Abstract
Objective
The aim of the present study is to assess the difference in the detection rates of small-bowel lesions in chronic abdominal pain (CAP) patients with irritable bowel syndrome (IBS) and non-IBS.
Patients
Ninety-nine CAP patients who were scheduled to undergo capsule endoscopy (CE) to investigate their abdominal symptoms were included in this study. Among the subjects, 34 patients fulfilled the Rome III criteria for IBS (IBS group); the remaining 65 patients were categorized as the non-IBS group. CE was performed in both groups and the total enteroscopy achievement rate, small-bowel lesion detection rate, and the presence of small-bowel lesions were evaluated. We also evaluated the patients' blood test results and the rate at which abdominal symptoms improved following internal medication.
Results
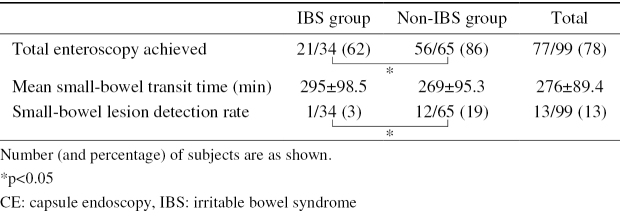
Total enteroscopy was achieved in 62% (21/34) and 86% (56/65) of the IBS and non-IBS patients, respectively. The total enteroscopy achievement rate was significantly higher in non-IBS patients. The small-bowel lesion detection rates were 3% (1/34) and 19% (12/65), respectively, and the detection rate was significantly higher in the non-IBS patients. In the non-IBS patients, mean C-reactive protein (CRP) was significantly higher in the patients with small-bowel lesions. The abdominal symptoms of 12 (92%) of the CAP patients with small-bowel lesions were improved by internal medication.
Conclusion
CE may be considered for non-IBS CAP patients with high levels of CRP.
Keywords: chronic abdominal pain, capsule endoscopy, small-bowel, irritable bowel syndrome
Introduction
Chronic abdominal pain (CAP) is the most common functional digestive disorder worldwide, with a prevalence of up to 21% in the general population. Several hypotheses have been suggested to explain the pathophysiology of CAP, including functional, organic, metabolic, toxicological, and psychiatric disorders (1-4). If the upper and lower endoscopy, blood test and transabdominal ultrasonography findings are normal, the symptoms are tend to be diagnosed as a functional disorder, such as irritable bowel syndrome (IBS). However, the small-bowel has not usually been examined, and it is possible that some CAP patients experience symptoms due to small-bowel disease. Until recently, however, it has been difficult to examine the entire small-bowel endoscopically because of its length and anatomical position. Capsule endoscopy (CE) (5-14) and balloon endoscopy (15-21) have enabled the endoscopic evaluation of the entire small-bowel. CE and BE have been reported to be useful in the diagnosis of small-bowel lesions (15-17). Although there have been some reports about small-bowel abnormalities in CAP patients (22-26), the conclusions have been controversial. In addition, the subjects of the reports included both patients with functional disorders and non-functional disorders. Previous studies to evaluate the diagnostic accuracy of the Rome criteria in the absence of the generally accepted alarm symptoms yielded a positive predictive value of 98% in distinguishing between IBS and organic disease by the Rome criteria (27-29). In addition, Ohlsson et al. reported that a pathological mucosal lesion to explain the symptoms of IBS cannot be identified by CE in the vast majority of patients whose symptoms meet the diagnostic criteria for IBS (23). Thus, it may be meaningless to perform CE for IBS patients. However there were few reports regarding small-bowel abnormalities in non-IBS CAP patients. The aim of this retrospective study is to assess whether CE is useful for detecting culprit small-bowel lesions in CAP patients, and to clarify the difference in the detection rates of small-bowel lesions in IBS and non-IBS in the CAP patients.
Materials and Methods
Subjects
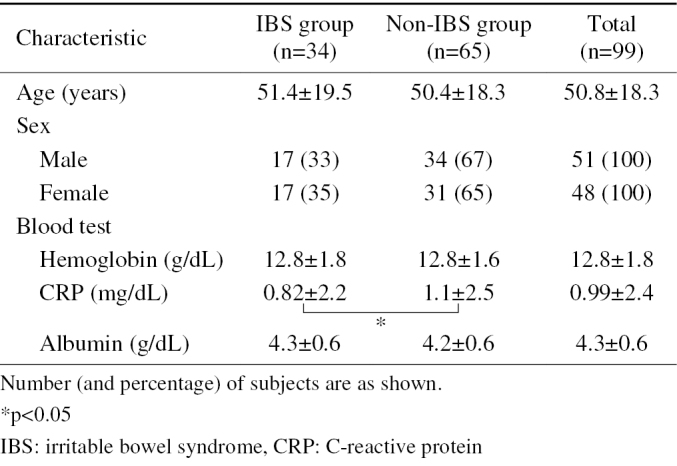
Ninety-nine CAP patients (men, n=51; women, n=48; mean age, 51 years) who were scheduled to undergo CE in order to investigate their symptoms at Hiroshima University Hospital between April 2006 and December 2014 were included in this study. All 99 patients underwent blood testing, esophagogastroduodenoscopy, colonoscopy, and transabdominal ultrasonography or computed tomography (CT). There was no evidence of lesions to explain the symptoms of any of the subjects. Thirty-four of the patients fulfilled the Rome III criteria for IBS (IBS group), while the remaining 65 did not (non-IBS group). The non-IBS patients had abdominal pain if at least 3 months' duration, which was continuous or nearly continuous, and not associated with, or infrequently associated with physiological events. The demographic information of the patients in the two groups is shown in Table 1. The study was approved by the Ethics Committee of Hiroshima University Hospital, and all of the patients provided their written informed consent to undergo CE.
Table 1.
Characteristics of Subjects per Study Group.
The IBS group consisted of 17 men and 17 women, who were from 15 to 81 years of age (mean, 51 years). All had undergone standard upper and lower gastrointestinal endoscopy, and none had shown any abnormalities. The non-IBS group consisted of 34 men and 31 women, who were from 14 to 90 years of age (mean age, 50 years).
CE procedure
A CE capsule (PillCam SB or SB2; Given Imaging, Yoqneam, Israel) was swallowed with a solution of dimethicone after an overnight fast, without any other preparation. Patients were allowed to drink clear liquids and eat a light meal at 2 and 4 hours after swallowing the capsule, respectively.
Study protocol and evaluation
We assessed the achievement of total enteroscopy by CE, the mean small-bowel transit time, the rate at which CE detected small-bowel lesions, the particular lesions that were detected, and improvement of abdominal symptoms by medication. In each of the two study groups, we compared the mean hemoglobin, mean C-reactive protein (CRP), mean albumin, the mean small-bowel transit time, the rate at which CE detected small-bowel lesions, and the particular lesions that were detected. In the present study, we defined small-bowel abnormalities as lesions that might have been the cause of the patients' symptoms; we excluded lesions such as red spots or erosions, as such lesions might not have contributed to these symptoms.
Statistical analysis
The continuous data are presented as the mean value, standard deviation (SD), and range. The categorical data are expressed as percentages. All of the statistical analyses were performed using the JMP software program (version 5.0.1J, SAS Institute, Cary, NC, USA). p values of <0.05 were considered to indicate statistical significance.
Results
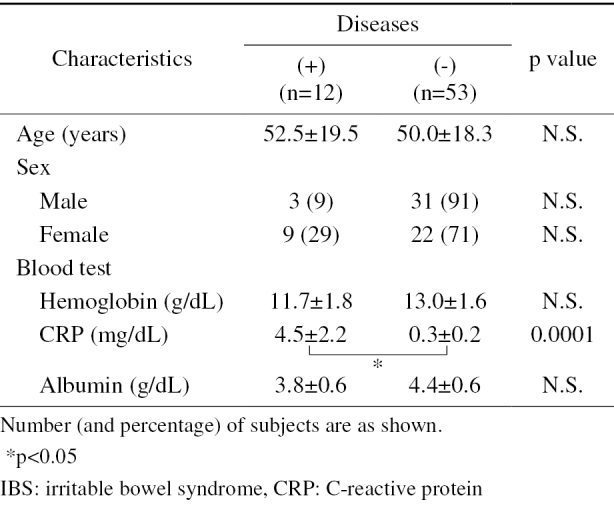
The main characteristics of the enrolled studies are shown in Table 1. There was no significant difference between the two groups with regards to age or sex. The mean CRP value of the non-IBS patients was significantly higher than in IBS patients. Among the non-IBS patients, the mean CRP value in patients with small-bowel lesions was significantly higher than that in patients without small-bowel lesions (Table 2).
Table 2.
Comparison of the Characteristics in Non-IBS Patients.
The total enteroscopy success rates and small-bowel transit times
CE achieved total enteroscopy in 78% (77/99) of the whole study population, 62% of the IBS group (21/34), and 86% (56/65) in the non-IBS group; the total enteroscopy achievement rate was significantly higher in the non-IBS patients (Table 3). For patients in whom total enteroscopy was achieved by CE, the mean small-bowel transit time was 276 minutes. The mean small-bowel transit time was 295 minutes in the IBS group, and 269 minutes in non-IBS group; the difference between the two groups was not significant.
Table 3.
Results of CE per Study Group.
Small-bowel lesion detection rates
The small-bowel lesion detection rates in the IBS and non-IBS groups were 3% (1/34) and 19% (12/65), respectively (Table 3); the detection rate in the non-IBS group was significantly higher than that in the IBS group. The only small-bowel lesions detected by CE in the IBS group were non-specific ulcers (n=1). In contrast, the small-bowel lesions detected by CE in the non-IBS patients included NSAID-induced ulcers (n=3), Crohn's disease (n=3), eosinophilic enteritis (n=3), IgA vasculitis (n=1), parasitic worms (n=1), and ischemic enteritis (n=1) (Table 4).
Table 4.
CE Findings per Study Group.
| Finding | IBS group (34) | Non-IBS group (65) | Total (99) |
|---|---|---|---|
| NSAIDs-induced ulcer | 0 (0) | 3 (5) | 3 (3) |
| Crohn’s disease | 0 (0) | 3 (5) | 3 (3) |
| Eosinophilic enteritis | 0 (0) | 3 (5) | 3 (3) |
| Non-specific ulcer | 1 (3) | 0 (0) | 1 (1) |
| IgA vasculitis | 0 (0) | 1 (2) | 1 (1) |
| Parasitic worm | 0 (0) | 1 (2) | 1 (1) |
| Ischemic enteritis | 0 (0) | 1 (2) | 1 (1) |
| Total | 1 (3) | 12 (21) | 13 (13) |
Number (and percentage) of subjects are shown.
IBS: irritable bowel syndrome, NSAIDs: non-steroidal anti-inflammatory drugs
The improvement of abdominal symptoms by internal medication
Of the patients with small-bowel lesions, the abdominal symptoms of 12 patients (92%) improved with medication (Table 5). Thus, the symptoms improved in 12% (12/99) of the subjects. Of the 3 patients with non-steroidal anti-inflammatory drug-induced ulcers, 2 patients were prescribed mucosal protectants, while 1 patient, who also had small-bowel stricture, underwent endoscopic dilatation. The patients with eosinophilic enteritis and IgA vasculitis were prescribed steroids, whereas the patients with Crohn's disease were prescribed 5-5-aminosalicylic acid. The patient with ischemic enteritis underwent surgical treatment, while the patient with parasitic worms was treated with an antibiotic.
Table 5.
Result of Improvement of Abdominal Pain by Medication per Small-bowel Disease.
| Patient no | Diagnosis | Treatment | Symptom improvement |
|---|---|---|---|
| 1 | NSAIDs-induced ulcer | Mucosal protectant | + |
| 2 | NSAIDs-induced ulcer | Mucosal protectant | + |
| 3 | NSAIDs-induced ulcer | Mucosal protectant | + |
| 4 | Eosinophilic enteritis | Steroid | + |
| 5 | Eosinophilic enteritis | Steroid | + |
| 6 | Eosinophilic enteritis | Steroid | + |
| 7 | Crohn’s disease | 5-ASA | + |
| 8 | Crohn’s disease | 5-ASA | + |
| 9 | Crohn’s disease | 5-ASA | + |
| 10 | Parasitic worm | Antibiotic | + |
| 11 | IgA vasculitis | Steroid | + |
| 12 | Ischemic enteritis | Mucosal protectant | + |
| 13 | Non-specific ulcer | Mucosal protectant | − |
IBS: irritable bowel syndrome, NSAID: non-steroidal anti-inflammatory drugx
5-ASA: 5-aminosalicylic acid
Discussion
Until recently, it has been difficult to perform endoscopy of the entire small bowel due to its length and anatomical position. The introduction of CE (5-14) and double-balloon endoscopy (DBE) (15-21) have made it possible to examine the entire small-bowel via endoscopy. CE is particularly useful in diagnosing small-bowel diseases, and has been found to be superior to other modalities, such as small-bowel radiography, push enteroscopy, CT, and angiography (5-15). Although CT and magnetic resonance imaging are useful for detecting small-bowel disease, they cannot easily detect small lesions or mucosal lesions easily. Jensen et al. reported that, in the diagnosis of Crohn's disease of the terminal ileum, the sensitivity and specificity of CE, magnetic resonance enterography and CT enterography were 100% and 91%, 81% and 86%, and 76% and 85%, respectively (30). They concluded that in patients without endoscopic or clinical suspicion of stenosis, CE should be the first line modality for the detection of small-bowel Crohn's disease, and we reported that CE and DBE have an almost equal ability to detect small-bowel lesions if the entire small bowel is observed (15). With the introduction of CE as a diagnostic tool for small-bowel disorders, it is necessary to establish the indications for CE. According to the published data, suspected mid-gastrointestinal bleeding is the most appropriate indication for this procedure (9). Published studies have reported diagnostic yields ranging from 48% to 76% with this indication (7-10). However, the benefits of CE as part of a diagnostic workup for other small-bowel disorders, such as abdominal pain, have remained unclear. Previous studies have examined the role of CE in patients with abdominal symptoms (22-26); Ohlsson et al. reported that, in the vast majority of patients who symptoms meet the criteria of IBS, no causative pathological mucosal lesion is found by CE (23). Another group concluded that CE should not be the first-line examination in patients with diarrhea or abdominal pain. However, we hypothesize that the subjects of these reports were IBS patients. The diagnosis of IBS is made when a patient's symptoms fulfill the Rome III criteria (27). Earlier studies have evaluated the diagnostic accuracy of the Rome criteria in the absence of ‘alarm’ symptoms. These studies showed a positive predictive value of 98% in distinguishing between IBS and organic diseases (28,29). In the present study, there was only 1 case (3%) in which a lesion might have caused the symptoms of small-bowel disease in an IBS patient; this is in line with previous reports. On the other hand, among CAP patients, the detection rate in the non-IBS group was significantly higher than that in the IBS group. In the non-IBS group, the mean CRP value was significantly higher among the patients with small-bowel lesions than it was among those without small-bowel lesions. Shim et al. reported that weight loss, inflammatory signs such as an elevated erythrocyte sedimentation rate or CRP level, and hypoalbuminemia were factors that were associated with positive CE findings (25). May et al. showed that the observation of signs of inflammation in addition to abdominal pain were the only signs that had a positive effect in obtaining a relevant CE finding in a prospective multicenter study (22). This result showed that CE cannot be the first choice in patients with IBS; we propose that non-IBS patients with high CRP values should undergo CE.
Among the patients with small-bowel lesions, the abdominal symptoms of 12 patients (92%) improved with medication; all of them were non-IBS patients. The symptoms of almost 20% of the non-IBS patients improved. This finding showed that CE was very useful for non-IBS patients.
Although there was no significant difference in the small bowel transit times, the total enteroscopy achievement rate was significantly higher in the non-IBS patients. Schmidt et al. reported small-bowel motility to be frequently but not universally abnormal in IBS patients (31). The transit time was abnormal in 35% of a series of patients who underwent CE for abdominal pain, suggesting that a motility abnormality was the origin of their symptoms (26). However, there is no consensus regarding small-bowel motility in IBS patients and further studies are required.
The present study is associated with some limitations. Our sample size was small and a larger prospective study would be desirable. Another limitation is that the difference in the detection rate of the two groups might have been due to the difference in the rate of total enteroscopy achievement. However, Ohlsson et al. reported that in the vast majority of patients whose symptoms meet the diagnostic criteria for IBS, no pathological mucosal lesion to explain the symptoms can be found by CE (23); we are therefore of the opinion that the lesion detection rate in non-IBS CAP patients would be high.
In conclusion, in the present study of CAP patients, the detection rate in the non-IBS group was significantly higher than that in the IBS group. Additionally, in the non-IBS group, the mean CRP was significantly higher in patients with small-bowel lesions. CE may therefore be considered for non-IBS CAP patients with high CRP values.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Zackowski SW. Chronic recurrent abdominal pain. Emerg Med Clin North Am 16: 877-894, 1998. [DOI] [PubMed] [Google Scholar]
- 2.DeBanto JR, Varilek GW, Haas L. What could be causing chronic abdominal pain? Anything from common peptic ulcers to uncommon pancreatic trauma. Postgrad Med 106: 141, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Guthrie E, Thompson D. Abdominal pain and functional gastrointestinal disorders. BMJ 325: 701, 2002. [PMC free article] [PubMed] [Google Scholar]
- 4.Srinivasan R, Greenbaum DS. Chronic abdominal wall pain: a frequent overlooked problem. Practical approach to diagnosis and management. Am J Gastroenterol 97: 824-830, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature 405: 417, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Meron GD. The development of the swallowable video capsule (M2A). Gastrointest Endosc 52: 817-819, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Lewis BS, Swain P. Capsule endoscopy in the evaluation of patients with suspected small intestinal bleeding: results of a pilot study. Gastrointest Endosc 56: 349-353, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Pennazio M, Santucci R, Rondonotti E, et al. . Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology 126: 643-653, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Apostolopoulos P, Liatsos C, Gralnek IM, et al. . The role of wireless capsule endoscopy in investigating unexplained iron deficiency anemia after negative endoscopic evaluation of the upper and lower gastrointestinal tract. Endoscopy 38: 1127-1132, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Apostolopoulos P, Liatsos C, Gralnek IM, et al. . Evaluation of capsule endoscopy in active, mild-to-moderate, overt, obscure GI bleeding. Gastrointest Endosc 66: 1174-1181, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Urbain D, De Looze D, Demedts I, et al. . Video capsule endoscopy in small-bowel malignancy: a multicenter Belgian study. Endoscopy 38: 408-411, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Bailey AA, Debinski HS, Appleyard MN, et al. . Diagnosis and outcome of small bowel tumors found by capsule endoscopy: a three-center Australian experience. Am J Gastroenterol 101: 2237-2243, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Hartmann D, Schmidt H, Bolz G, et al. . A prospective two-center study comparing wireless capsule endoscopy with intraoperative enteroscopy in patients with obscure GI bleeding. Gastrointest Endosc 61: 826-832, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Imagawa H, Oka S, Tanaka S, et al. . Improved detectability of small-bowel lesions via capsule endoscopy with computed virtual chromoendoscopy: a pilot study. Scand J Gastroenterol 46: 1133-1137, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukumoto A, Tanaka S, Shishido T, Takemura Y, Oka S, Chayama K. Comparison of detectability of small-bowel lesions between capsule endoscopy and double-balloon endoscopy for patients with suspected small-bowel disease. Gastrointest Endosc 69: 857-865, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Kameda N, Higuchi K, Shiba M, et al. . A prospective, single-blind trial comparing wireless capsule endoscopy and double-balloon enteroscopy in patients with obscure gastrointestinal bleeding. J Gastroenterol Hepatol 43: 434-440, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Shishido T, Oka S, Tanaka S, et al. . Outcome of patients who have undergone total enteroscopy for obscure gastrointestinal bleeding. World J Gastroenterol 18: 666-672, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukumoto A, Tanaka S, Yamamoto H, et al. . Diagnosis and treatment of small-bowel stricture by double balloon endoscopy. Gastrointest Endosc 66 (3 Suppl): S108-S112, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto H, Sekine Y, Sato Y, et al. . Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc 53: 216-220, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto H, Sugano K. A new method of enteroscopy: the double-balloon method. Can J Gastroenterol 17: 273-274, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Domagk D, Mensink P, Aktas H, et al. . Single- vs. double-balloon enteroscopy in small-bowel diagnostics: a randomized multicenter trial. Endoscopy 43: 472-476, 2011. [DOI] [PubMed] [Google Scholar]
- 22.May A, Manner H, Schneider M, Ipsen A, Ell C. Prospective multicenter trial of capsule endoscopy in patients with chronic abdominal pain, diarrhea and other signs and symptoms (CEDAP-Plus Study). Endoscopy 39: 606-612, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Ohlsson B, Bengtsson M, Nielsen J, Toth E. A prospective evaluation of the diagnostic value of video capsule endoscopy in patients initially classified as irritable bowel syndrome. Eur J Intern Med 20: 48-52, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Fry LC, Carey EJ, Shiff AD, et al. . The yield of capsule endoscopy in patients with abdominal pain or diarrhea. Endoscopy 38: 498-502, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Shim KN, Kim YS, Kim KJ, et al. . Abdominal pain accompanied by weight loss may increase the diagnostic yield of capsule endoscopy: a Korean multicenter study. Korean Gut Image Study Group. Scand J Gastroenterol 41: 983-988, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Mele C, Infantolino A, Conn M, Kowalski T, Cohen S, DiMarino A. The diagnostic yield of wireless capsule endoscopy in patients with unexplained abdominal pain. Am J Gastroenterol 98: S298, 2003. [Google Scholar]
- 27.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis 15: 237-241, 2006. [PubMed] [Google Scholar]
- 28.Vanner SJ, Depew WT, Paterson WG, et al. . Predictive value of the Rome criteria for diagnosing the irritable bowel syndrome. Am J Gastroenterol 94: 2912-2917, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Olden KW. Diagnosis of irritable bowel syndrome. Gastroenterology 122: 1701-1714, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Jensen MD, Nathan T, Rafaelsen SR, Kjeldsen J. Diagnostic accuracy of capsule endoscopy for small bowel Crohn's disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol 9: 124-129, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt T, Hackelsberger N, Widmer R, Meisel C, Pfeiffer A, Kaess H. Ambulatory 24-hour jejunal motility in diarrhea-predominant irritable bowel syndrome. Scand J Gastroenterol 31: 581-589, 1996. [DOI] [PubMed] [Google Scholar]