health behavior covariates. Shorter actigraphy-assessed total sleep time was a second significant predictor of flatter cortisol slopes (F(1, 411) = 25.27, B = −.0002, p < .0001). Total sleep time partially accounted for the relationship between race and diurnal slope [c.i. = .05 (lower = .014, upper .04) or c.i. = .01 (lower = .01, upper .045)].
Conclusions
African Americans have flatter diurnal cortisol slopes than their White counterparts, an effect that may be partially attributable to race differences in nightly sleep duration. Sleep parameters should be considered in further research on race and cortisol.
Keywords: cortisol, race, sleep, hypothalamic-pituitary-adrenocortical -axis, health disparities
Substantial health disparities persist between African Americans and Whites in the U.S., with African Americans experiencing earlier mortality and greater morbidity for a number of disease outcomes (Williams & Mohammed, 2009). Researchers are increasingly interested in race differences in both health behaviors and physiological responses, and how these, in turn, contribute to disproportionate morbidity and mortality among African Americans (Myers, 2009; Pascoe & Smart Richman, 2009; Wyatt et al., 2003). Cortisol, a hormone secreted from the adrenal cortex, is a marker of hypothalamic-pituitary-adrenocortical (HPA) axis activation. Dysregulation of diurnal cortisol rhythm is related to mortality (Kumari, Shipley, Stafford, & Kivimaki, 2011; Sephton, Sapolsky, Kraemer, & Spiegel, 2000). In the past decade, multiple studies investigating race differences in cortisol have reported healthier diurnal cortisol profiles among Whites compared to African Americans (Adam, Hawkley, Kudielka, & Cacioppo, 2006; Cohen et al., 2006; Fuller-Rowell, Doan, & Eccles, 2012; Hajat et al., 2010). Of various indices of diurnal cortisol patterns, racial disparities are most consistent with respect to cortisol slope (Garvin, Eller, & Harris, 2012).
Cortisol fluctuates rhythmically, sharply rising after waking and declining throughout the day (Nicholson, 2008). More sharply declining cortisol slopes are thought to represent well-regulated HPA-axis functioning (Adam & Kumari, 2009), and flatter diunal cortisol slopes are linked to chronic stress and hypocortisolemia (Miller, Cohen, & Ritchey, 2002; Saxbe, 2008). While the mechanistic relations linking flatter cortisol slope and poor health are not fully understood, a growing body of research demonstrates that flatter diurnal cortisol slopes are related to a number of negative health outcomes. Specifically, African American and White adults with a flatter diurnal cortisol slope have more calcification in the coronary arteries, a marker of cardiovascular disease risk (Matthews et al., 2006), and breast cancer patients with flatter slope have earlier mortality (Sephton et al., 2000). More recent research indicates that flatter diurnal cortisol slopes are related to an increased risk of all-cause mortality in nonclinical populations as well, with these deaths being primarily from cardiovascular causes (Kumari et al., 2011).
Multiple studies examining race differences in diurnal cortisol slope have found flatter slopes among African Americans when compared to Whites (Adam et al., 2006; Cohen et al., 2006; Fuller-Rowell et al., 2012; Hajat et al., 2010). Research by Fuller-Rowell and colleagues (2012) demonstrated that race differences persisted when controlling for demographic variables, socioeconomic status (SES), discrimination, and health behaviors (e.g., smoking), with saliva samples collected 4 times a day over the course of 4 days. Cohen and colleagues (2006) also reported that African Americans had significantly flatter slopes when accounting for psychosocial and health behavior variables (e.g., self-reported physical activity and monthly hours of sleep), with salivary cortisol samples collected 6 times a day on 1 day. In the Multi-Ethnic Study of Atherosclerosis, adults provided cortisol samples 6 times a day over the course of 3 days (Hajat et al., 2010). Results indicated that African Americans had flatter slopes than Whites while controlling for SES, hostility, depression, emotional support, chronic burden, body mass index (BMI), smoking status, and self-reported physical activity. Similar race differences in diurnal cortisol slope have been found across the developmental spectrum, including among preadolescent youth (ages 9–12; Martin, Bruce, & Fisher, 2012), adolescents (ages 16–18; DeSantis et al., 2007), young adults (ages 19–22; Skinner, Shirtcliff, Haggerty, Coe, & Catalano, 2011), adults (all around age 32; Adam et al., 2015), and older adults (ages 50–68; Adam et al., 2006). Taken together, the research shows a pattern of race differences in diurnal cortisol and provides evidence that psychosocial factors and health behaviors associate with diurnal slope, but this research has yet to identify whether particular health behaviors (e.g., sleep) may mediate this relationship.
Sleep and HPA-functioning are related (Lutgendorf & Costanzo, 2003), can influence one another (Bucky & Schatzbrg, 2005; Zeiders, Doane, & Adam, 2011), and emerging research suggests that sleep, in particular shorter duration of sleep, may be associated with a flatter cortisol slope the following day (Kumari et al., 2009; Omisade, Buxton, & Rusak, 2010). Lab-based research suggests that shorter sleep time interferes with next-day HPA-recovery and resiliency (Leproult, Copinschi, Buxton, & Van Cauter, 1997), and chronic sleep loss may result in flatter diurnal slopes due to impairments in the negative feedback regulation associated with the HPA-axis (reviewed in Balbo et al., 2010). Given the role of the HPA-axis and glucocorticoid receptors in the regulation of inflammatory and metabolic processes relevant to cardiovascular and other chronic diseases, it is plausible that impaired regulation of this system could, over time, contribute to chronic disease risk (Miller, Rohleder, & Cole, 2009; Miller, et al., 2002). Habitual sleep patterns may contribute to race differences in cortisol slope, as African Americans tend to have less sleep duration and worse sleep quality than Whites (Grandner, Williams, Knutson, Roberts, & Jean-Louis, 2016; Petrov & Lichstein, 2015). Elucidating such relationships could be useful from a theoretical as well as an intervention perspective. Given that sleep is malleable, understanding the role of sleep in cortisol dynamics could inform opportunities for testing risk prevention interventions in a vulnerable population.
Studies assessing race differences in cortisol slope have not found evidence that sleep mediates race differences in cortisol rhythm. However, these studies have either not included assessments of sleep (Fuller-Rowell et al., 2012; Hajat et al., 2010) or have relied on self-report assessments of sleep (Adam et al., 2006; Cohen et al., 2006; DeSantis et al., 2007; Martin et al., 2012; Skinner et al., 2011). Sleep behavior assessed via self-report is subject to estimation heuristics, and as a non-conscious behavior, participants are limited to reporting sleep quality or number of hours slept, estimates with less sensitivity compared to objective measures (Hilliard, 2013; Stone, 2000). Compared to laboratory assessment of sleep, wearable actigraphy devices allow for sleep time assessment in a natural environment (Martin & Hakim, 2011), reduce participant reporting burden (De Weerd, 2014), and emerging research among adolescents and young adults indicates that objective sleep tends to be more predictive of physiological cardiovascular risk than self-reported sleep (Matthews & Pantesco, 2015). We sought to expand previous research on race differences in cortisol slope by employing sleep actigraphy in addition to self-report measures as markers of habitual patterns of sleep.
In the present study, we examined diurnal cortisol slope differences in a sample of 424 African American and White adults. The current work extends existing research in three ways. First, cortisol sampling procedures (i.e., slopes calculated using 4 saliva samples a day over the course of 4 days) were more extensive than in many previous samples, enhancing our ability to capture trait-like characteristics and increasing reliability (Adam & Kumari, 2009). Second, we collected a comprehensive battery of psychosocial measures in order to help us assess the factors potentially accounting for any race-related effects. Third, we supplemented self-report assessment of sleep with monitor-based trait measures of sleep. We predicted a main effect for race such that African Americans would have a flatter cortisol slope than Whites. We also examined factors that might account for this effect, hypothesizing that sleep characteristics, particularly shorter sleep duration, might partially account for race differences in cortisol slope.
Method
Participants
Participants were drawn from the Adult Health and Behavior Project – Phase 2 (AHAB-II), a study of psychosocial, behavioral, and biological risk factors for cardiovascular disease. AHAB-II participants were recruited from the Pittsburgh metropolitan area between March 2008 and October 2011 through mass mailings of recruitment letters to individuals selected from voter registration lists and other public domain lists.
To be eligible to participate in AHAB-II, individuals had to be between the ages of 30 – 54 years and working at least 25 hours per week outside of the home (a substudy involving this cohort was focused on occupational stress). Individuals were excluded from participation if they (a) had a history of cardiovascular disease, schizophrenia or bipolar disorder, chronic hepatitis, renal failure, neurological disorder, lung disease requiring drug treatment, or stage 2 hypertension (SBP/DBP ≥ 160/100 mm Hg); (b) excessively consumed alcohol (≥5 portions 3–4 times per week); (c) used fish-oil supplements (because of the requirements for another substudy); (d) were prescribed medications with autonomic effects or used insulin, glucocorticoid, antiarrhythmic, antihypertensive, lipid-lowering, psychotropic, or prescription weight loss medications; (e) were pregnant; or (f) were shift workers. Of the 530 individuals who were scheduled for monitoring, 36 withdrew or were withdrawn during the monitoring period, yielding 494 participants in the sample (93% monitoring completion rate).
The current analyses included a subsample of 480 individuals who identified as African American or White. An additional 56 participants were not included in final adjusted models because of other missing data (education n = 7; family income n = 7; discrimination n = 13; perceived stress n = 13; negative and positive affect n = 9; smoking status n = 1; monitor-measured physical activity n = 14; self-report sleep n = 7; monitor-measured sleep and wake time or less than 3 days of actigraphy sleep data n = 23). Participants with data missing from the final model reported higher discrimination, worse self-reported sleep, had higher BMI, and were more likely to be African American (ps < .05). There were a total of 424 participants in the final model. Participants were on average 42.8 years of age (SD = 7.30) and the majority of the sample was White (n = 361; 84.2%) and female (n = 230; 53.6%). The study was approved by the University of Pittsburgh Institutional Review Board. Participants provided informed consent and received compensation up to $410, depending on extent of participation in visits and compliance with the protocol.
Procedure
Participants completed seven visits, some of which are not relevant to the current report. Demographic variables, trait psychosocial measures, and trait health behaviors were assessed at Visits 1, 3, and 4. The monitoring period for cortisol, and monitor-assessed physical activity and sleep occurred between Visits 2 and 3, spanning 4 – 8 days. Diurnal cortisol was assessed using best-practices for trait assessment including measuring over the course of several days and providing participants with distinct instructions and procedures to enhance adherence (Adam & Kumari, 2009). Cortisol was measured over 4 days within the 4 – 8 monitoring period, i.e., 3 working days and 1 nonworking day, typically broken into two 2-day monitoring periods, one at the beginning of the work week and another at the end of the work week, with at least one non-monitoring day in between. During each of the 4 cortisol monitoring days, participants collected cortisol samples 5 times a day by mouth using a cotton salivette (Sarstedt, Inc). Participants were prompted to complete samples immediately upon waking, and, again, 30 minutes, 4 hours, and 9 hours after waking and also before bed, following a programmed prompt from a study-issued personal data assistant (PDA). Participants were instructed to gently chew on the cotton swab for approximately 2 minutes and place it into a salivette tube. Following each sampling prompt, the PDA displayed a unique 4-digit code that remained on the screen for 5 minutes. To improve compliance and time stamp each sample, participants were instructed to copy the code onto the salivette label after collection. Participants were then instructed to store the salivettes in their refrigerator prior to returning to the lab. In addition, all participants were instrumented with armband SenseWear monitors for the assessment of physical activity/energy expenditure (Body Media, Pittsburgh, PA; SenseWear Pro3) and watch-based actigraphy devices for the assessment of sleep (Mini-Mitter Inc., Bend, OR; Actiwatch 16) during the period between Visits 2 and 3 (4 – 8 days). Participants also employed other ambulatory assessments not discussed here.
Measures
Cortisol
At the lab, saliva samples were frozen and stored at −20°C until analysis. After thawing, salivettes were centrifuged at 3,000 rpm for 5 min, which resulted in a clear supernatant of low viscosity. Salivary concentrations, expressed as nmol/L, were measured using commercially available chemiluminescence immunoassay with high sensitivity (IBL International, Hamburg, Germany). The intra and interassay coefficients for cortisol were below 8%. Samples that fell below the lowest reliably detected levels (.3 nmol/L) or outliers above 60 nmol/L (determined from examining preliminary distributions) were excluded. Invalid cortisol data included missing samples, out of range cortisol values, and samples for which the timing of assessment could not be determined. Within the sample that completed no more than 4 monitoring days (n = 389), on average, 93.7% of each participant’s samples were successfully collected and available for analysis. Some participants (n = 40) completed more than 4 monitoring days at the request of the researchers because of problems with some of their assessments. After excluding invalid data and outliers, and including extra monitoring, on average 95.1% of each participant’s samples were available for analysis.
Replicating established approaches (see Matthews et al., 2006), the second cortisol value of the day was excluded from calculations to eliminate the influence of the cortisol awakening response on slope calculations, resulting in four samples: waking cortisol (sample 1), 4 hours after waking (sample 2), 9 hours after waking (sample 3), and bedtime (sample 4). Cortisol values were log-transformed and slope was calculated only for those days in which there was a) a valid waking cortisol reading, and b) at least a valid 9-hour (sample 3) or a bedtime (sample 4) cortisol reading (see Matthews et al., 2006). The majority of the sample collected cortisol to allow for slope calculation across 4 or more days (n = 388; 80.83%), and the remaining participants had slope values available across 3 (n = 80; 16.67%) or 2 (n = 12; 2.50%) days. For each day on which the required cortisol readings were available, we regressed the log-transformed cortisol readings on number of minutes since awakening. The resulting slope values were averaged across all available monitoring days. Diurnal cortisol slope declines across the day, therefore more negative numbers (i.e., further from 0) signified a steeper decline in slope.
Demographics
At Visit 1, participants self-reported their age, race/ethnicity, and sex. Participants reported their highest level of education completed, which was coded into four levels: 1 = high school diploma or less, 2 = associate’s degree or some college, 3 = college graduate, 4 = graduate degree. Participants reported their family income across 15 income ranges (1 = less than $10,000 a year to 15 ≥ $185,000 a year), which was adjusted for household size by dividing the midpoint of each range by the number of individuals in the household.
Psychosocial indicators
Participants reported their general experience of discrimination at Visit 3 using the Brief Perceived Ethnic Discrimination Questionnaire – Community Version (Brondolo et al., 2005). The scale consists of 17 items that assess the experience of racial or ethnic discrimination in the participants’ life (e.g., “Because of your ethnicity/race how often…have others hinted that you were dishonest,” with scale responses ranging from 1 = never to 5 = very often). Item responses were summed (α = .92), with higher numbers representing greater perceived discrimination.
Participants reported their general perceived stress at Visit 1 using the Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1983). The scale consists of 10 items that assess the level of stress participants have experienced in the past month (e.g., “In the last month, how often have you found that you could not cope with all the things that you had to do?” with scale responses ranging from 0 = never, to 4 = very often). Item responses were summed (α = .57), with higher numbers representing greater perceived stress.
Mood was assessed using the Positive and Negative Affect Scale (PANAS) at Visit 4 (Thompson, 2007). Positive affect was captured using the 10-item subscale of the PANAS assessing the general mood of the participant (e.g., “Generally, I feel inspired” with scale responses ranging from 1 = very slightly or not at all to 5 = extremely). Negative affect was assessed with the same response scale using items assessing negative mood (e.g., “Generally, I feel upset”). Items responses were summed, with higher numbers signifying greater positive affect (α = .89) or negative affect (α = .88).
Health behaviors
Participants self-reported their smoking status (non-smoker, ex-smoker, current smoker, and other tobacco user; recoded as current smoker 1 vs. 0) and number of drinks of alcohol in the month prior to data collection (note that those drinking 35 or more drinks per week were excluded from the sample). Body mass index (BMI) was calculated based on height and weight measured in the clinic (lbs/inches2 X 703).
Physical activity
Participants self-reported their physical activity for the past week at Visit 1 using the Paffenbarger Physical Activity Questionnaire, a widely used self-report questionnaire with adequate retest reliability (Washburn, Adams, & Haile, 1987) and predictive validity for chronic health outcomes (Paffenbarger, Wing, & Hyde, 1978).
A SenseWear armband monitor captured total energy expenditure during the monitoring period. The SenseWear is a multichannel device, which assesses heat flux, galvanic skin response, skin temperature, and near-body temperature in addition to movement (it contains a 3-axis accelerometer). Software algorithms calculate energy expenditure in metabolic equivalent units (METS) for each minute using pattern recognition algorithms that detect classes of activities (e.g., walking, road bike) as well as body parameters (Johannsen et al., 2010). SenseWear monitors have demonstrated validity for assessing daily total energy expenditure (Johannsen et al., 2010; Mackey et al., 2011; St-Onge, Mignault, Allison, & Rabasa-Lhoret, 2007) and have been shown to assess total energy expenditure more accurately than traditional actigraphy devices (Berntsen et al., 2010). Monitor-measured physical activity was assessed continuously between Visits 2 and 3. During this period, participants wore ambulatory blood pressure (ABP) monitoring cuffs on the majority of their monitoring days, MET values were averaged across each minute across each of the ABP days for which 6 or more hours of physical activity were sampled. Data were excluded on 15 participants who had complete data on fewer than 4 days of assessment.
Sleep and wake time
Sleep was assessed using both participants’ self-reported sleep quality and actigraphy-derived total sleep time. At Visit 6 participants completed the Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989), a widely used and well-validated instrument (Carpenter & Andrykowski, 1998) that assesses sleep quality and disturbance over the past month. The PSQI has good test-retest reliability (r =.85), high internal consistency (Cronbach’s α = .83), and has been shown to distinguish good sleepers from sleep-disordered and depressed patients (Backhaus, Junghanns, Broocks, Riemann, & Hohagen, 2002; Buysse et al., 1989). A global PSQI score > 5 indicates poor sleep quality. General sleep quality was calculated based on the global sleep score, which captures sleep quality, duration, efficiency, disturbances (e.g., snoring), use of sleeping medication and daytime dysfunction.
Monitor-measured sleep was assessed using the Actiwatch-16 (Bend, OR: Philips Electronics). Participants were instructed to wear the watch 24-hours a day for at least 7 days (between Visits 2 and 3; range 1 – 11, mean = 7.01, mode = 7.00, SD = 1.33). Actigraphy data were stored in 1-minute epochs. Data were scored with Actiware software (v5.59) using automated, standard medium thresholds. Sleep onset was defined as a period lasting at least 10 consecutive minutes with activity counts < 40 per epoch. Wake onset was defined as 10 consecutive minutes of ≥ 40 activity counts per epoch and operationalized as seconds past midnight. Total sleep time was operationalized as the total number of minutes of sleep between sleep onset and wake onset, as determined by the Actiware software (i.e., excluding minutes that met threshold to be considered waking activity). Data quality was maintained by trained data managers visually checking the automated sleep epochs, and clarifying any questions about sleep onset and wake time with the participants at equiptment collection visit. The results presented are based on the average total sleep time across all monitoring nights.
Statistical Analyses
Race/ethnicity was recoded into a dummy variable representing African American race (1 vs. 0), and gender was recoded into a dummy variable representing women (1 vs. 0). Race differences in cortisol slope and all covariates were tested using t-tests and chi-square statistics. Multiple linear regressions (PROC GLM) were used to conduct all primary analyses. All models included race (White, African American) as a predictor and were first adjusted for age, sex, and wake time (minimal-adjusted model, Model 1), second, for psychosocial indicators that were associated with significant race differences (i.e., education, household-adjusted family income, discrimination, stress, and positive and negative affect; psychosocial-adjusted model, Model 2), and third, for health behavior indicators that differed significantly by race (i.e., BMI, alcohol use, smoking, self-report and monitor-measured physical activity and self-report and monitor-measured sleep; health behavior/full-adjusted model, Model 3). Models were additive, with Models 2 and 3 including all covariates from preceding models. Analyses were conducted using SAS statistical software (version 9.3; SAS Institute, Cary, NC). In follow up analyses, we employed RMediation (Tofighi & MacKinnon, 2011) to examine whether any psychosocial or health behavior covariates might be putative mediators of race differences in diurnal cortisol slope (p < .05). All inferential statistics were run using observations represented in the final model (n = 424).
Results
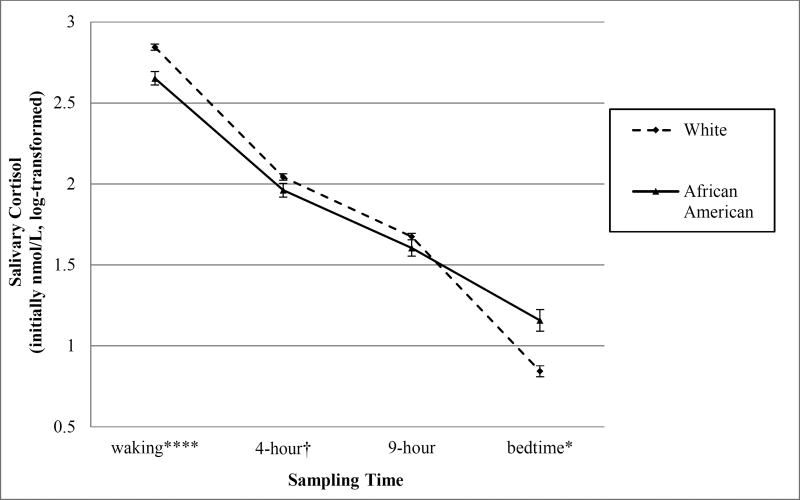
The average cortisol slope was −.12 (SD = .04) over the course of the averaged monitoring days. Whites had significantly steeper cortisol declines than African Americans (see Table 1). To further examine the race differences in diurnal slope, we ran t-tests comparing composite cortisol (nmol/L) levels from each sampling time of day. Results indicated that Whites had significantly higher waking cortisol and marginally higher 4-hour cortisol levels than African Americans. There were no significant race differences in 9-hour cortisol levels and African Americans had significantly higher bedtime cortisol levels than Whites (see Table 1, Figure 1).1
Table 1.
Characteristics of study population and race differences.
| Variable Mean (Standard Deviation) |
Total sample (N = 480) |
White (82.7%, n = 397) |
African American (17.3%, n = 83) |
|---|---|---|---|
|
|
|||
| Diurnal Cortisol Slope (Δ nmol/L/min) | −0.12 (.04) | −0.13 (.04)*** | −0.10 (.04)*** |
| Wake time (seconds after midnight)a | 23467.3 (4180.04) | 23323.3 (3902.90) | 24198.9 (4988.80) |
| Age (years) | 42.8 (7.33) | 42.4 (7.38)** | 44.7 (6.83)** |
| Female (n, %) | 255 (53.1%) | 200 (78.4%)** | 55 (21.6%)** |
| Educationab | 2.96 (.95) | 3.1 (.89)*** | 2.3 (.95)*** |
| Household-adjusted Family Incomea | 33,842.02 (23,715.86) | 35,725.0 (26,346.4)*** | 24,835.3 (19919.3)*** |
| Discriminationa | 21.5 (7.10) | 19.6 (4.85)*** | 30.8 (8.55)*** |
| Perceived Stressa | 15.8 (4.00) | 15.6 (4.01) | 16.4 (3.95) |
| Negative Affecta | 15.5 (5.09) | 15.6 (5.00)† | 14.6 (5.47)† |
| Positive Affecta | 34.1 (5.92) | 33.9 (5.88) | 34.8 (6.12) |
| BMI (kg/m2) | 27.0 (5.26) | 26.4 (4.95)*** | 29.7 (5.88)*** |
| Alcohol (drinks past month) | 11.6 (17.64) | 12.2 (16.79) | 8.8 (21.15) |
| Current smoker (n, %)a | 75 (15.7%) | 63 (15.6%)* | 20 (26.7%)* |
| Physical Activity Self- report: Paffenbargher | 2845.7 (2242.20) | 2851.8 (2073.40) | 2816.9 (2933.70) |
| Physical Activity Monitor- measured: SenseWear (METS)a | 2.1 (.42) | 2.09 (.42)* | 2.20 (.45)* |
| Sleep Self-report: Pittsburgh Sleep Quality Indexa | 5.0 (2.68) | 4.9 (2.56)* | 5.8 (3.08)* |
| Sleep Monitor-measured: Actiwatch (total sleep time min.) | 356.8 (52.85) | 360.2 (49.95)** | 339.5 (63.21)** |
| Cortisol 1, awakening (nmoL)d | 17.8 (6.33) | 18.3 (6.31)*** | 15.2 (5.75)*** |
| Cortisol 2, 4-hour (nmoL)d | 8.2 (3.66) | 8.4 (3.80)† | 7.6 (2.87)† |
| Cortisol 3, 9-hour (nmoL)d | 5.8 (2.59) | 5.8 (2.61) | 5.5 (2.48) |
| Cortisol 4, bed (nmoL)d | 3.2 (2.82) | 3.0 (2.84)* | 3.8 (2.64)* |
p < .1;
p < .05;
p < .01;
p < .0001
n < 480
Highest level of education, continuous (1 = high school diploma or less to 4 = graduate degree).
Independent sample t-tests were run on log-transformed variables, but non-logged means and standard deviations are presented here to aid in interpretability.
Figure 1.
Time-sampled cortisol values by race.
† p < .1; * p < .05; ** p < .01; *** p < .001; **** p < .0001
To examine variables that could help to explain the race differences in slope, we explored race differences for all potential psychosocial and health behavior variables. Results demonstrated that the African Americans in the sample were significantly less educated, had lower household-adjusted family income, reported greater discrimination, had higher BMI, included a higher proportion of smokers, exhibited greater monitor-measured physical activity, worse self-report sleep quality, and less monitor-measured total sleep time (all ps < .05, see Table 1); all of these variables were included in further analyses. There were no significant race differences in perceived stress, positive or negative affect, alcohol use, or self-report physical activity (ps > .05, see Table 1), and these variables were not included in additional analyses.
We examined whether the significant race difference in slope was accounted for by race differences in demographic variables, psychosocial constructs, or health behaviors. Race differences emerged in the minimal-adjusted model (Model 1), with flatter slopes among African Americans (F(1, 419) = 18.34, B = .02, p < .0001) when controlling for age, sex, and wake time (see Table 2). In the psychosocial-adjusted model (Model 2), race differences persisted, with African Americans having a flatter slope than Whites (F(1, 416) = 10.69, B = .02, p = .001) when including education, household-adjusted family income, and discrimination in the model2. Race differences remained in the health behavior/full–adjusted model (F(1, 411) = 10.45, B = .02, p < .0001), and monitor-measured total sleep time emerged as a significant predictor of steeper cortisol slope (F(1, 411) = 25.27, B = −.0002, p < .0001), independent of race and accounting for an additional 5.08% of the variance in cortisol slope (see Table 2)3.
Table 2.
Parameter estimates, standard errors, and significance levels for models predicting flatter (less steep) diurnal cortisol slope (N = 424).
| Variable | Model 1 Minimal Adjusted Model |
Model 2 Psychosocial Adjusted Model |
Model 3 Health Behavior/ Full Adjusted Model |
|---|---|---|---|
| R2 | .06 | .07 | .13 |
|
| |||
| Wake time (seconds after midnight) | −.00001 (.000001) p = .59 | −.00001 (.000001) p = .58 | −.00001 (.000001) p = .40 |
| Age (years) | .0005 (.0003) p = .09 | .0005 (.0003) p = .10 | .0005 (.0003) p = .11 |
| Sex (Male = 0; Female = 1) | .005 (.004) p = .21 | .005 (.004) p = .23 | .009 (.005) p = .07 |
| Race (White = 0; African American = 1) | .024 (.006) p < .0001 | .023 (.007) p = .001 | .023 (.007) p = .001 |
| Educationa | −.0006 (.002) p = .80 | .002 (.002) p = .54 | |
| Household-adjusted Family Income | −.00001 (.000001) p = .93 | −.00001 (.000001) p = .99 | |
| Discrimination | .00005 (.0004) p = .90 | −.0003 (.0004) p = .51 | |
| BMI(kg/m2) | −.0005 (.0004) p = .27 | ||
| Smoking status (0 = non smoker; 1 = current smoker) | .01 (.006) p = .10 | ||
| Physical Activity Monitor-measured: SenseWear (METS) | −.002 (.006) p = .82 | ||
| Sleep Self-report: Pittsburgh Sleep Quality Index | .0009 (.0008) p = .27 | ||
| Sleep Monitor-measured: Actiwatch (total sleep time in min) | −.0002 (.00004) p < .0001 | ||
Note. Diurnal cortisol slope (sample M = −.12, SD = .04) declines across the day, therefore more negative numbers (i.e., further from 0) signify steeper slope.
Highest level of education, continuous (1 = high school diploma or less to 4 = graduate degree).
We investigated whether monitor-measured total sleep time accounted for the relationship between race and cortisol slope. We used RMediation open-source software, which allows for distribution-of-the-product mediation testing of models with significantly related predictor (race) and mediator (monitor-measured total sleep time) variables, given that monitor-measured total sleep time was higher among Whites in the sample. For the effect of race on sleep (â = −26.66; SE = 6.46) and sleep on slope when controlling for race (b̂ = −.001; .0001), the indirect effect estimate was 0.027 (SE = 0.007). The distribution of the product of coefficients did not contain 0 at either the .05 (lower = .014, upper .04) or .01 (lower = .01, upper .045) alpha levels. These results indicate that flatter cortisol slope among African Americans can be partially accounted for by race differences in total sleep time4. Sleep time explained 21.52% of the variance in the effects of race on cortisol slope5.
Several exploratory analyses were conducted. First, we conducted several analyses substituting actigraphy-assessed measures of sleep quality for total sleep time. Analyses were conducted examining whether actigraphy-assessed sleep efficiency (the proportion of total time asleep between going to bed to fall asleep and waking up), sleep latency (number of minutes between going to bed and falling asleep), or waking-after-sleep onset (number of minutes awake after falling asleep and before waking up) were associated with cortisol slope. Analyses revealed that none of these indices were significantly associated with cortisol slope (ps > .23). Second, we examined whether self-report sleep minutes (derived as minutes between bed and wake time self-reported by the participant each morning) averaged over the nights of the monitoring period associated with cortisol slope. The main models were reanalyzed substituting self-report total sleep time for actigraphy-assessed total sleep time. Analyses revealed that self-report sleep time did not significantly associate with cortisol slope (p = .24).
Discussion
Findings from the current study support the hypothesis that African Americans have flatter diurnal cortisol slopes when compared to their White counterparts, and these race differences persisted when controlling for trait psychosocial variables, health behaviors, and objective measures of sleep and physical activity. African Americans’ flatter cortisol slope was partially explained by race differences in actigraphy-assessed total sleep time. We did not find evidence that any other psychosocial indicators, self-reported health behaviors, or monitor-measured physical activity explained the race differences in cortisol slope. This is the first study showing that not only do healthy individuals showing smaller total sleep times in the natural environment tend to show flatter diurnal slopes, but that race differences in slope may also be explained, in part, by such effects.
The current study examined race differences in cortisol functioning using comprehensive measures to assess trait cortisol slope, as well as controlling for wake time, theoretically-relevant psychosocial covariates, and diverse measures of health behavior. The results are consistent with prior research demonstrating race differences in cortisol slope (Adam et al., 2016; Adam et al., 2006; Cohen et al., 2006; Fuller-Rowell et al., 2012; Hajat et al., 2010). Additionally the results replicate previous research demonstrating African Americans’ lower waking cortisol levels (Cohen et al., 2006; DeSantis et al., 2007; Fuller-Rowell et al., 2012; Hajat et al., 2010; Martin et al., 2012; Skinner et al., 2011), and higher end-of-day cortisol levels (Cohen et al., 2006; DeSantis et al., 2007; Fuller-Rowell et al., 2012; Skinner et al., 2011).
One important finding of the present research is that monitor-measured total sleep time was predictive of flatter slope in the sample as a whole. Little research has investigated associations between individual differences in sleep characteristics and cortisol slope, but research that has emerged suggests that shorter sleep duration, rather than worse sleep quality, may blunt cortisol slope. Some studies failed to find a relationship between sleep characteristics and cortisol indices (reviewed in Balbo et al., 2010), but our finding that shorter total sleep time associates with a flatter diurnal slope is consistent with laboratory and observation studies. Acute sleep restriction has been shown to induce a flatter decline in cortisol throughout the following day (Omisade et al., 2010) and both self-reported and actigraphy-based shorter sleep duration have been linked to flatter diurnal slope (Hansen et al., 2012; Kumari et al., 2009), with one study reporting the effect of shorter sleep persisting after controlling for subjective sleep difficulties (Castro-Diehl et al., 2015). Sleep deprivation also appears to elevate nocturnal cortisol levels over time, suggesting that chronic sleep loss may lead to impairments in the regulatory feedback loop of the HPA-axis, contributing to a flatter diurnal slope (Balbo et al., 2010). Because the HPA-axis and sleep can also influence each other reciprocally (Buckley & Schatzberg, 2005) as evidenced in both ecological (Zeiders et al., 2011) and lab-based (Spath-Scwalbe et al., 1991) studies, future research should further explore the bidirectional nature of cortisol dynamics and total sleep time.
We found no evidence that psychosocial factors explain race differences in cortisol slope. Despite African Americans reporting greater discrimination than Whites, individual differences in perceived discrimination neither predicted slope nor accounted for race differences. These null effects are similar to one previous investigation that showed no relationship between discrimination and cortisol slope among a sample of Whites and African Americans (Cohen et al., 2006), and contrast with findings of another study showing that greater discrimination (measured as non-attributed unfair treatment) was associated with steeper cortisol slopes among older adult African Americans and flatter cortisol slopes among older adult Whites (Fuller-Rowell et al., 2012). Part of the inconsistency in results linking discrimination and diurnal cortisol among African Americans may stem from the static timeframe through which discrimination has been investigated. Recent research suggests that discrimination experienced among African Americans in adolescence is associated with flatter slopes in adulthood (Adam et al., 2015), providing evidence for life-course effects or critical developmental periods. Given the strong evidence of our study and others showing consistent race differences in slope (Adam et al., 2015; Adam et al., 2006; Cohen et al., 2006; Fuller-Rowell et al., 2012; Hajat et al., 2010), it would be beneficial for future research to consider life-course perspectives while investigating psychosocial or health behavior mechanisms linking race with diurnal cortisol slope.
The present findings suggest that total sleep time partially accounts for race differences in diurnal cortisol. African Americans in the sample experienced less sleep time when compared to Whites, and this replicates a growing literature demonstrating that African Americans tend to have worse sleep than other racial groups (Grandner et al., 2016; Petrov & Lichstein, 2015). This disparity in sleep takes on increased importance given that Africans Americans in the present sample had flatter cortisol slopes, which the current data suggest might be partially attributable to shorter sleep duration. While African Americans often self-report same or fewer sleep complaints than Whites, African Americans tend to have less total sleep time and less sleep efficiency (Petrov & Lichstein, 2015). Our findings demonstrated race differences in both subjective and objective sleep measures, however objectively recorded total sleep time, rather than self-report or objective sleep quality, partially accounted for differences in cortisol slope. These findings parallel emerging research suggesting that shorter actigraphy-assessed sleep duration, rather than subjective or actigraphy-assessed sleep quality, associate with inflammation biomarkers (e.g., C-reactive protein; Park et al., 2016). While the explanatory factors regarding race differences in objective sleep are not clearly understood, Petrov and Lichstein (2015) provide a framework that includes possible biological (e.g., circadian period), psycho-behavioral (e.g., perceived stress, attitudes towards disordered sleep), as well as sociocultural (e.g., occupational, differences in childhood adversity) mediators that may explain race differences in sleep.
Limitations
There are a number of limitations associated with the current report. First, although the monitoring procedures in this study spanned a number of days, the analysis is cross-sectional. More research is needed to determine if less sleep in African Americans, relative to Whites, precedes differences in diurnal cortisol functioning. Second, and relatedly, the indirect effects analyses do not warrant causal interpretation. Third, the effect of sleep is significant and explains 21.52% of the variance in the effects of race on cortisol slope, but other unmeasured factors could account for fluctuations in cortisol rhythm. Future research should consider race differences in environmental (e.g., noise), neighborhood (e.g., exposure to violence, community stability; Skinner et al., 2011), other sleep-related (e.g., prevalence of sleep apnea syndrome; Chen et al., 2015) variables, racism vigilance (anticipatory stress; Hicken, Lee, Morenoff, House, & Williams, 2014), or evolutionary/life history biological HPA-axis functioning (see the Adaptive Callibration Model of stress responsivity; Del Giudice, Ellis, & Shirtcliff, 2011) as potential contributers to race differences in cortisol dynamics.
Study variables in the present investigation were assessed concurrently, though recent research linking discrimination in adolescence to flatter cortisol slope in adulthood (Adam et al., 2015) may suggest that sleep time captured during critical developmental periods (e.g. Zeiders et al., 2010) could account for an even larger amount of racial differences in adult cortisol slope. Future research would benefit from investigating total sleep time both concurrently and in earlier periods of development. Fourth, our sample included fewer African Americans than Whites, and power is constrained by the smaller group. Fifth, African Americans and Whites were generally well-educated, and participants who were not working or who worked overnight/shift-work schedules were excluded. Future research should capture sleep-related breathing disorders and attempt to replicate these results in larger samples, with a wider and more balanced range of socioeconomic, ethnic, and occupational status representation.
Implications/Future Research
This study lends additional support to the body of literature demonstrating race differences in diurnal cortisol slope, a marker of HPA-axis functioning. Findings here are also consistent with a small literature linking sleep duration and diurnal cortisol slope. Finally, this research uncovered a health behavior that may partially account for race differences in cortisol slope. Replication with lifespan data collection may help to determine whether race disparities in total sleep time influence future cortisol slope, or if cortisol slope influences future sleep disparities, as emerging research provides evidence of the bidirectional relationship of cortisol slope and sleep (e.g., Zeiders et al., 2011). Additionally, research on cortisol and race needs to be expanded to include participants of other races as well as multiracial participants. Sleep parameters should be considered in research on diurnal cortisol functioning, and research aimed at eliminating racial health disparities should further investigate the clinical significance of the influence of total sleep time on HPA-dynamics and its modification as a potential target for health behavior interventions.
Acknowledgments
The authors would like to acknowledge Nathan Pugh for his assistance with data analysis and Maeve Malloy and Janet Monroe for their comments on earlier drafts.
Support: National Institutes of Health Grants P01 HL040962 and T32 HL007560.
Footnotes
Independent sample t-tests were run comparing the log-transformed cortisol values. Functional results (i.e., significant predictors and direction of prediction) did not differ when we repeated these analyses while controlling for age, sex, and wake time, and, therefore, we report the means from the t-tests.
We conducted analyses to investigate whether discrimination relationships with cortisol slope were moderated by race. Analyses revealed that race did not moderate the discrimination and cortisol slope relationship (p = .91); stratifying the models revealed that discrimination did not have a significant relationship with cortisol slope for either the African American or the White subsample (ps > .24).
We conducted analyses to investigate whether to collapse cortisol slope measures across work days and non-work days. Cortisol slope did not significantly differ within participants on work days versus non-work days, and there was no significant day type × race interaction on cortisol slope (ps > .30). The main models reported here were reanalyzed examining the main effect of demographic, psychosocial, and health behavior variables on both work day cortisol slope and non-work day cortisol slope. Functional results did not differ for work days and non-work days and thus, slope calculations were collapsed across all four days for the reported analyses.
We conducted several supplemental analyses in regards to sleep. First, we examined whether the amount of available actigraphy sleep data may have influenced the results. The main models were reanalyzed excluding participants who had 1) less than 7 and 2) less than 5 days of actigraphy. Functional results of the model did not differ when participants with fewer days of actigraphy were excluded from the analyses, and, therefore, we did not exclude participants on the basis of missing actigraphy data. Second, we conducted analyses examining whether bedtime may have influenced the results. The main models were reanalyzed controlling for bedtime in addition to waketime and substituting bedtime for waketime; bedtime did not emerge as significantly associated with diurnal slope (ps > .57). Bedtime and waketime were correlated (p < .0001), and therefore we included only waketime in the model to reduce the number of covariates. Finally, we investigated whether chronotype (Morningness-Eveningness Questionnaire; Horne & Ostberg, 1976) was associated with cortisol slope when included in the model. Chronotype was not significantly associated with cortisol slope (p = .27).
We conducted supplemental analyses investigating whether race differences emerged for alternative diurnal cortisol indices, specifically area-under-the-curve and awakening response. Replicating the main models revealed that alternative cortisol indices did not significantly vary by race (ps > .28).
Contributor Information
Laurel M. Peterson, Department of Psychology, Bryn Mawr College, lmpeterson@brynmawr.edu.
Karissa G. Miller, Department of Psychology, University of Pittsburgh, kgm18@pitt.edu
Patricia M. Wong, Department of Psychology, University of Pittsburgh, paw43@pitt.edu
Barbara A. Anderson, Department of Psychology, University of Pittsburgh, baa1@pitt.edu.
Thomas W. Kamarck, Departments of Psychology and Psychiatry, University of Pittsburgh, tkam@pitt.edu.
Karen A. Matthews, Department of Psychiatry, University of Pittsburgh School of Medicine; Department of Epidemiology, University of Pittsburgh Graduate School of Public Health; Department of Psychology, University of Pittsburgh, MatthewsKA@upmc.edu.
Clemens Kirschbaum, Department of Psychology, Technical University, clemens.kirschbaum@tu-dresden.de.
Stephen B. Manuck, Department of Psychology, University of Pittsburgh, manuck@pitt.edu.
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences. 2006;103(45):17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Heissel JA, Zeiders KH, Richeson JA, Ross EC, Ehrlich KB, Eccles JS. Developmental histories of perceived racial discrimination and diurnal cortisol profiles in adulthood: A 20-year prospective study. Psychoneuroendocrinology. 2015;62:279–291. doi: 10.1016/j.psyneuen.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34(10):1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. Journal of Psychosomatic Research. 2002;53(3):737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Balbo M, Leproult R, Van Cauter E, Balbo M, Leproult R, Van Cauter E. Impact of sleep and its disturbances on Hypothalamo-Pituitary-Adrenal Axis activity. International Journal of Endocrinology. 2010:e759234. doi: 10.1155/2010/759234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsen S, Hageberg R, Aandstad A, Mowinckel P, Anderssen SA, Carlsen K-H, Andersen LB. Validity of physical activity monitors in adults participating in free-living activities. British Journal of Sports Medicine. 2010;44(9):657–664. doi: 10.1136/bjsm.2008.048868. [DOI] [PubMed] [Google Scholar]
- Brondolo E, Kelly KP, Coakley V, Gordon T, Thompson S, Levy E, Contrada RJ. The Perceived Ethnic Discrimination Questionnaire: Development and preliminary validation of a community version. Journal of Applied Social Psychology. 2005;35(2):335–365. [Google Scholar]
- Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: Normal HPA axis activity and circadian rhythm, exemplary sleep disorders. The Journal of Clinical Endocrinology & Metabolism. 2005;90(5):3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ., III The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. Journal of Psychosomatic Research. 1998;45(1):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- Castro-Diehl C, Roux AVD, Redline S, Seeman T, Schrager S, Shea S. Association of sleep duration and quality with alterations in the hypothalamic-pituitary adrenocortical axis: the Multi-Ethnic Study of Atherosclerosis (MESA) The Journal of Clinical Endocrinology & Metabolism. 2015;100(8):3149–3158. doi: 10.1210/jc.2015-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, Redline S. Racial/ethnic differences in sleep disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38(6):877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosomatic Medicine. 2006;68(1):41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neuroscience and Biobehavioral Reviews. 2011;35(7):1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. The Journal of Adolescent Health. 2007;41(1):3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- De Weerd AW. Actigraphy, the alternative way? Frontiers in Psychiatry. 2014;5 doi: 10.3389/fpsyt.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Rowell TE, Doan SN, Eccles JS. Differential effects of perceived discrimination on the diurnal cortisol rhythm of African Americans and Whites. Psychoneuroendocrinology. 2012;37(1):107–118. doi: 10.1016/j.psyneuen.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin P, Eller NH, Harris A. In: The Role of Saliva Cortisol Measurement in Health and Disease. Kristenson M, Garvin P, Lundberg U, editors. Bentham Science Publishers; 2012. pp. 17–42. [Google Scholar]
- Grandner MA, Williams NJ, Knutson KL, Roberts D, Jean-Louis G. Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Medicine. 2016;18:7–18. doi: 10.1016/j.sleep.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux A, Franklin TG, Seeman T, Shrager S, Ranjit N, Kirschbaum C. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2010;35(6):932–943. doi: 10.1016/j.psyneuen.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen ÅM, Thomsen JF, Kaergaard A, Kolstad HA, Kaerlev L, Mors O, Mikkelsen S. Salivary cortisol and sleep problems among civil servants. Psychoneuroendocrinology. 2012;37(7):1086–1095. doi: 10.1016/j.psyneuen.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Hicken MT, Lee H, Morenoff J, House JS, Williams DR. Racial/ethnic disparities in hypertension prevalence: Reconsidering the role of chronic stress. American Journal of Public Health. 2014;104(1):117–123. doi: 10.2105/AJPH.2013.301395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M. Principles of health behavior measurement. In: Riekert KA, Ockene JK, Pbert L, editors. The handbook of health behavior change. 4. New York, NY: Springer Publishing; 2013. pp. 465–482. [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4:97–110. [PubMed] [Google Scholar]
- Johannsen DL, Calabro MA, Stewart J, Franke W, Rood JC, Welk GJ. Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Medicine & Science in Sports & Exercise. 2010;42(11):2134–2140. doi: 10.1249/MSS.0b013e3181e0b3ff. [DOI] [PubMed] [Google Scholar]
- Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. The Journal of Clinical Endocrinology and Metabolism. 2009;94:4801–4809. doi: 10.1210/jc.2009-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Shipley M, Stafford M, Kivimaki M. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: Findings from the Whitehall II study. The Journal of Clinical Endocrinology and Metabolism. 2011;96(5):1478–1485. doi: 10.1210/jc.2010-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leproult R, Copinschi G, Buxton OM, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20(10):865–870. [PubMed] [Google Scholar]
- Lutgendorf SK, Costanzo ES. Psychoneuroimmunology and health psychology: An integrative model. Brain, Behavior, and Immunity. 2003;17(4):225–232. doi: 10.1016/s0889-1591(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Mackey DC, Manini TM, Schoeller DA, Koster A, Glynn NW, Goodpaster BH, Cummings SR. Validation of an armband to measure daily energy expenditure in older adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66(10):1108–1113. doi: 10.1093/gerona/glr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CG, Bruce J, Fisher PA. Racial and ethnic differences in diurnal cortisol rhythms in preadolescents: The role of parental psychosocial risk and monitoring. Hormones and Behavior. 2012;61(5):661–668. doi: 10.1016/j.yhbeh.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514–1527. doi: 10.1378/chest.10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Pantesco EJ. Sleep characteristics and cardiovascular risk in children and adolescents: An enumerative review. Sleep Medicine. 2016;18:36–49. doi: 10.1016/j.sleep.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA Study. Psychosomatic Medicine. 2006;68(5):657–661. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology. 2002;21(6):531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Miller G, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro-and anti-inflammatory signaling pathways six months later. Psychosomatic Medicine. 2009;71:57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers HF. Ethnicity- and socio-economic status-related stresses in context: An integrative review and conceptual model. Journal of Behavioral Medicine. 2009;32(1):9–19. doi: 10.1007/s10865-008-9181-4. [DOI] [PubMed] [Google Scholar]
- Nicolson NA. Measurement of cortisol. In: Luecken LJ, Gallo LC, editors. Handbook of physiological research methods in health psychology. Los Angelos, CA: Sage Publications; 2008. pp. 37–74. [Google Scholar]
- Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiology & Behavior. 2010;99(5):651–656. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. American Journal of Epidemiology. 1978;108(3):161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- Park H, Tsai KM, Dahl RE, Irwin MR, McCreath H, Seeman TE, Fuligni AJ. Sleep and inflammation during adolescence. Psychosomatic Medicine. 2016;78:677–685. doi: 10.1097/PSY.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe EA, Smart Richman L. Perceived discrimination and health: A meta-analytic review. Psychological Bulletin. 2009;135(4):531–554. doi: 10.1037/a0016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov ME, Lichstein KL. Differences in sleep between Black and White adults: An update and future directions. Sleep Medicine. 2015;18:74–81. doi: 10.1016/j.sleep.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute. 2000;92(12):994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Saxbe DE. A field (researcher’s) guide to cortisol: Tracking HPA axis functioning in everyday life. Health Psychology Review. 2008;2(2):163–190. [Google Scholar]
- Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, Catalano RF. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Development and Psychopathology. 2011;23(4):1167–1186. doi: 10.1017/S095457941100054X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Späth-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biological Psychiatry. 1991;29(6):575–584. doi: 10.1016/0006-3223(91)90093-2. [DOI] [PubMed] [Google Scholar]
- Stone AA. Self-reporting of physical symptoms. In: Stone AA, Turkkan JS, Bachrach CA, Jobe JB, Kurtzman HS, Cain VS, editors. The science of self-report: Implications for research and practice. Mahwah, NJ: Lawrence Erlbaum; 2000. pp. 297–299. [Google Scholar]
- St-Onge M, Mignault D, Allison DB, Rabasa-Lhoret R. Evaluation of a portable device to measure daily energy expenditure in free-living adults. The American Journal of Clinical Nutrition. 2007;85(3):742–749. doi: 10.1093/ajcn/85.3.742. [DOI] [PubMed] [Google Scholar]
- Thompson ER. Development and validation of an internationally reliable short-form of the Positive and Negative Affect Schedule (PANAS) Journal of Cross-Cultural Psychology. 2007;38(2):227–242. [Google Scholar]
- Tofighi D, MacKinnon DP. RMediation: An R package for mediation analysis confidence intervals. Behavior Research Methods. 2011;43(3):692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn RA, Adams LL, Haile GT. Physical activity assessment for epidemiologic research: The utility of two simplified approaches. Preventive Medicine. 1987;16(5):636–646. doi: 10.1016/0091-7435(87)90047-8. [DOI] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA. Discrimination and racial disparities in health: Evidence and needed research. Journal of Behavioral Medicine. 2009;32(1):20–47. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt SB, Williams DR, Calvin R, Henderson FC, Walker ER, Winters K. Racism and cardiovascular disease in African Americans. The American Journal Of The Medical Sciences. 2003;325(6):315–331. doi: 10.1097/00000441-200306000-00003. [DOI] [PubMed] [Google Scholar]
- Zeiders KH, Doane LD, Adam EK. Reciprocal relations between objectively measured sleep patterns and diurnal cortisol rhythms in late adolescence. Journal of Adolescent Health. 2011;48(6):566–571. doi: 10.1016/j.jadohealth.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]