Abstract
Objective
This study aimed to examine the association between the changes in an overall healthy lifestyle, as quantified by the number of unhealthy lifestyle factors and obesity status, and the incidence of proteinuria in the general Japanese population.
Methods
A retrospective cohort study was conducted among 99,404 (men, 36.9%) participants aged from 40-74 years of age who underwent two health check-ups with a 1-year interval in Japan between 2008 and 2009. Any participants with chronic kidney disease at baseline were excluded. The smoking status, body mass index, physical activity, alcohol consumption, and healthy eating habits were combined into a simple overall healthy lifestyle score ranging from 0 to 5. The changes in overall healthy lifestyle scores from baseline (range, -5 to +5) and the incidence of proteinuria, defined by a dipstick urinalysis (score ≥1+), were assessed at the second check-up. A logistic regression analysis was used to examine the association between the changes in overall healthy lifestyle scores and the incidence of proteinuria.
Results
After one year of follow-up, 3.9% of men and 2.4% of women developed proteinuria. Each increase (or decrease) in the changes in overall healthy lifestyle scores was associated with a reduced (or increased) risk of proteinuria in both men (odds ratio (OR) 0.87; 95% confidence interval (CI), 0.81-0.94) and women (OR 0.87; 95%CI, 0.80-0.94) after adjusting for age, baseline lifestyle scores, hypertension, diabetes mellitus, and hypercholesterolemia. Stratified analyses based on age, the presence or absence of hypertension, or diabetes mellitus revealed similar results.
Conclusion
Overall lifestyle changes, even within a year, were found to influence the incidence of proteinuria.
Keywords: CKD, epidemiology, multiple behaviour intervention, obesity, proteinuria
Introduction
The prevention of proteinuria/albuminuria is highly desirable in both clinical and public health settings, because proteinuria/albuminuria is a prognostic marker of kidney disease, as well as an independent risk factor for cardiovascular morbidity and mortality (1-6). The odds of developing proteinuria/albuminuria are heightened by poor lifestyle behaviours and the obesity status both independently and in combination (7-11). Because multiple lifestyle behaviours coexist and may interact, studying the combined impact of lifestyle factors instead of their individual impact on proteinuria/albuminuria is highly relevant. Furthermore, applying a simple lifestyle index is useful for decision-makers and political governments, and for health professionals, and it may also motivate people to change their lifestyles in a healthier direction, particularly if the message is simple.
An overall unhealthy lifestyle, quantified as the number of unhealthy life-related factors and obesity status, is associated with the incidence of microalbuminuria (10) and proteinuria (11). A community-based prospective cohort study reported that an overall unhealthy lifestyle, including current smoking, obesity (body mass index (BMI) of ≥30 kg/m2), and a poor diet quality as defined by a low Dietary Approaches to Stop Hypertension (DASH)-type diet score, was significantly associated with the incidence of microalbuminuria over a 15-year period, after adjusting for age, sex, family history of kidney disease, education, baseline hypertension, and diabetes (10). Another community-based cohort study reported that an overall unhealthy lifestyle, including current smoking, obesity (BMI of ≥25 kg/m2), heavy alcohol consumption, lack of physical activity, and unhealthy eating habits, was significantly associated with the incidence of proteinuria (11). These studies, however, were limited in that they failed to address how changes in lifestyle-related factors are associated with the incidence of proteinuria. Lifestyles change throughout life, even in the elderly stages (12).
In the above context, this study aimed to examine the association between changes in an overall healthy lifestyle and the incidence of proteinuria in the general Japanese population.
Materials and Methods
Study population
This retrospective cohort study used data from the Specific Health Checkups and Guidance System in Japan (SHC) in 2008 and 2009. The SHC has been described elsewhere (13,14). In brief, the participants were individuals aged from 40-74 years who underwent the SHC throughout Japan. The participants answer a self-administered questionnaire that covers their medical history, smoking habits, alcohol intake, exercise habits, and eating habits. Trained staff measures the height, weight, and blood pressure of each participant. Serum and spot urine samples were collected. The present study included participants who underwent two health check-ups with a 1-year interval in Japan between 2008 and 2009. Any participants with chronic kidney disease (CKD) at baseline were excluded. CKD was defined as glomerular filtration rate (GFR) <60 mL/min/1.73 m2 as calculated using the estimated GFR (eGFR) formula for Japanese individuals (15), ≥1+ proteinuria on urinalysis, or both (16).
All participants remained anonymous and the study complied with the Declaration of Helsinki and Ethical Guidelines for Epidemiological Studies published by the Ministry of Education, Science and Culture and the Ministry of Health, Labour and Welfare of Japan. The study protocol was approved by the ethics committee of Fukushima Medical University (IRB No. 1485).
Changes in overall healthy lifestyle scores and covariates
Overall healthy lifestyle scores were calculated at baseline and at the one-year follow-up by adding up the total number of lifestyle factors for which the participants were at low risk. For each lifestyle factor (smoking, BMI, alcohol intake, exercise habits, and eating habits), we created a binary low-risk variable, in which the participants were given a score of 1 if they met the criteria for low risk, and 0 if otherwise, in accordance with a previous study (11,13,17-27). The a priori definition of low risk was based not only on the current literature and recommended guidelines, but also on the levels that were considered to be realistically obtainable within the general population (11,13). The overall healthy lifestyle scores ranged from 0 (least healthy) to 5 (most healthy). For smoking, a low risk was defined as not currently smoking. An optimal body weight was defined as a BMI <25 kg/m2, the standard World Health Organization cutoff for healthy weight. For alcohol, low risk was defined as an average daily alcohol consumption of less than 20 g. For exercise habits, two questions were posed: ‘Are you in the habit of exercising to a light sweat for more than 30 minutes at a time, 2 times weekly, for over a year?’ and ‘In daily life, do you walk or do any equivalent amount of physical activity more than one hour a day?’ Those who answered ‘Yes’ to both questions were considered ‘low risk’ based on the current Japanese guidelines (11,13,28). Regarding eating habits, the following two questions were posed: ‘Do you skip breakfast more than 3 times per week?’ and ‘Do you eat snacks after supper more than 3 times a week?’. Those who answered ‘No’ to both questions were considered ‘low risk’ (11,13). We also calculated changes in overall healthy lifestyle scores during a one-year period (range, -5 to +5) and classified the participants according to score changes into three categories: improved (increased from lower to higher scores), deteriorated (decreased from higher to lower scores), and unchanged (the same scores) (29).
Diabetes was defined in accordance with the American Diabetes Association guidelines (30) as a fasting plasma glucose (FPG) concentration of 126 mg/dL or higher, HbA1c of 6.5% or higher, or self-reported use of anti-hyperglycaemic drugs. The value of haemoglobin A1c (HbA1c) was estimated as a National Glycohemoglobin Standardization Program equivalent value calculated with the following equation (31): HbA1c (%) = HbA1c (%) + 0.4%. Hypertension was defined as the use of antihypertensive medication, a systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥90 mmHg, or both. Hypercholesterolemia was defined as the use of cholesterol-lowering medication, a low-density lipoprotein (LDL) cholesterol level ≥140 mg/dL, or both.
Outcome measurement
The primary end-point was the incidence of proteinuria, defined as a dipstick urinalysis score of 1+ or greater (equivalent to ≥30 mg/dL) due to the difficulty of discriminating between negative and trace positive dipstick readings (32,33), at the SHC in 2009.
Statistical analyses
Data were analysed separately by sex, and presented as the mean (standard deviation) for continuous variables and the number of subjects (percent) for categorical variables. Descriptive statistics of clinical characteristics across the categories of overall healthy lifestyle score at baseline were compared using the chi-square test for trend for categorical data, and an analysis of variance (ANOVA) or the Jonckheere-Terpstra test for continuous variables. A logistic regression was used to assess the relationship between changes in overall healthy lifestyle scores and the incidence of proteinuria after adjusting for a priori identified potential covariates. The initial model tested the main effect of changes in overall healthy lifestyle scores as a continuous variable on the incidence of proteinuria, after adjusting for age (per 10-year increase) and overall healthy lifestyle scores at baseline (continuous variable). The second, fully adjusted model added a term for hypertension (yes/no), diabetes (yes/no), and hypercholesterolemia (yes/no).
To assess the robustness of the main results, sensitivity analyses were performed. First, we performed a multiple logistic regression analysis with baseline overall healthy lifestyle scores as a categorical variable. For women, baseline overall healthy lifestyle scores of 0 (least healthy) and 1 were combined into one category because there were few cases. Second, an age-stratified analysis was performed, since the age distribution of the study participants was dispersed. We included a continuous term for age in the age-stratified subgroup analysis. Baseline overall healthy lifestyle scores of 0-1 for men, and 0-2 for women, were treated as one category, because there were few cases. Third, subgroup analyses were performed based on the presence or absence of diabetes or hypertension. Finally, we ran separate models predicting the incidence of proteinuria for each individual lifestyle factor with the healthy lifestyle factor as a categorical variable. p<0.05 was considered to be statistically significant, and all tests were two-tailed. All statistical analyses were performed with SPSS for Windows statistical package (Version 18.0; SPSS, Chicago, IL, USA) and the Stata/MP software program (Version 12.1; Stata Corp, College Station, TX, USA).
Results
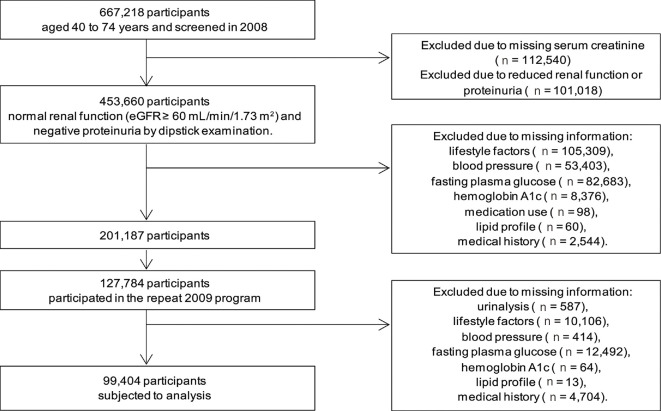
Of the 667,218 individuals aged 40-74 years who participated in the SHC in 2008, we excluded those for whom serum creatinine (n=112,540) measurements were unavailable (Fig. 1). The serum creatinine level is not included as a mandatory item of the SHC, but it is included in some areas. Among these individuals, 453,660 had normal renal function (eGFR ≥60 mL/min/1.73 m2) and negative proteinuria on dipstick examination. Among these individuals, those with missing information (n=252,473) were excluded. Of the individuals who were not missing any information (n=201,187), 127,784 were re-examined in the 2009 SHC program. Again, those with missing information were excluded (n=28,380), thus resulting in a final sample size of 99,404. Compared with those who met the inclusion criteria, those who were excluded who did not have CKD in 2008 were more likely to be male, younger, have hypertension, diabetes, a lower baseline healthy lifestyle score, and a history of stroke, heart disease, or renal disease (Supplementary Table 1).
Figure 1.
Flowchart of the participant selection.
Baseline characteristics of excluded and included participants without chronic kidney disease
Tables 1 and 2 show the clinical characteristics as a function of healthy lifestyle scores by sex. Participants with higher healthy lifestyle scores at baseline were more likely to be older, have a history of stroke, lower BMI, lower triglyceride levels, lower fasting glucose, lower eGFR, and less likely to have hypertension and diabetes in both men and women. Men with higher healthy lifestyle scores at baseline were more likely to have a history of heart disease and higher high-density lipoprotein (HDL)-cholesterol levels, while women with higher healthy lifestyle scores at baseline were more likely to have a history of renal failure.
Table 1.
Clinical Characteristics of Male Participants by Healthy Lifestyle Scores (n=36,703).
| Healthy lifestyle scores at baseline | p for trend |
||||||
|---|---|---|---|---|---|---|---|
| 0 [n=134 (0.4%)] |
1 [n=1,654 (4.5%)] |
2 [n=5,967 (16.3%)] |
3 [n=11,535 (31.4%)] |
4 [n=12,302 (33.5%)] |
5 [n=5,111 (13.9%)] |
||
| Baseline data | |||||||
| Age (years) | 55.8 (8.8) | 57.8 (9.3) | 60.8 (8.8) | 63.1 (8.4) | 64.8 (7.6) | 66.7 (6.3) | <0.001 |
| History of stroke (%) | 3.7 | 2.8 | 3.5 | 4.0 | 4.4 | 4.6 | <0.001 |
| History of heart disease (%) | 5.2 | 4.6 | 5.5 | 6.6 | 7.6 | 7.2 | <0.001 |
| History of renal failure (%) | 0 | 0.1 | 0.3 | 0.2 | 0.2 | 0.2 | 0.65 |
| Comorbidities (%) | |||||||
| Hypertension | 50.7 | 49.0 | 48.4 | 47.7 | 43.3 | 42.2 | <0.001 |
| Diabetes | 15.7 | 14.6 | 13.5 | 12.0 | 12.1 | 12.9 | 0.03 |
| Hypercholesterolemia | 39.6 | 34.9 | 32.8 | 33.0 | 32.6 | 32.8 | 0.16 |
| BMI (kg/m2) | 27.3 (2.2) | 25.5 (3.2) | 24.5 (3.3) | 23.8 (3.0) | 22.8 (2.4) | 22.2 (1.8) | <0.001 |
| Systolic BP (mmHg) | 131 (15) | 130 (17) | 131 (17) | 130 (17) | 129 (16) | 129 (16) | 0.02 |
| Diastolic BP (mmHg) | 82 (11) | 80 (11) | 79 (11) | 78 (10) | 77 (10) | 76 (10) | <0.001 |
| HDL cholesterol (mg/dL) | 55 (14) | 55 (15) | 57 (15) | 58 (15) | 59 (16) | 61 (15) | <0.001 |
| LDL cholesterol (mg/dL) | 125 (33) | 121 (32) | 121 (31) | 121 (29) | 121 (28) | 121 (27) | 0.13 |
| Triglycerides (mg/dL) | 147 [103, 217] | 134 [93, 202] | 118 [84, 171] | 106 [76, 151] | 97 [70, 136] | 88 [65, 122] | <0.001 |
| FPG (mg/dL) | 106 (23) | 103 (26) | 101 (22) | 100 (21) | 100 (20) | 99 (19) | <0.001 |
| Haemoglobin A1c(%) | 5.9 (0.7) | 5.8 (0.9) | 5.8 (0.7) | 5.7 (0.7) | 5.7 (0.7) | 5.7 (0.6) | 0.01 |
| Serum Cr (mg/dL) | 0.77 (0.11) | 0.78 (0.11) | 0.78 (0.11) | 0.78 (0.11) | 0.79 (0.10) | 0.80 (0.10) | <0.001 |
| eGFR (mL/min/1.73 m2) | 85 (15) | 82 (15) | 81 (14) | 79 (14) | 78 (13) | 76 (12) | <0.001 |
| Follow-up data | |||||||
| Healthy lifestyle scores in 2009 (%) |
|||||||
| 0 | 33.6 | 3.9 | 0.3 | 0.1 | 0.0 | 0.0 | |
| 1 | 43.3 | 47.6 | 8.3 | 1.1 | 0.2 | 0.0 | |
| 2 | 17.2 | 35.2 | 51.4 | 14.1 | 2.6 | 0.4 | |
| 3 | 6.0 | 11.2 | 31.5 | 56.1 | 20.1 | 5.9 | |
| 4 | 0.0 | 1.9 | 7.6 | 25.2 | 61.6 | 32.1 | |
| 5 | 0.0 | 0.1 | 0.9 | 3.4 | 15.5 | 61.6 | |
| Incidence of proteinuria (%) | 6.7 | 5.7 | 4.7 | 4.2 | 3.3 | 3.1 | <0.001 |
Numbers are mean (standard deviation) or proportion. For triglycerides, median and the 25th and 75th percentile are shown.
BMI: body mass index, BP: blood pressure, HDL: high-density lipoprotein, LDL: low-density lipoprotein, FPG: fasting plasma glucose, Cr: creatinine, eGFR: estimated glomerular filtration rate
Table 2.
Clinical Characteristics of Female Participants by Healthy Lifestyle Scores (n=62,701).
| Healthy lifestyle scores at baseline | p for trend |
||||||
|---|---|---|---|---|---|---|---|
| 0 [n=26 (0.0%)] |
1 [n=347 (0.6%)] |
2 [n=3,363 (5.4%)] |
3 [n=14,318 (22.8%)] |
4 [n=31,771 (50.7%)] |
5 [n=12,876 (20.5%)] |
||
| Baseline data | |||||||
| Age (years) | 56.4 (8.4) | 56.2 (9.1) | 60.0 (8.8) | 62.4 (8.1) | 63.9 (7.4) | 65.9 (5.9) | <0.001 |
| History of stroke (%) | 0.0 | 1.7 | 2.7 | 2.4 | 2.2 | 2.1 | 0.03 |
| History of heart disease (%) | 3.8 | 2.9 | 4.7 | 4.9 | 4.5 | 4.5 | 0.31 |
| History of renal failure (%) | 0.0 | 0.3 | 0.4 | 0.4 | 0.3 | 0.2 | 0.001 |
| Comorbidities (%) | |||||||
| Hypertension | 53.8 | 39.5 | 43.1 | 43.9 | 37.0 | 37.0 | <0.001 |
| Diabetes | 11.5 | 7.8 | 9.3 | 8.3 | 5.5 | 6.6 | <0.001 |
| Hypercholesterolemia | 38.5 | 44.1 | 51.9 | 52.6 | 49.8 | 51.1 | 0.05 |
| BMI (kg/m2) | 27.2 (1.9) | 24.6 (4.2) | 25.5 (4.0) | 24.4 (3.8) | 21.8 (2.7) | 21.4 (2.1) | <0.001 |
| Systolic BP (mmHg) | 127 (16) | 127 (17) | 128 (18) | 128 (17) | 127 (17) | 127 (17) | 0.97 |
| Diastolic BP (mmHg) | 75 (12) | 75 (10) | 76 (11) | 75 (10) | 74 (10) | 74 (10) | 0.44 |
| HDL cholesterol (mg/dL) | 65 (13) | 64 (17) | 63 (15) | 64 (16) | 67 (16) | 68 (16) | 0.06 |
| LDL cholesterol (mg/dL) | 127 (42) | 125 (35) | 131 (32) | 131 (30) | 129 (29) | 129 (29) | 0.42 |
| Triglycerides (mg/dL) | 128 [91, 209] | 113 [79, 166] | 104 [76, 144] | 96 [71, 132] | 88 [66, 119] | 85 [65, 115] | <0.001 |
| FPG (mg/dL) | 101 (15) | 97 (16) | 97 (20) | 95 (17) | 93 (14) | 94 (14) | 0.001 |
| Haemoglobin A1c(%) | 5.7 (0.6) | 5.6 (0.6) | 5.7 (0.6) | 5.7 (0.6) | 5.7 (0.5) | 5.7 (0.5) | 0.51 |
| Serum Cr (mg/dL) | 0.57 (0.10) | 0.58 (0.09) | 0.59 (0.09) | 0.59 (0.08) | 0.60 (0.08) | 0.60 (0.08) | 0.01 |
| eGFR (mL/min/1.73 m2) | 87 (17) | 84 (17) | 81 (15) | 80 (14) | 79 (14) | 77 (13) | <0.001 |
| Follow-up data | |||||||
| Healthy lifestyle scores in 2009 (%) | |||||||
| 0 | 30.8 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 1 | 23.1 | 44.7 | 3.4 | 0.3 | 0.0 | 0.0 | |
| 2 | 38.5 | 38.6 | 47.3 | 7.8 | 0.7 | 0.1 | |
| 3 | 3.8 | 13.8 | 37.9 | 57.9 | 12.8 | 3.5 | |
| 4 | 3.8 | 2.3 | 10.4 | 30.3 | 71.4 | 35.3 | |
| 5 | 0.0 | 0.0 | 1.0 | 3.8 | 15.1 | 61.1 | |
| Incidence of proteinuria (%) | 0.0 | 4.3 | 3.4 | 3.1 | 2.2 | 1.9 | <0.001 |
Numbers are mean (standard deviation) or proportion. For triglycerides, median and the 25th and 75th percentile are shown.
BMI: body mass index, BP: blood pressure, HDL: high-density lipoprotein, LDL: low-density lipoprotein, FPG: fasting plasma glucose, Cr: creatinine, eGFR: estimated glomerular filtration rate
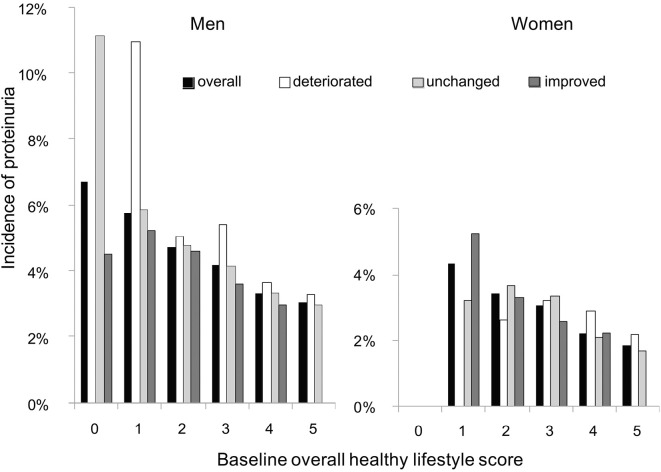
After one year of follow-up, 23.1% of men and 18.4% of women had a higher overall healthy lifestyle score (the ‘improved’ category), whereas 19.4% of men and 16.9% of women had a lower overall healthy lifestyle scores (the ‘deteriorated’ category); 57.5% of men and 64.7% of women had the same scores as those at baseline (the ‘unchanged’ category). The onset of proteinuria was noted in 1,434 of 36,703 men (3.9%) and 1,514 of 62,701 women (2.4%). Overall, a clearly inverse dose-dependent relationship was observed between the baseline overall healthy lifestyle scores and the incidence of proteinuria in both men and women (p<0.001 for trend, Fig. 2). The incidence of proteinuria tended to be higher among the participants in the deteriorated category, but lower in the improved category, compared with those in the unchanged category.
Figure 2.
Incidence of proteinuria as stratified by the baseline scores and changes in overall healthy lifestyle scores. Changes in the overall healthy lifestyle scores (non-smoking, healthy weight, adequate alcohol drinking, physically active, and healthy eating habits) were categorized as ‘improved’ (increased from lower to higher scores), ‘deteriorated’ (decreased from higher to lower scores), and ‘unchanged’ (the same scores). Overall, a clearly dose-dependent relationship was observed between the baseline overall healthy lifestyle scores and the incidence of proteinuria in both men and women (■: p<0.001 for trend).
Table 3 shows the results of a logistic regression analysis estimating the odds of the incidence of proteinuria after a one-year follow-up by sex. These results indicate that having hypertension, diabetes, or lower baseline overall healthy lifestyle scores, and decreases in healthy lifestyle scores were all associated with greater odds of developing proteinuria in both men and women. Each increase in healthy lifestyle factors at baseline was associated with 18% (95% confidence interval (CI), 13-22%) and 24% (95% CI, 18-29%) lower adjusted odds ratios (OR) for incident proteinuria in men and women, respectively. A 1-healthy lifestyle score increase (or decrease) after one year was independently associated with a 13% (95% CI, 6-19%) and 13% (95% CI, 6-20%) reduction (or increase) in OR for incident proteinuria in men and women, respectively.
Table 3.
Prediction of Incidence of Proteinuria by Changes in Healthy Lifestyle Score.
| Variable | Male (n=36,703) | Female (n=62,701) | |||
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p value | Odds Ratio (95% CI) | p value | ||
| Age, y | 1.00 (1.00, 1.01) | 0.27 | 1.01 (1.00, 1.01) | 0.11 | |
| Hypertension | 1.71 (1.53, 1.91) | <0.001 | 1.59 (1.43, 1.77) | <0.001 | |
| Diabetes | 1.71 (1.50, 1.96) | <0.001 | 1.66 (1.41, 1.96) | <0.001 | |
| Hypercholesterolemia | 1.02 (0.91, 1.14) | 0.77 | 1.08 (0.98, 1.20) | 0.13 | |
| Healthy lifestyle score change | 0.87 (0.81, 0.94) | <0.001 | 0.87 (0.80, 0.94) | 0.001 | |
| Healthy lifestyle score at baseline | 0.82 (0.78, 0.87) | <0.001 | 0.76 (0.71, 0.82) | <0.001 | |
Covariates included in the model were age, healthy lifestyle score at baseline, hypertension, diabetes, and hypercholesterolemia.
CI: confidence interval
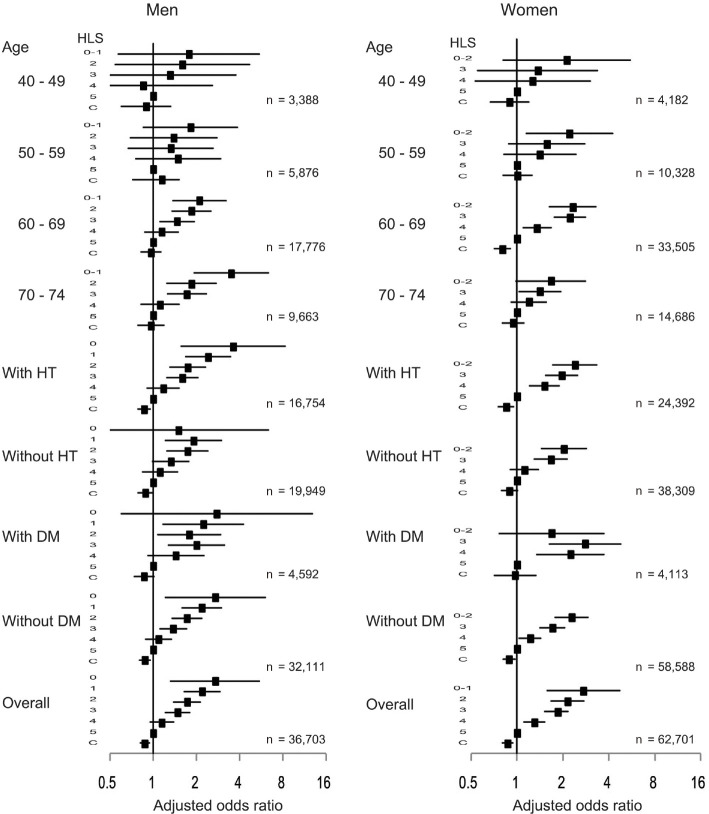
Logistic regression analyses were repeated with baseline overall healthy lifestyle scores as a categorical variable instead of a continuous variable, yielding similar results (Fig. 3). Each increase (or decrease) in changes in healthy lifestyle scores was associated with a reduced (or increased) OR for incident proteinuria in both men (OR, 0.87; 95% CI, 0.81-0.94) and women (OR, 0.87; 95% CI, 0.80-0.94). When stratified by age categories, the associations were generally similar with those of the entire study population, although they were no longer significant, except in the female age category of 60-69 years of age. Stratified analyses based on the presence or absence of hypertension revealed that the associations were generally similar with those of the entire study population. Furthermore, the associations were consistent among the participants without diabetes; among the participants with diabetes, no significant association was observed in both men (OR, 0.89; 95% CI, 0.79-1.00) and women (OR, 0.97; 95% CI, 0.71-1.33).
Figure 3.
Forest plot showing odds ratios with 95% confidence intervals for the association between baseline healthy lifestyle scores or changes in healthy lifestyle scores and the incidence of proteinuria in the subgroups and the entire study population. All analyses were adjusted for the following covariates (except for the variables used to define the subgroup in each case): age, hypertension, diabetes, and hypercholesterolemia. C: changes in healthy lifestyle scores, DM: diabetes mellitus, HT: hypertension, HLS: healthy lifestyle scores
We also performed an analysis using separate models predicting the incidence of proteinuria for each individual lifestyle factor with the healthy lifestyle factor as a categorical variable (Table 4). Compared with the category of individuals with no adherence to healthy lifestyle factors at baseline (Y0) or one-year follow-up (Y1) (‘Neither Y0 Y1’), improved changes in alcohol consumption were significantly associated with reduced OR for incident proteinuria in men (OR, 0.73; 95% CI, 0.58-0.93). On the other hand, quitting smoking was associated with an increased OR (OR, 2.05; 95% CI, 1.21-3.46) in women, relative to the Neither Y0 Y1 category. The associations were not significant in positive changes in other individual lifestyle factors during the one-year follow-up period. Relative to the Neither Y0 Y1 category, negative changes in each individual lifestyle factor during the one-year follow-up period (‘Yes Y0, No Y1’) were not significantly associated with an increased OR for the incidence of proteinuria.
Table 4.
Odds Ratios (95% CI) and p Values for Categorically Defined Individual Healthy Lifestyle Factor Components Predicting the Incidence of Proteinuria, with ’Neither Y0 Y1’ as the Reference, in Men and Women.
| Healthy Lifestyle Score component |
Male (n=36,703) | Female (n=62,701) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Neither Y0 Y1 |
No Y0, Yes Y1 |
Yes Y0, No Y1 |
Yes Y1, Yes Y1 |
Neither Y0 Y1 |
No Y0, Yes Y1 |
Yes Y0, No Y1 |
Yes Y1, Yes Y1 |
||
| Not smoking | ref | 1.10 (0.81, 1.49); p=0.54 |
0.99 (0.63, 1.56); p=0.99 |
0.81 (0.71, 0.92); p=0.001 |
ref |
2.05 (1.21, 3.46); p=0.01 |
1.20 (0.54, 2.69); p=0.65 |
1.02 (0.77, 1.35); p=0.91 |
|
| BMI<25 kg/m2 | ref | 0.76 (0.57, 1.02); p=0.06 |
0.86 (0.64, 1.15); p=0.31 |
0.72 (0.64, 0.81); p<0.001 |
ref | 0.98 (0.74, 1.30); p=0.88 |
0.73 (0.53, 1.02); p=0.06 |
0.62 (0.55, 0.70); p<0.001 |
|
| Low alcohol | ref |
0.73 (0.58, 0.93); p=0.01 |
0.87 (0.68, 1.10); p=0.24 |
0.89 (0.79, 1.01); p=0.07 |
ref | 0.92 (0.50, 1.69); p=0.79 |
0.66 (0.32, 1.37); p=0.27 |
0.92 (0.63, 1.34); p=0.66 |
|
| Regular exercise | ref | 0.92 (0.77, 1.09); p=0.35 |
0.85 (0.70, 1.03); p=0.10 |
0.92 (0.80, 1.04); p=0.18 |
ref | 0.95 (0.80, 1.12); p=0.51 |
0.95 (0.79, 1.14); p=0.56 |
0.87 (0.76, 1.00); p=0.05 |
|
| Healthy eating habits | ref | 0.84 (0.66, 1.06); p=0.15 |
1.05 (0.84, 1.31); p=0.66 |
0.76 (0.65, 0.89); p=0.001 |
ref | 0.87 (0.69, 1.09); p=0.22 |
1.07 (0.87, 1.33); p=0.52 |
0.77 (0.65, 0.90); p=0.001 |
|
Covariates included in the model were age, hypertension, diabetes, hypercholesterolemia, smoking status, BMI, alcohol intake, regular exercise, and healthy eating habits (except for the variable used to define the subgroup in each case).
CI: confidence interval, Y0: year 0 (at baseline), Y1: year 1 (1 year follow-up)
Discussion
This nationwide, large retrospective cohort study revealed that about one fifth of all participants improved their overall healthy lifestyle scores and another fifth of the participants demonstrated a deterioration in their scores after a one-year follow-up, suggesting that lifestyle deterioration was as common as lifestyle improvement in the general population without CKD. Furthermore, this study demonstrated that changes in the overall lifestyle scores, even within a duration as short as one year, were independently associated with the incidence of proteinuria after adjusting for age, baseline lifestyle scores, hypertension, diabetes mellitus, and hypercholesterolemia for both men and women without CKD at baseline. A 1-healthy lifestyle score increase (or decrease) after one year was independently associated with a 13% reduction (or increase) in OR for incident proteinuria in both sexes. These findings indicate that lifestyle changes, even within a year, can significantly influence incident proteinuria. This simple message is therefore considered to be informative for health professionals to motivate people to change their lifestyle in a healthier direction.
Consistent with previous studies, a significant, graded, inverse association was observed between the overall healthy lifestyle scores at baseline and incident proteinuria. However, with regard to the individual components of the healthy lifestyle score, negative and positive (with the exception of alcohol intake in men) changes in each individual lifestyle factor after one year were not significantly associated with an increased OR for the incidence of proteinuria. These results suggest that although the individual effects of each factor might be weak or insignificant, multiple healthy lifestyle behaviours might have an additive or synergistic positive influence on health. Previous observational studies support this possibility by showing linear relationships between risk reduction and the number of healthy lifestyle behaviours for several diseases, such as coronary heart disease (17,18), type 2 diabetes mellitus (19), stroke (20), dementia (21), sudden cardiac death (22), and cancer (23-25), as well as a reduction in total mortality (26).
In addition to the additive or synergistic positive influence on health, targeting multiple risk behaviours offers the potential to increase health benefits. Multiple behaviour intervention, defined as efforts to treat two or more health behaviours either simultaneously or sequentially within a limited time period, have shown substantial and real effects on behaviour changes. For example, the impact of multiple behaviour intervention for weight management had three times the effect of single behaviour intervention (34). Smokers treated for two or three behaviours were as effective in being abstinent at long-term follow-up as those treated for only smoking (35). Success in changing one or more lifestyle behaviours may also increase one's confidence or self-efficacy to improve risk behaviours for which individuals have little motivation to alter (36). Although more studies on multiple behaviour intervention are needed, we would like to emphasize the desirability of a unified simple approach that promotes common steps for preventing several diseases and conditions including proteinuria.
The present study revealed an unexpected association between quitting smoking and an increased OR for the incidence of proteinuria in women. This finding is contradictory to previous reports, which demonstrated that cigarette smoking was independently associated with an increased risk of proteinuria for both men and women (7,37), and that the discontinuation of smoking substantially reduced the risk of proteinuria in the healthy middle-aged working population (38). Regarding the reason for this surprising result, the possibility of reverse causality could be suggested, i.e., poor health associated with proteinuria might have led to smoking cessation.
Our study is associated with several limitations. First, proteinuria was measured by a urine dipstick only once. However, a previous Japanese cohort study revealed that proteinuria measured by urine dipstick only once significantly predicted end-stage kidney disease (1). Thus, the outcome of our study is still meaningful. Second, the overall healthy lifestyle scores, except for BMI, were determined based on self-reporting and thus may not be accurate. We also did not evaluate nutrition, given the lack of information on this aspect. Furthermore, optimal body weight was defined as BMI <25 kg/m2 in agreement with previous studies, and a lower limit of BMI was not set. Because being underweight is associated with the incidence of proteinuria in women (39), this definition may weaken the true associations between overall healthy lifestyle scores and the incidence of proteinuria. Third, we did not have any information regarding antihypertensive drug classes, such as angiotensin-converting-enzyme inhibitors or angiotensin receptor blockers, that might influence the incidence of proteinuria. However, our results were generally similar with or without hypertension. Fourth, despite adjusting for potential confounding factors, residual confounding factors may still have influenced these findings. For example, we did not adjust for socioeconomic status because this information was not available. Finally, the study duration was relatively short for detecting the incidence of proteinuria in the general population. Despite this, our study revealed that healthy lifestyle changes could result in significant effects on incident proteinuria, even within a short time frame. We believe that the large sample size of this study may have compensated for this limitation.
Despite these limitations, this study also has strengths worth noting. First, the study was a large-scale retrospective cohort study with participants from throughout Japan. Second, to the best of our knowledge, this is the first report demonstrating the influence of changes in overall healthy lifestyle on the incidence of proteinuria in the general Japanese population. Our findings suggest the possibility that multiple behaviour intervention might be effective in preventing several chronic diseases, such as CKD.
In conclusion, overall healthy lifestyle changes, even within the short span of a year, can significantly influence the incidence of proteinuria. Our data highlight the importance of an overall healthy lifestyle for preventing proteinuria, and also highlight the influence of lifestyle deterioration in accelerating the incidence of proteinuria, even within a year. These findings therefore suggest that it is never too late to change one's lifestyle.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
This study was supported by a Health and Labour Sciences Research Grant for “Design of the comprehensive health care system for chronic kidney disease based on the individual risk assessment by Specific Health Checkups” from the Ministry of Health, Labour and Welfare of Japan, and in part by a Grant-in-Aid for Research on Advanced Chronic Kidney Disease, Practical Research Project for Renal Diseases from the Japan Agency for Medical Research and Development, AMED.
References
- 1.Iseki K, Iseki C, Ikemiya Y, Fukiyama K. Risk of developing end-stage renal disease in a cohort of mass screening. Kidney Int 49: 800-805, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Stampfer MJ, Castelli WP, Verter J. The prognostic significance of proteinuria: the Framingham study. Am Heart J 108: 1347-1352, 1984. [DOI] [PubMed] [Google Scholar]
- 3.Grimm RH Jr, Svendsen KH, Kasiske B, Keane WF, Wahi MM. Proteinuria is a risk factor for mortality over 10 years of follow-up. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Kidney Int 63 (Suppl): S10-S14, 1997. [PubMed] [Google Scholar]
- 4.Culleton BF, Larson MG, Parfrey PS, Kannel WB, Levy D. Proteinuria as a risk factor for cardiovascular disease and mortality in older people: a prospective study. Am J Med 109: 1-8, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Wen CP, Cheng TY, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 371: 2173-2182, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Scheven L, Van der Velde M, Lambers Heerspink HJ, De Jong PE, Gansevoort RT. Isolated microalbuminuria indicates a poor medical prognosis. Nephrol Dial Transplant 28: 1794-1801, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Yamagata K, Ishida K, Sairenchi T, et al. Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int 71: 159-166, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Tozawa M, Iseki K, Iseki C, Oshiro S, Ikemiya Y, Takishita S. Influence of smoking and obesity on the development of proteinuria. Kidney Int 62: 956-962, 2002. [DOI] [PubMed] [Google Scholar]
- 9.White SL, Polkinghorne KR, Cass A, Shaw JE, Atkins RC, Chadban SJ. Alcohol consumption and 5-year onset of chronic kidney disease: the AusDiab study. Nephrol Dial Transplant 24: 2464-2472, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Chang A, Van Horn L, Jacobs DR Jr, et al. Lifestyle-related factors, obesity, and incident microalbuminuria: the CARDIA (Coronary Artery Risk Development in Young Adults study. Am J Kidney Dis 62: 267-275, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakasugi M, Kazama JJ, Yamamoto S, Kawamura K, Narita I. A combination of healthy lifestyle factors is associated with a decreased incidence of chronic kidney disease: a population-based cohort study. Hypertens Res 36: 328-333, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Zanjani FA, Schaie KW, Willis SL. Age group and health status effects on health behavior change. Behav Med 32: 36-46, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Wakasugi M, Kazama JJ, Narita I, et al. Association between combined lifestyle factors and non-restorative sleep in Japan: a cross-sectional study based on a Japanese health database. PloS one 9: e108718, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato Y, Yano Y, Fujimoto S, et al. Glycohemoglobin not as predictive as fasting glucose as a measure of prediabetes in predicting proteinuria. Nephrol Dial Transplant 27: 3862-3868, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982-992, 2009. [DOI] [PubMed] [Google Scholar]
- 16.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39: S1-S266, 2002. [PubMed] [Google Scholar]
- 17.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 343: 16-22, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation 114: 160-167, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 345: 790-797, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Kurth T, Moore SC, Gaziano JM, et al. Healthy lifestyle and the risk of stroke in women. Arch Intern Med 166: 1403-1409, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Elwood P, Galante J, Pickering J, et al. Healthy lifestyles reduce the incidence of chronic diseases and dementia: evidence from the Caerphilly cohort study. PloS one 8: e81877, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiuve SE, Fung TT, Rexrode KM, et al. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA 306: 62-69, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Platz EA, Willett WC, Colditz GA, Rimm EB, Spiegelman D, Giovannucci E. Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control 11: 579-588, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Jiao L, Mitrou PN, Reedy J, et al. A combined healthy lifestyle score and risk of pancreatic cancer in a large cohort study. Arch Intern Med 169: 764-770, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasazuki S, Inoue M, Iwasaki M, et al. Combined impact of five lifestyle factors and subsequent risk of cancer: the Japan Public Health Center Study. Prev Med 54: 112-116, 2012. [DOI] [PubMed] [Google Scholar]
- 26.van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ 337: a1440, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu K, Daviglus ML, Loria CM, et al. Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age: the Coronary Artery Risk Development in (Young) Adults (CARDIA) study. Circulation 125: 996-1004, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Office for Lifestyle-Related Diseases Control GAD, Health Service Bureau, Ministry of Health, Labour and Welfare of Japan. Exercise and Physical Activity Guide for Health Promotion 2006- To Prevent Lifestyle-related Diseases-<Exercise Guide 2006> Prepared in August, 2006. [Internet]. [cited 2011 Sep 4]. Available from: http://www.nih.go.jp/eiken/programs/pdf/exercise_guide.pdf
- 29.Spring B, Moller AC, Colangelo LA, et al. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation 130: 10-17, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Diabetes A Diagnosis and classification of diabetes mellitus. Diabetes Care 34 (Suppl 1): S62-S69, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seino Y, Nanjo K, Tajima N, et al. ; Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes M Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 1: 212-228, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrison NA, Rainford DJ, White GA, Cullen SA, Strike PW. Proteinuria--what value is the dipstick? Br J Urol 63: 202-208, 1989. [DOI] [PubMed] [Google Scholar]
- 33.White SL, Yu R, Craig JC, Polkinghorne KR, Atkins RC, Chadban SJ. Diagnostic accuracy of urine dipsticks for detection of albuminuria in the general community. Am J Kidney Dis 58: 19-28, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Johnson SS, Paiva AL, Cummins CO, et al. Transtheoretical model-based multiple behavior intervention for weight management: effectiveness on a population basis. Prev Med 46: 238-246, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prochaska JJ, Velicer WF, Prochaska JO, Delucchi K, Hall SM. Comparing intervention outcomes in smokers treated for single versus multiple behavioral risks. Health Psychol 25: 380-388, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Prochaska JJ, Prochaska JO. A review of multiple health behavior change interventions for primary prevention. Am J Lifestyle Med 2011. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon HJ, Park M, Yoon H, Son KY, Cho B, Kim S. The differential effect of cigarette smoking on glomerular filtration rate and proteinuria in an apparently healthy population. Hypertens Res 32: 214-219, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Noborisaka Y, Ishizaki M, Yamada Y, et al. The effects of continuing and discontinuing smoking on the development of chronic kidney disease (CKD) in the healthy middle-aged working population in Japan. Environ Health Prev Med 18: 24-32, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jang CM, Hyun YY, Lee KB, Kim H. The association between underweight and the development of albuminuria is different between sexes in relatively healthy Korean subjects. Nephrol Dial Transplant 29: 2106-2113, 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics of excluded and included participants without chronic kidney disease