Abstract
Localized small rectal neuroendocrine tumors (NETs) without any vascular involvement rarely metastasize, and their resection alone is considered curative. We herein report a case of localized rectal NET (10×8 mm) without vascular involvement. Although resected initially, it recurred as liver metastasis 30 years later. For rectal NETs smaller than 10 to 20 mm, surveillance for 12 months is considered sufficient. However, this case suggests that such tumors can recur even 30 years after curative resection. The interval of recurrence is the longest among reported cases.
Keywords: rectal neuroendocrine tumor, liver metastasis, recurrence
Introduction
The incidence of rectal neuroendocrine tumors (NETs) has increased because of more frequent colonic investigations (1,2). Some 75-85% of rectal NETs are localized at the time of diagnosis (3). An increased size and vascular involvement are associated with greater metastasis risk (4). Tumors smaller than 10 or 20 mm that are confined to the submucosa and with no vascular involvement rarely metastasize (5,6). The 5-year survival rate for localized rectal NETs is 90-100%, and resection alone is considered to be sufficient to obtain a cure (1,7,8). Therefore, 12 months' follow-up is enough after resection of localized rectal NET (9).
We herein report a case with recurrent liver metastasis of localized small rectal NET 30 years after curative resection. To our knowledge, this case represents the longest reported interval from resection to liver metastasis among cases of localized rectal NET.
Case Report
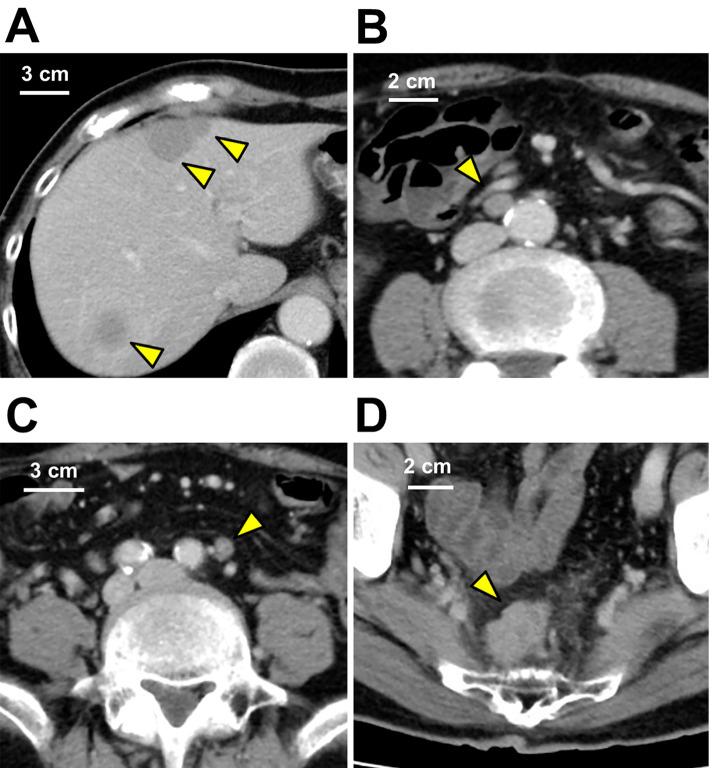
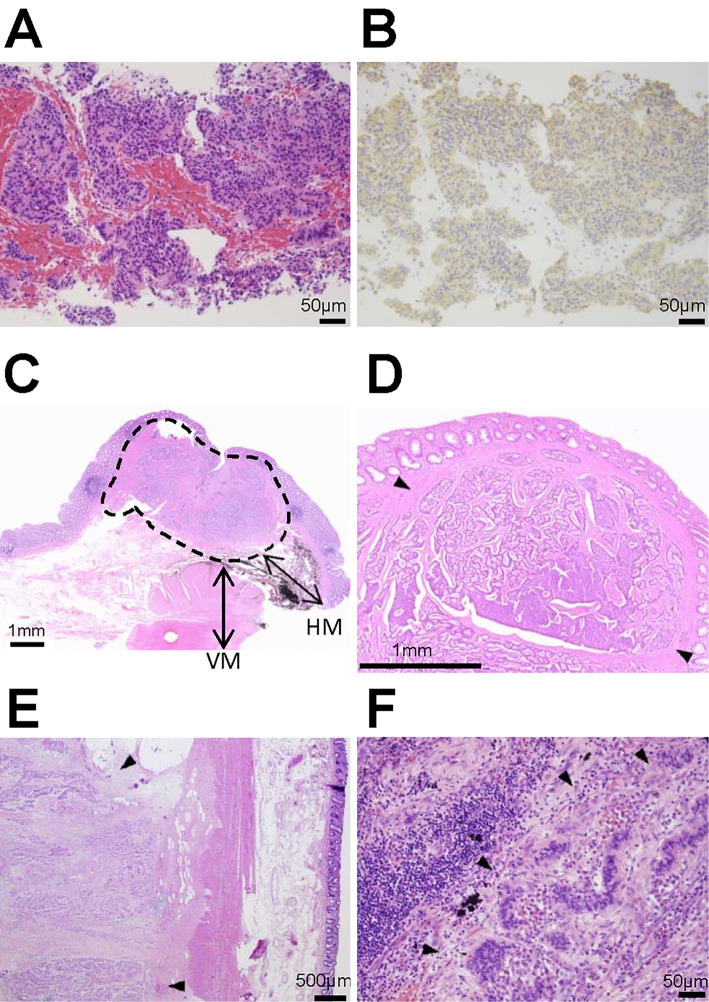
A 78-year-old asymptomatic Japanese man underwent an annual abdominal ultrasound examination for a suspicious liver hemangioma in the upper posterior segment. The lesion was identified during a medical checkup two years prior, and an increase in the tumor size had been noticed (Fig. 1). For a more detailed examination, contrast-enhanced computed tomography (CT) of the whole body, from the chest to the pelvic region, was conducted, revealing multiple low-density areas in the liver that showed lower enhancement than the normal liver during the contrast phase. In addition, swollen lymph nodes (LNs) around the aorta and inferior mesenteric artery (IMA) and a nodule in the paraproctium of the upper rectum were observed (Fig. 2). Gadoxetic-acid-enhanced magnetic resonance imaging (MRI) of the upper and lower abdomen provided little additional information. Upper and lower gastrointestinal endoscopy revealed no relevant findings. On positron emission tomography of the whole body, slightly increased fluorodeoxyglucose (FDG) uptake in the nodule located in the paraproctium was observed, and we did not obtain significant findings to indicate primary site. A liver biopsy of the tumors showed characteristic “organoid” arrangements with nesting, trabecular, or gyriform patterns, whose cells were uniform with a round nucleus and scant acidophilic cytoplasm; an immunohistochemical analysis revealed the tumor cells to be positive for chromogranin A (Fig. 3A, B). The Ki-67 labeling index of the tumor was 10%. Thus, the liver tumors were diagnosed as intermediate-grade (G2) NET.
Figure 1.
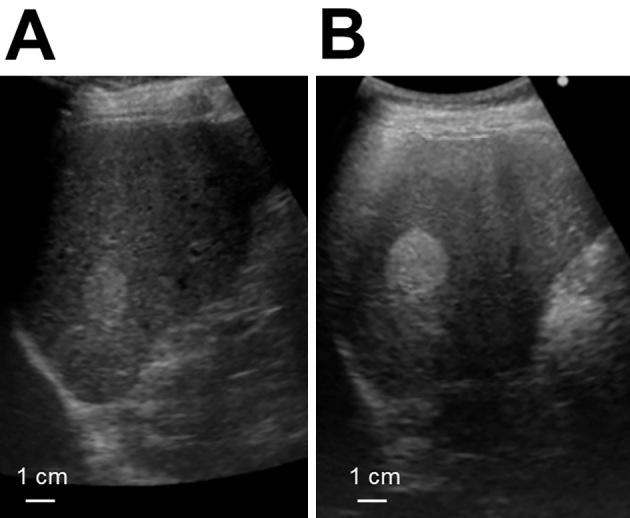
Abdominal ultrasound images of the liver. (A) Abdominal ultrasound revealed a hyperechoic lesion 23×19 mm in size in the posterior lateral segment of liver. (B) Four months later, the size of the lesion had increased to 29×23 mm. Although the appearance was compatible with hemangioma, the increase in size over a short time interval was unusual.
Figure 2.
Contrast-enhanced CT of the abdomen. (A) Multiple low-density areas without capsules were observed in the liver (yellow arrows). The lesions were homogeneous and showed lower density than the normal liver during the contrast phases. (B) In the para-aortic lymph node (yellow arrow) and (C) inferior mesenteric lymph node (yellow arrow), the contrast enhancement patterns were similar to those of the liver lesions, and the size had increased to 10×7 mm and 9×6 mm, respectively. (D) A nodule (yellow arrow) 25×20 mm in size was observed in the paraproctium of the upper rectum. These findings suggested that the liver lesions were metastasis from the rectal lesion.
Figure 3.
A histopathological analysis of the biopsy and surgical specimen. (A) The liver biopsy specimen revealed that the tumor displayed the classical histologic architecture of trabecular or ribbon-like cell clusters with little or no cellular pleomorphism and a few mitoses. (B) An immunohistochemical analysis showed that the tumor cells expressed chromogranin A in the cytoplasm. (C) A cross-section of the primary rectal neuroendocrine tumor showed that the lesion was localized in the submucosa (area encircled by a broken line), and the vertical and horizontal margins were negative for the tumor cells. VM: vertical margin, HM: horizontal margin. (D) The histological appearance of the primary rectal neuroendocrine tumor (arrows) that was diagnosed 30 years ago showed solid, ribbon-like and acinar growth patterns. (E) In the paraproctium, the tumor deposit (arrows) showed an acinar pattern of growth. (F) In the para-aortic lymph node, tumor cells (arrows) that were similar to the liver tumor were observed. Given these findings, the NETs in the paraproctium, lymph nodes and the liver were considered to be recurrence of rectal NET.
The patient's medical history revealed that he had experienced NET located in the rectum below the peritoneal reflection without any metastasis at the age of 48 years (30 years prior), and transsacral posterior proctotomy had been performed. The NET had presented as a submucosal tumor with slight depression and lack of surface ulceration in the central region covered by regenerative epithelium. The excised tumor size had been 10×8 mm and confined to the submucosa (Fig. 3C). Elastica van Gieson stain and immunohistochemistry using CD31 and D2-40 had been performed, confirming negative vessel involvement in the tumor. The tumor had shown ribbon-like or solid growth patterns (Fig. 3D). The mitotic index of the tumor had been approximately 4 per 10 high-power fields, and the Ki-67 labeling index had been 5%, suggesting the tumor was of intermediate grade. In addition, the margin had been pathologically negative; implying a low risk of recurrence. We also reviewed the pathological sections and obtained the same findings.
Given these findings, low anterior resection with D3 plus para-aortic LNs dissection and right hepatectomy and partial hepatectomy of the left upper posterior segment were performed. In the pathological examination, more than 30 tumors, with sizes ranging from 1 mm to 50 mm, were detected in the liver. The para-aortic LN (one out of seven dissected LNs) and an inferior mesenteric LN (one out of five dissected LNs) had metastatic tumors. A 25×20 mm tumor deposit without LN structure was also observed in the paraproctium with venous invasion. There were no metastatic LNs around the rectum. The histological appearances of the tumors were similar to those on the liver biopsy, and the findings were consistent with recurrent metastatic rectal NET (Fig. 3E, F). A year after the surgery, liver metastasis of the NET recurred. A contrast CT scan of the whole body at the time showed no tumors in other areas, and the findings further suggested that the metastatic NETs were likely derived from the rectal NET that had been diagnosed 30 years prior. The patient underwent radiofrequency ablation and repeat liver resection. At the time of drafting this report, the patient remains alive and has maintained a good performance status.
Discussion
We reported a case of rectal localized NET with a liver metastasis 30 years after curative surgery. Although the definitive route of recurrence is not clear, the NET located in the paraproctium as a tumor deposit was considered to be derived from an extramural venous invasion of the primary tumor. This is because the deposit had massive vessel invasions and no LN structures. The LN and liver metastases might have been derived from this tumor deposit. The primary tumor had been small and confined to the submucosa without any evidence of vascular involvement; recurrence of such a tumor with a long interval from curative surgery is quite rare (10,11). A case of liver metastasis 13 years after resecting a rectal NET smaller than 10 mm has been reported (12). Our case shows a longer time period from the primary operation to recurrence. In addition to the tumor size (not less than 10 mm), lymphovascular invasion, muscularis propria invasion and the mitotic index are associated with metastasis in rectal NET (10-14). We noted none of these risk factors in our case except for the tumor size (10×8 mm). The National Comprehensive Cancer Network recommended a postoperative proctoscopic examination be conducted at 6 and 12 months for rectal NETs of size 10-20 mm (9); however, our exceptional case demonstrated that this follow-up period is insufficient.
Interestingly, in this case, the histological grade and mitotic index of the lesion changed only slightly over three decades. Transformation from well-differentiated NETs into poorly differentiated neuroendocrine carcinoma (NEC) has only been reported exceptionally; some tumors show features of well-differentiated NETs containing areas showing a much higher proliferative rate with more atypical cytological features (15). The relatively good prognosis of NETs indicates that they are distinct from poorly differentiated NEC (15). A recent genomic investigation also revealed that poorly differentiated NEC does not show the same genetic progression of a low- or intermediate-grade well-differentiated NET (16).
In conclusion, this was a rare case of a recurrent metastatic tumor occurring a long time after the curative surgery of a primary rectal NET. We may therefore conclude the possibility of metastatic NET when unusual liver tumors are diagnosed in a patient with a history of localized rectal NET.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images, laboratory data and pathological data.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Toshiya Nagasaki for constructive comments on our manuscript.
References
- 1.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 97: 934-959, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Tsikitis VL, Wertheim BC, Guerrero MA. Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: a seer analysis. J Cancer 3: 292-302, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caplin M, Sundin A, Nillson O, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology 95: 88-97, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Kasuga A, Chino A, Uragami N, et al. Treatment strategy for rectal carcinoids: a clinicopathological analysis of 229 cases at a single cancer institution. J Gastroenterol Hepatol 27: 1801-1807, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Mani S, Modlin IM, Ballantyne G, Ahlman H, West B. Carcinoids of the rectum. J Am Coll Surg 179: 231-248, 1994. [PubMed] [Google Scholar]
- 6.Naunheim KS, Zeitels J, Kaplan EL, et al. Rectal carcinoid tumors: treatment and prognosis. Surgery 94: 670-676, 1983. [PubMed] [Google Scholar]
- 7.Kim MS, Hur H, Min BS, Baik SH, Lee KY, Kim NK. Clinical outcomes for rectal carcinoid tumors according to a new (AJCC 7th edition) TNM staging system: a single institutional analysis of 122 patients. J Surg Oncol 107: 835-841, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Chagpar R, Chiang YJ, Xing Y, et al. Neuroendocrine tumors of the colon and rectum: prognostic relevance and comparative performance of current staging systems. Ann Surg Oncol 20: 1170-1178, 2013. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Neuroendocrine Tumors Version 1. 2015 [Internet]. [cited 2016 Feb 21]. Available from: http://www.iqanda-cme.com/assets/pdf/NCCN%20Guidelines_Neuroendocrine%20Tumors.pdf
- 10.Konishi T, Watanabe T, Nagawa H, et al. Treatment of colorectal carcinoids: a new paradigm. World J Gastrointest Surg 2: 153-156, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konishi T, Watanabe T, Kishimoto J, Kotake K, Muto T, Nagawa H. Prognosis and risk factors of metastasis in colorectal carcinoids: results of a nationwide registry over 15 years. Gut 56: 863-868, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwaan MR, Goldberg JE, Bleday R. Rectal carcinoid tumors: review of results after endoscopic and surgical therapy. Arch Surg 143: 471-475, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Jernman J, Välimäki MJ, Louhimo J, Haglund C, Arola J. The novel WHO 2010 classification for gastrointestinal neuroendocrine tumours correlates well with the metastatic potential of rectal neuroendocrine tumours. Neuroendocrinology 95: 317-324, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Shields CJ, Tiret E, Winter DC. Carcinoid tumors of the rectum: a multi-institutional international collaboration. Ann Surg 252: 750-755, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Tang LH, Untch BR, Reidy DL, et al. Well-differentiated neuroendocrine tumors with a morphologically apparent high-grade component: a pathway distinct from poorly differentiated neuroendocrine carcinomas. Clin Cancer Res 22: 1011-1017, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yachida S, Vakiani E, White CM, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol 36: 173-184, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]