Abstract
There is increasing evidence that growth hormone (GH) and insulin-like growth factor 1 (IGF-1) signaling (collectively referred to as somatotropic signaling) during development has a profound influence on aging and longevity. Moreover, the absence of GH action was shown to modify responses of adult mice to calorie restriction (CR) and other antiaging interventions. It was therefore of interest to determine whether GH resistance in GH receptor knockout (GHR-KO) mice would modify the effects of mild pre-weaning CR imposed by increasing the number of pups in a litter (the so-called litter crowding). In addition to the expected impact on body weight, litter crowding affected glucose homeostasis, hepatic expression of IGF-1 and genes related to lipid metabolism, and expression of inflammatory markers in white adipose tissue, with some of these effects persisting until the age of 2 years. Litter crowding failed to further extend the remarkable longevity of GHR-KO mice and, instead, reduced late life survival of GHR-KO females, an effect opposite to the changes detected in normal animals. We conclude that the absence of GH actions alters the responses to pre-weaning CR and prevents this intervention from extending longevity.
Keywords: GHR-KO, Growth hormone, Litter crowding, Aging
Introduction
The biological basis of aging is not “programmed” in the sense of being driven by evolutionarily selected genes that promote aging (reviewed in Bartke et al. 2016). However, there is increasing evidence that early-life events can shape the trajectory of aging and influence longevity. Both undernutrition and overnutrition during pregnancy can profoundly alter adult metabolic phenotypes, risk of various chronic diseases (particularly cardiovascular disease and diabetes), and longevity (Barker et al. 1993; Beauchamp et al. 2015; Berends et al. 2013; Blackmore and Ozanne 2013; Blackmore et al. 2012; Petry et al. 2001). In animals with genetic defects in endocrine function, hormonal replacement therapy during the early postnatal development period can alter longevity (Bartke et al. 2016; Panici et al. 2010; Podlutsky et al. 2017; Sonntag et al. 2005; Tarantini et al. 2016). Alterations in nutrient availability during the pre-weaning period produced by manipulating litter size can also influence adult phenotypic characteristics related to age-related disease and aging (Jennings et al. 1999; Martin-Gronert et al. 2008). Of particular interest was the report that mild restriction of nutrient availability to suckling pups produced by adding extra pups to a litter (so-called “litter crowding”) resulted in significant extension of longevity (Sun et al. 2009).
Mice with growth hormone (GH) resistance produced by targeted disruption of the GH receptor/binding protein gene (GHR/GHBP-KO, hereafter referred to as GHR-KO mice) (Zhou et al. 1997) are characterized by normal prenatal growth, major retardation of postnatal growth and adult body size, and a major extension of longevity (Coschigano et al. 2003). These animals thrive under a standard feeding regimen, but as adults fail to benefit from a regimen of calorie restriction (CR) that significantly extends longevity of their normal siblings (Bonkowski et al. 2009; Bonkowski et al. 2006). It was therefore of interest to determine if the longevity of GHR-KO mice is affected by a mild pre-weaning nutrient restriction produced by litter crowding. Here, we demonstrate that pre-weaning nutrient restriction does not affect longevity of male GHR-KO mice, whereas it shortens longevity in female GHR-KO mice (in contrast to the extension of longevity observed in their normal female littermates). We also show that body weight as well as gene expression of hepatic IGF-1 and inflammatory markers in white adipose tissue are altered, even after 2 years since the mild CR produced by litter crowding.
Materials and methods
Animals
GHR-KO mice were produced by mating homozygous mutant males with heterozygous females. Siblings of GHR-KO mice are therefore phenotypically normal and are used as controls. Normal and GHR-KO mice used in the present study were originally derived from animals generated by the Kopchick laboratory from 129Ola embryonic stem cells and include genetic contribution from BALB/c, C57BL/6, and C3H/J inbred strains. In our closed breeding colony, we avoided brother × sister mating. The relative contribution of genetic material from the contributing strains and the degree of heterozygosity in our colony of GHR-KO mice were assessed in our earlier study (Panici et al. 2009). Both GHR-KO and normal mice are weaned at the age of 21 days and are subsequently housed at five mice per cage with ad libitum access to water and food (LabDiet 5001, with 13% calories from fat, 29% calories from protein, and 56% calories from carbohydrate) in a room with controlled temperature (20–23 °C) and illumination (12 h light: 12 h dark).
In the current study, litter size was adjusted within the first day of birth by adding pups from another litter or (rarely) by removing pups to produce either “small” (S) litters consisting of eight pups (i.e., the number of pups identical to the size of S litters in the study of Sun et al. (Sun et al. 2009) or “large” (L) litters consisting of 12 pups. The mice set aside for longevity determination were not subjected to any invasive procedures and were undisturbed except for occasional body weight measurements. These animals were checked daily, and the dates of deaths were recorded. Animals that appeared moribund (non-reactive, cold to touch) or in distress (e.g., bleeding from tumors) were killed, and the date of euthanasia was considered as the date of death. We believe this procedure did not introduce significant bias to the data because nearly all of the animals died from natural causes, and those that were killed were very unlikely to survive longer than a few days. Some of the mice were killed at the age of 21 days for measurements of gene expression or subjected to various tests outlined below, and others were killed at the age of approximately 2 years for collection of organs for gene expression analysis (these mice were not included in the longevity analysis). All animal protocols were approved by the Southern Illinois University School of Medicine Animal Care and Use Committee.
Glucose and insulin tolerance tests
Glucose and insulin tolerance tests were performed as previously described (Bonkowski et al. 2006; Darcy et al. 2016a; Fang et al. 2013). For a glucose tolerance test (GTT), mice were fasted overnight for 16 h. Following an initial blood glucose measurement from the tail vein (time 0), glucose was injected intraperitoneally (i.p.) at a dose of 1 g per kg of body weight. Sequential glucose measurements were taken at 15, 30, 45, 60, and 120 min following the injection with glucose. For the insulin tolerance test (ITT), blood glucose was measured similarly in non-fasted mice. Following an initial blood glucose measurement from the tail vein (time 0), porcine insulin was injected i.p. at a dose of 1 international unit (IU) per kg of body weight. Sequential glucose measurements were taken at 15, 30, 45, 60, and 120 min following the injection with insulin.
Indirect calorimetry
Indirect calorimetry was performed as previously described (Darcy et al. 2016b; Hill et al. 2016; Westbrook et al. 2009) using the PhysioScan Metabolic System, which uses zirconia and infrared sensors to measure oxygen and carbon dioxide, respectively, inside chambers where mice are singly housed. After a 24-h acclimation period, mice were monitored in a metabolic chamber for 24 h, with ad libitum access to food and water. Oxygen and carbon dioxide levels were analyzed every 10 min. All comparisons are based on animals studied simultaneously to minimize the effects of environmental and calibration variations.
RT-PCR
RT-PCR was performed as previously described (Darcy et al. 2016b) using the StepOne System and SYBR Green MasterMix. In short, tissue was homogenized, and RNA was extracted using an RNeasy Lipid Tissue Kit for adipose tissue following the manufacturer’s instructions. A standard laboratory Trizol extraction protocol was used for all other organs. RNA was reverse transcribed using an iScript cDNA synthesis Kit following the manufacturer’s instructions. Forty cycles of amplification were performed of denaturation, annealing, and elongation. All data presented used B2M as a housekeeping gene, while Actin was used as a second housekeeping gene for confirmation of results. Relative expression was calculated as previously described (Masternak et al. 2005).
Statistical analysis
For the longevity study, a log-rank test followed by a Tukey post hoc analysis was used to determine statistical significance (males and females separately). This procedure resulted in an overall test for any differences in the four survival curves (genotype x litter size), as well as tests exploring specific differences between groups. In addition, a secondary set of analyses were conducted to evaluate the differences between specific curves beyond the median, using either the pooled median or the individual medians of each group. For the ITT, GTT, and respiratory quotient (RQ), the area under the curve for each group was compared using a two-way ANOVA. For all other analyses, a two-way ANOVA was used. All figures are represented as mean ± SEM. All statistical analyses were done using GraphPad Prism 6.
Results
Litter crowding did not further extend lifespan of GHR-KO mice
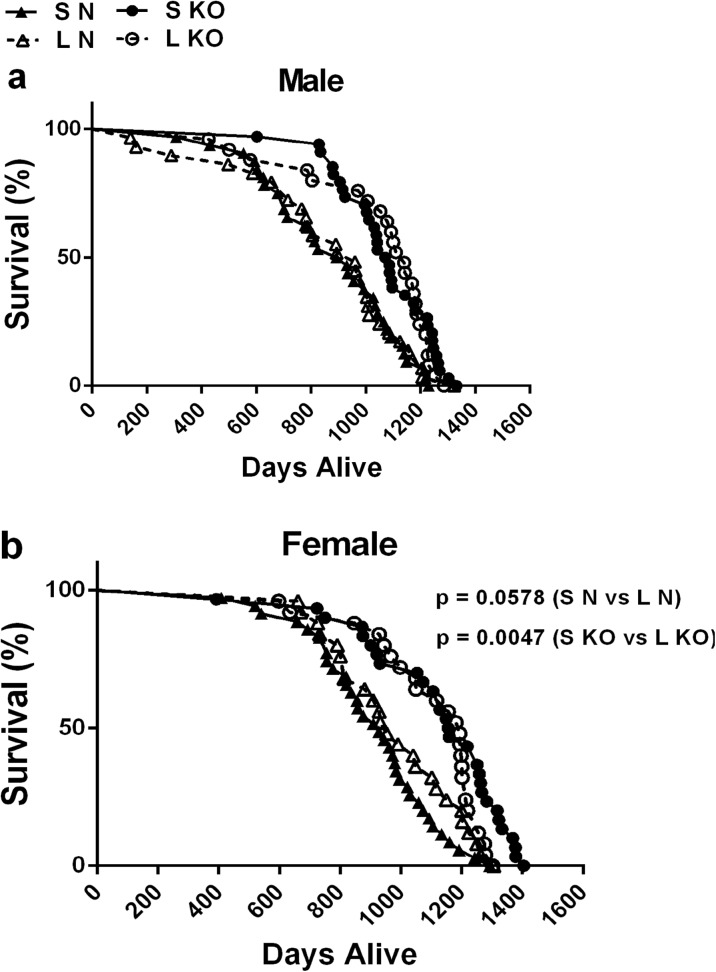
Contrary to our hypothesis, litter crowding had no effect on the average longevity of male mice used in the present study (Fig. 1a). However, in females, litter crowding did influence mortality in the second half of lifespan (Fig. 1b). Intriguingly, the effects of litter crowding in females were opposite in GHR-KO mice as compared to normal mice, where litter crowding appeared to increase longevity in normal mice, but shortened longevity in GHR-KO mice. Analysis of the longevity of animals that lived longer than the median for their group revealed that GHR-KO females from L litters lived significantly shorter than GHR-KO females from S litters (P = 0.0047), while normal females from L litters tended to live longer than normal females from S litters, which approached statistical significance (P = 0.0578) (Table 1).
Fig. 1.
Litter crowding does not further extend lifespan of GHR-KO mice. a Survival curve for normal (N) and GHR-KO (KO) male mice from small (S) and large (L) litters (n = 32 for SN, 34 for SKO, 29 for LN, and 25 for LKO). b Survival curve for N and KO female mice from S and L litters (n = 35 for SN, 30 for SKO, 25 for LN, and 25 for LKO)
Table 1.
Analysis of lifespan for female GHR-KO raised in S or L litter
| Litter size/sex/phenotype | n | Median lifespan in 2nd half of survival curve | Wilcoxon P value for 2nd half of survival curve |
|---|---|---|---|
| S fN | 35 | 1044 | 0.0578 |
| L fN | 25 | 1149 | |
| S fKO | 30 | 1282*** | 0.0047 |
| L fKO | 25 | 1213 |
Data represented as mean ± SEM. P values were calculated by Wilcoxon test between S fN (female normal mice from small litter) and L fN (female normal mice from large litter) or between S fKO (female GHR-KO mice from small litter) and L fKO (female GHR-KO mice from large litter) in 2nd half of survival curve
***P ≤ 0.001
Pre-weaning undernutrition differentially effected body size of male and female GHR-KO mice
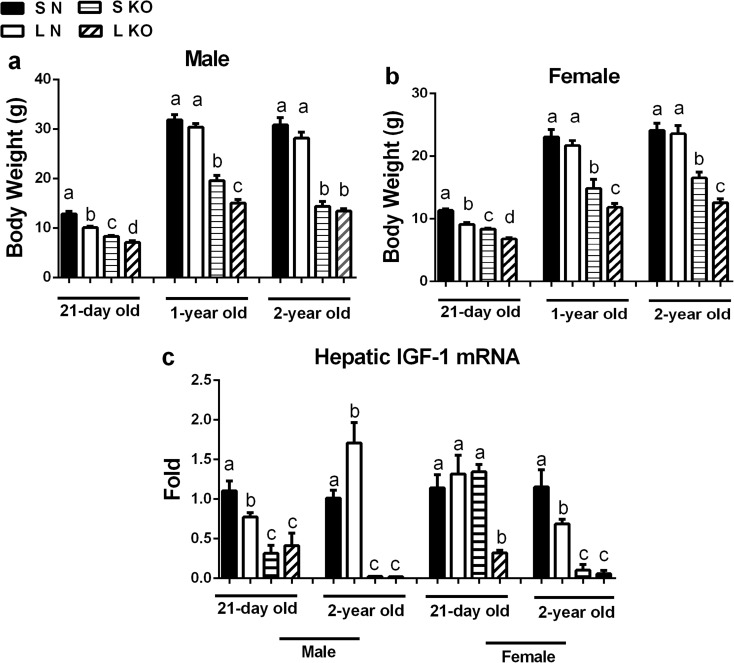
As expected, both sexes of GHR-KO and normal pups from L litters had a lower body weight at weaning than the corresponding animals from S litters (Fig. 2a and b). In normal mice, both male and female mice from L litters weighed about 20% less than animals from S litters. In GHR-KO mice, the difference between the body weight of pups raised in S and L litters was 15% in males and 20% in females. Interestingly, this decrease in body weight did not reflect a decrease in the hepatic gene expression of IGF-1 in male GHR-KO mice, whereas litter crowding reduced hepatic expression of IGF-1 mRNA in normal male mice and both genotypes of female mice (Fig. 2c). In normal mice, the differences in body weight diminished after weaning (Fig. 2a and b); however, the reduction in body weight in female GHR-KO mice from L litters persisted up to the age of 2 years (Fig. 2b), which may in part be due to the strikingly lower expression of hepatic IGF-1 mRNA during the litter crowding period (Fig. 2c). In contrast to female GHR-KO mice, males from L litters had a reduction in body weight that only existed at the age of 1 year, but was no longer evident at the age of 2 years (Fig. 2a).
Fig. 2.
Pre-weaning undernutrition differentially effects body size of male and female GHR-KO mice. a Body weights of normal (N) and GHR-KO (KO) male mice from small (S) and large (L) litters. b Body weights of N and KO female mice from S and L litters (a and b, n = 9–13). c Hepatic IGF-1 mRNA expression between N and KO mice (both sexes) from S and L litters (n = 4–7). Values not marked with the same superscript (a–c) are significantly different (P < 0.05)
Litter crowding improved glucose homeostasis in male GHR-KO mice
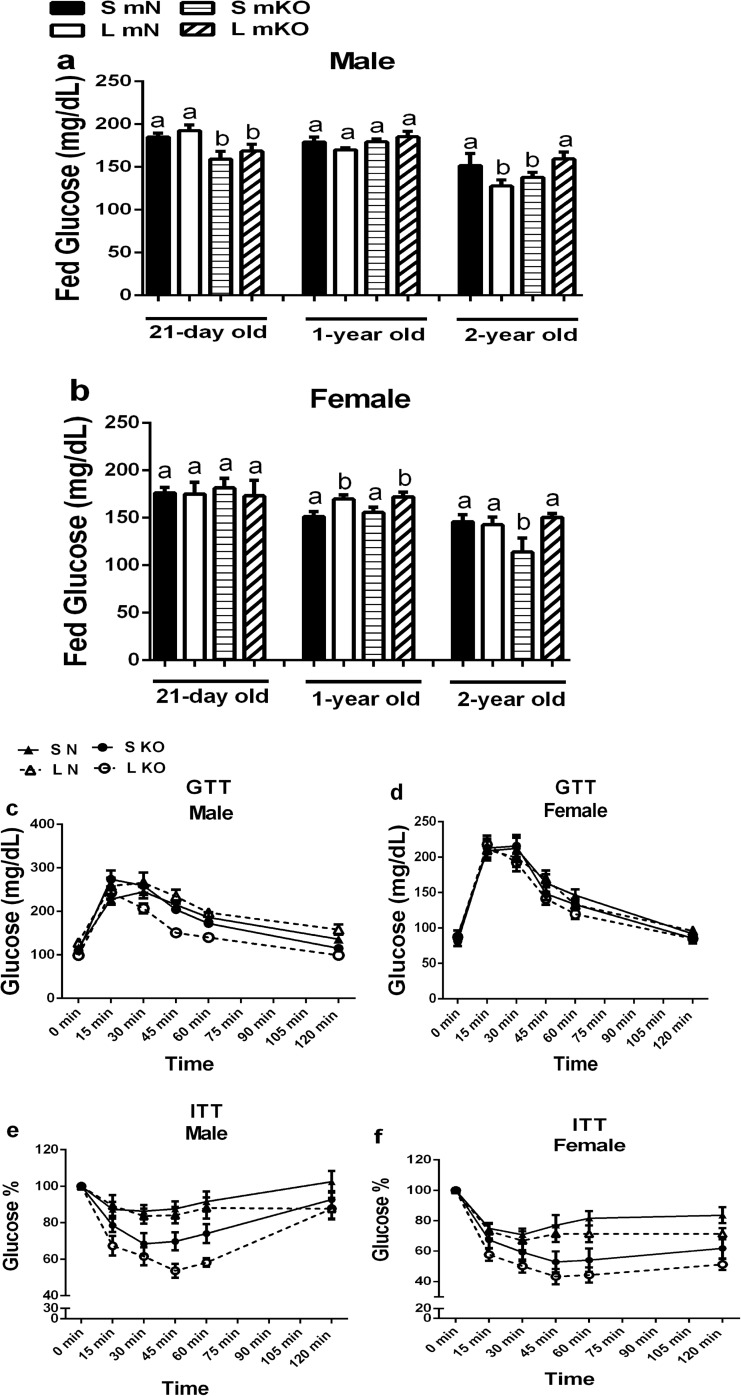
Compared to normal animals, GHR-KO mice typically have improved glucose metabolism with very low insulin levels and increased insulin sensitivity (Coschigano et al. 2003). Because litter crowding is essentially a mild early-life calorie restriction, it was of interest to see if the glucose metabolism in these mice was altered. In the present study, fed glucose levels in normal males were not affected by litter crowding (Fig. 3a), whereas normal females from L litters had elevated fed glucose levels at 1 year of age (Fig. 3b). Both sexes of GHR-KO mice from L litters had increased fed glucose at 2 years of age (Fig. 3a and b). Surprisingly, this did not correlate with glucose tolerance and insulin sensitivity as measured by a GTT and an ITT, respectively. Middle-aged GHR-KO males from L litters were more glucose tolerant (Fig. 3c) and insulin sensitive (Fig. 3e) than their normal littermates, whereas no such changes were detected in GHR-KO females of the same age (Fig. 3d and f). Average fed glucose levels were reduced in GHR-KO as compared to normal mice only in 21-day-old males and in 2-year-old females raised in S litters.
Fig. 3.
Litter crowding improves glucose homeostasis in male GHR-KO mice. a Fed glucose levels in normal (N) and GHR-KO (KO) male mice from small (S) and large (L) litters. b Fed glucose levels in N and KO female mice from S and L litters. c Glucose tolerance test (GTT) of N and KO male mice from S and L litters. d Glucose tolerance test of N and KO female mice from S and L litters. e Insulin tolerance test (ITT) of N and KO male mice from S and L litters. f Insulin tolerance test of N and KO female mice from S and L litters (a–f, n = 9–13). Values not marked with the same superscript (a–d) are significantly different (P < 0.05)
Litter crowding improved lipid metabolism in male GHR-KO mice
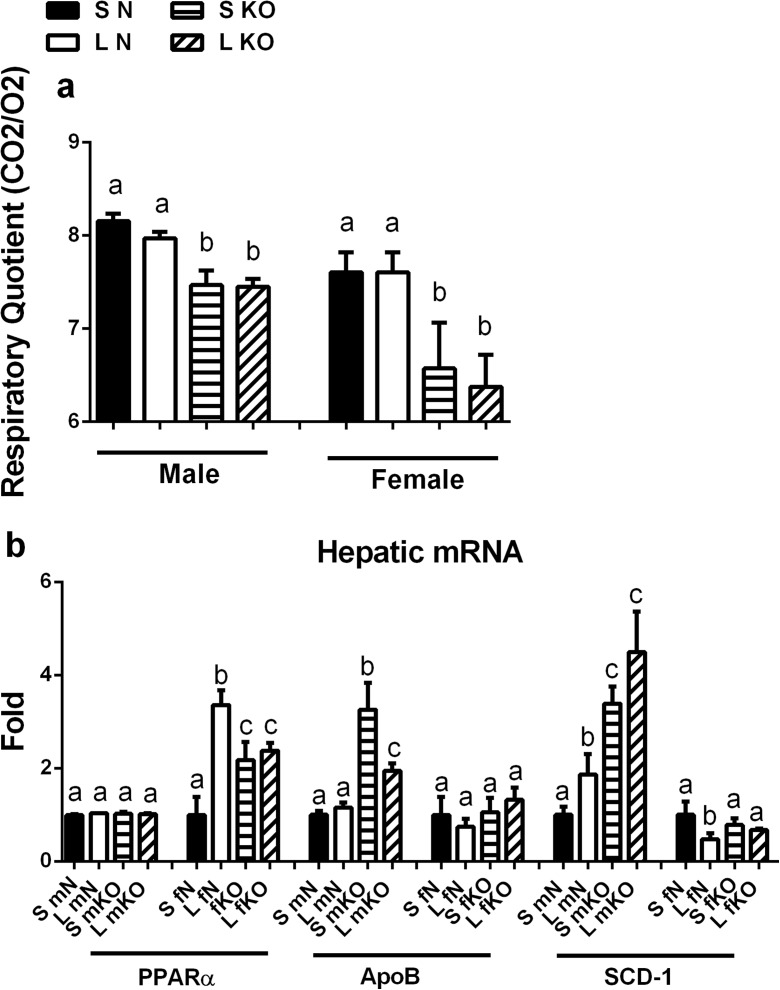
Plasma lipid analyses showed that there were no significant differences in the circulating levels of triglycerides, cholesterol, or free fatty acids between GHR-KO and normal mice from S or L litters (both males and females) (data not shown). This was also reflected in a lack of change in respiratory quotient (RQ) between S and L litters (it is established that GHR-KO mice have a reduced RQ compared to their normal littermates (Westbrook et al. 2009)) (Fig. 4a). However, there were some alterations in the hepatic expression of the genes we measured that relate to lipid metabolism. Normal females had an increase in PPARα mRNA expression following litter crowding, while normal males had an increase in ApoB mRNA expression, and both normal and GHR-KO males had an increase SCD-1 mRNA expression following litter crowding (Fig. 4b).
Fig. 4.
Litter crowding improves lipid metabolism in male GHR-KO mice. a Respiratory quotient (RQ) of normal (N) and GHR-KO (KO) mice (both sexes) from small (S) and large (L) litters (n = 7). b Hepatic mRNA expression of lipid metabolism genes in N and KO mice (both sexes) from S and L litters (n = 4–7). Values not marked with the same superscript (a–d) are significantly different (P < 0.05)
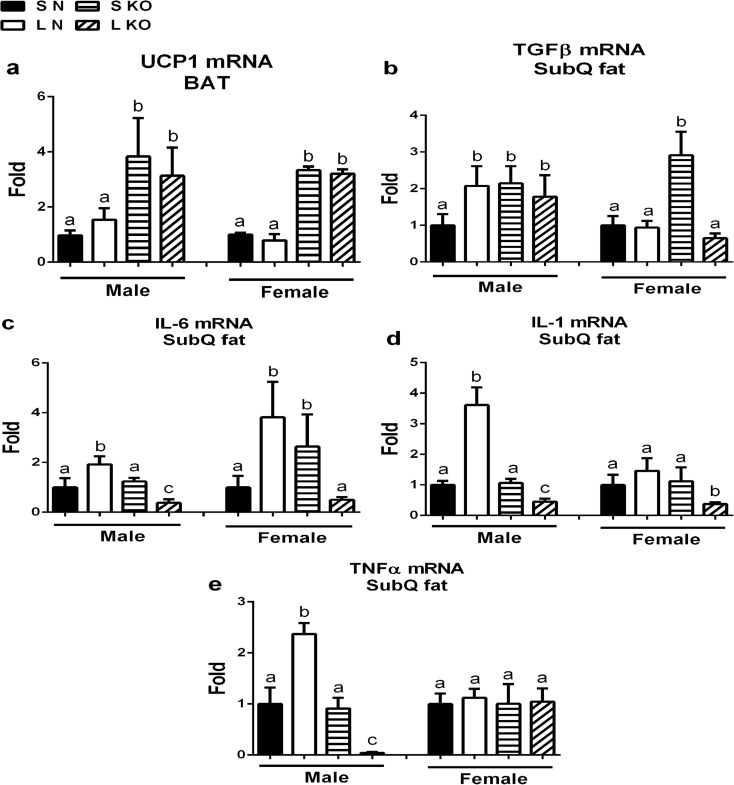
Litter crowding altered gene expression in adipose tissue of both male and female GHR-KO mice
We observed no effect of litter size on UCP-1 expression in brown adipose tissue (BAT) in either sex or genotype (Fig. 5a). The effects of litter crowding on inflammatory gene expression in subcutaneous adipose tissue were opposite in normal and GHR-KO mice. As a result of litter crowding, TGF-β gene expression increased in normal males and decreased in GHR-KO females (Fig. 5b), IL-6 gene expression increased in both male and female normal mice and decreased in both male and female GHR-KO mice (Fig. 5c), IL-1 gene expression increased in normal males and decreased in both male and female GHR-KO mice (Fig. 5d), and TNF-α increased in normal males and decreased in male GHR-KO mice (Fig. 5e).
Fig. 5.
Litter crowding altered gene expression in adipose tissue of both male and female GHR-KO mice. a UCP1 mRNA levels in brown adipose tissue (BAT) from normal (N) and GHR-KO (KO) mice (both sexes) from small (S) and large (L) litters at 2-years age. b–e Expression of inflammatory genes in subcutaneous adipose tissue from N and KO mice (both sexes) from S and L litters (a–e, n = 4–7) at 2 years age. Values not marked with the same superscript (a–d) are significantly different (P < 0.05)
Discussion
The main finding from the present study is that mild pre-weaning nutrient restriction produced by litter crowding not only fails to further extend the remarkable longevity of GHR-KO mice but it also compromises old age survival of GHR-KO females. However, these findings are not entirely unexpected when viewed in the light of the documented failure of calorie restriction started in adulthood to increase the longevity of GHR-KO mice (Bonkowski et al. 2006). Perhaps in GHR-KO mice, which are characterized by major reductions in growth rate and adult body size, a further reduction of growth produced by nutrient restriction can be harmful rather than beneficial. In support of this interpretation, longevity was reduced in GHR-KO females in which a reduction of body weight produced by litter crowding persisted until the age of 2 years and not in males in which body weight difference disappeared during the second half of life. Increased late life mortality of GHR-KO females raised in large litters contrasts sharply with the pronounced beneficial effect of litter crowding on longevity of normal mice studied by Sun et al. (Sun et al. 2009) and also differs from the somewhat less clear benefit seen in normal females in the present study. We have no explanation for the much smaller longevity benefit of litter crowding in normal animals in the present study in comparison to the mice studied by Sun et al. (Sun et al. 2009). Differences in the genetic background may have been involved, but recent studies in the Miller laboratory in UM-HET animals (produced by the same crosses as those used by Sun et al.) also showed only small and sex-specific extension of longevity in response to litter crowding (data not shown).
Regardless of these discrepancies, the indication that the effect of pre-weaning nutritional restriction on old age survival of female mice was opposite in GHR-KO as compared to normal animals is of considerable interest. It is reminiscent of the opposite effects of visceral fat removal on glucose signaling (Masternak et al. 2012) and the opposite effects of rapamycin treatment (Fang and Bartke, unpublished data) in GHR-KO and normal mice. While it is not possible to identify the underlying mechanisms from the present data, it is intriguing that the effects of litter crowding on the expression inflammation-related genes in white adipose tissue are generally opposite in GHR-KO and normal mice: specifically, litter crowding tends to increase several of these mRNAs in normal mice, whereas the expression of these mRNAs decreases in GHR-KO mice. A comprehensive analysis of this idea will require assessment of these inflammatory proteins (not merely the corresponding mRNAs) in fat tissue and in plasma, as well as extension to a wider range of inflammatory mediators produced both by adipocytes and macrophages within the fat depots.
In the present study, the trends for improved glucose tolerance in males and insulin tolerance in both sexes of mice from L litters did not reach statistical significance. This difference from the results obtained by Sagagurski et al. (Sadagurski et al. 2014) may have been due to a strain difference and unexpected increase in the expression of pro-inflammation markers. Although enhanced insulin sensitivity is believed to represent one of the mechanisms of extended longevity of GHR-KO mice, normal males from L litters did not live longer, whereas normal females did. Apparently, the effects of early nutritional restriction on longevity of these animals are not mediated by alterations of glucose homeostasis during adult life, or the effects of altering insulin signaling are overridden by other effects of nutrient restriction during pre-weaning development. However, these conclusions are tentative without additional studies of glucose management at various stages of the life history.
The effects of litter crowding on inflammatory gene expression in subcutaneous adipose tissue at 2-years of age are interesting. The levels of TNF-α and IL-6 become elevated as humans or animals age (Masternak and Bartke 2012), and high levels of IL-6 expression are associated with impaired glucose metabolism (Pradhan et al. 2001). In our study, the levels of IL-6 in subcutaneous adipose tissue were increased by litter crowding in both sexes of normal mice (Fig. 5c), which may explain why glucose homeostasis was not significantly improved by mild early-life calorie restriction unlike the results from a litter crowding study (Sadagurski et al. 2014). The levels of TNF-α and IL-1 were increased in normal males, but not in females from L litter (Fig. 5e), which could be one of reasons why normal males from L litters did not live longer as was observed in normal females from L litters (Fig. 1). On the other hand, litter crowding increased the levels of TGF-β (anti-inflammatory marker) in normal females from L litters, whereas it was decreased in GHR-KO females from L litters (Fig. 5), which may contribute to the impact on lifespan, in which normal females from L litters lived longer, while GHR-KO females from L litters lived shorter.
The present results indicate that genetic differences in somatotropic signaling are likely to influence the intricate balance between early nutrition, growth, adult metabolic phenotype, aging, and longevity.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) grants AG031736, AG0119899, and AG051869 (to A.B.) and AG048264 and AG050531 (to L.Y.S.). This work is solely the view of the authors and does not reflect those of the NIH.
Compliance with ethical standards
All animal protocols were approved by the Southern Illinois University School of Medicine Animal Care and Use Committee.
References
- Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-A. [DOI] [PubMed] [Google Scholar]
- Bartke A, Sun L, Fang Y, Hill C. Growth hormone actions during development influence adult phenotype and longevity. Exp Gerontol. 2016;86:22–27. doi: 10.1016/j.exger.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp B et al (2015) Undernutrition during pregnancy in mice leads to dysfunctional cardiac muscle respiration in adult offspring. Biosci Rep 35. doi:10.1042/BSR20150007 [DOI] [PMC free article] [PubMed]
- Berends LM, Fernandez-Twinn DS, Martin-Gronert MS, Cripps RL, Ozanne SE. Catch-up growth following intra-uterine growth-restriction programmes an insulin-resistant phenotype in adipose tissue. Int J Obes. 2013;37:1051–1057. doi: 10.1038/ijo.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore HL, Ozanne SE. Maternal diet-induced obesity and offspring cardiovascular health. J Dev Orig Health Dis. 2013;4:338–347. doi: 10.1017/S2040174412000761. [DOI] [PubMed] [Google Scholar]
- Blackmore HL, Piekarz AV, Fernandez-Twinn DS, Mercer JR, Figg N, Bennett M, Ozanne SE. Poor maternal nutrition programmes a pro-atherosclerotic phenotype in ApoE-/- mice. Clin Sci (Lond) 2012;123:251–257. doi: 10.1042/CS20110487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski MS, et al. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One. 2009;4:E4567. doi: 10.1371/journal.pone.0004567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- Darcy J, Fang Y, Hill CM, McFadden S, Sun LY, Bartke A. Original Research: metabolic alterations from early life thyroxine replacement therapy in male Ames dwarf mice are transient. Exp Biol Med (Maywood) 2016;241:1764–1771. doi: 10.1177/1535370216650292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcy J, McFadden S, Fang Y, Huber JA, Zhang C, Sun LY, Bartke A. Brown adipose tissue function is enhanced in long-lived, male Ames dwarf mice. Endocrinology. 2016;157:4744–4753. doi: 10.1210/en.2016-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, et al. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab. 2013;17:456–462. doi: 10.1016/j.cmet.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CM, Fang Y, Miquet JG, Sun LY, Masternak MM, Bartke A (2016) Long-lived hypopituitary Ames dwarf mice are resistant to the detrimental effects of high-fat diet on metabolic function and energy expenditure. Aging Cell. doi:10.1111/acel.12467 [DOI] [PMC free article] [PubMed]
- Jennings BJ, Ozanne SE, Dorling MW, Hales CN. Early growth determines longevity in male rats and may be related to telomere shortening in the kidney. FEBS Lett. 1999;448:4–8. doi: 10.1016/S0014-5793(99)00336-1. [DOI] [PubMed] [Google Scholar]
- Martin-Gronert MS, Tarry-Adkins JL, Cripps RL, Chen JH, Ozanne SE. Maternal protein restriction leads to early life alterations in the expression of key molecules involved in the aging process in rat offspring. Am J Physiol Regul Integr Comp Phys. 2008;294:R494–R500. doi: 10.1152/ajpregu.00530.2007. [DOI] [PubMed] [Google Scholar]
- Masternak MM, Bartke A (2012) Growth hormone, inflammation and aging. Pathobiol Aging Age Relat Dis 2. doi:10.3402/pba.v2i0.17293 [DOI] [PMC free article] [PubMed]
- Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Bonkowski MS, Panici JA, Przybylski GK, Bartke A. Caloric restriction results in decreased expression of peroxisome proliferator-activated receptor superfamily in muscle of normal and long-lived growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2005;60:1238–1245. doi: 10.1093/gerona/60.10.1238. [DOI] [PubMed] [Google Scholar]
- Masternak MM, et al. Metabolic effects of intra-abdominal fat in GHRKO mice. Aging Cell. 2012;11:73–81. doi: 10.1111/j.1474-9726.2011.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panici JA, et al. Is altered expression of hepatic insulin-related genes in growth hormone receptor knockout mice due to GH resistance or a difference in biological life spans? J Gerontol A Biol Sci Med Sci. 2009;64:1126–1133. doi: 10.1093/gerona/glp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010;24:5073–5079. doi: 10.1096/fj.10-163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry CJ, Ozanne SE, Hales CN. Programming of intermediary metabolism. Mol Cell Endocrinol. 2001;185:81–91. doi: 10.1016/S0303-7207(01)00627-X. [DOI] [PubMed] [Google Scholar]
- Podlutsky A et al (2017) The GH/IGF-1 axis in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes: implications for the developmental origins of cancer. Geroscience. doi:10.1007/s11357-017-9966-x [DOI] [PMC free article] [PubMed]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Sadagurski M, et al. Long-lived crowded-litter mice exhibit lasting effects on insulin sensitivity and energy homeostasis. Am J Phys Endocrinol Metab. 2014;306:E1305–E1314. doi: 10.1152/ajpendo.00031.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, et al. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- Sun L, Sadighi Akha AA, Miller RA, Harper JM. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J Gerontol A Biol Sci Med Sci. 2009;64:711–722. doi: 10.1093/gerona/glp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, et al. IGF-1 deficiency in a critical period early in life influences the vascular aging phenotype in mice by altering miRNA-mediated post-transcriptional gene regulation: implications for the developmental origins of health and disease hypothesis. Age (Dordr) 2016;38:239–258. doi: 10.1007/s11357-016-9943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J Gerontol A Biol Sci Med Sci. 2009;64:443–451. doi: 10.1093/gerona/gln075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U S A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]