Abstract
A 61-year-old Japanese man developed nephrotic syndrome (NS) due to idiopathic membranous glomerulonephritis (MGN). He received immunosuppressive therapy for two years, including prednisolone, cyclophosphamide, and cyclosporine A, but the NS persisted. Low-density lipoprotein apheresis (LDL-A) was initiated at a frequency of twice a month and continued for 9 years (203 sessions in total). His proteinuria reduced to less than 1 g daily after 9 years. LDL-A was stopped, and the NS has not relapsed for five years. This case suggests that long-term LDL-A therapy may be a treatment option for idiopathic MGN refractory to immunosuppressive therapy or short-term LDL-A.
Keywords: long-term LDL apheresis, idiopathic membranous glomerulonephritis, nephrotic syndrome, diabetes mellitus
Introduction
Immunosuppressive therapy employing medications such as prednisolone (PSL), cyclophosphamide (CPA), and cyclosporine A (CyA) is generally considered to be the most important therapeutic option for nephrotic syndrome (NS) caused by focal segmented sclerosis (FSGS), minimal change nephropathy syndrome (MCNS), or idiopathic membranous glomerulonephritis (MGN). However, nephrologists frequently encounter patients in whom NS is refractory to these medications. In recent years, low-density lipoprotein apheresis (LDL-A) has been reported to be effective for such patients (1). In Japan, LDA-A is usually performed twice a week for less than three months in total, but a more effective regimen is required (2).
We herein report a patient with Type 2 diabetes mellitus (DM) who developed idiopathic MGN that was resistant to standard immunosuppressive therapy but responded to long-term LDL-A over a nine-year period.
Case Report
A 61-year-old Japanese man was admitted to our hospital for the evaluation of leg edema and nephrotic-range proteinuria.
At 51 years of age, abnormal glucose tolerance was detected by a health check. At 53 years of age, Type 2 DM was diagnosed by the oral glucose tolerance test, and treatment with glic1azide (40 mg/day) was started. There was no history of diabetic ketoacidosis, retinopathy, or polyneuropathy. He had smoking history for 11 years and had drunk three shots of whiskey a day from the age of 18 years. There was no family history of kidney disease or DM.
On admission, the patient was 168 cm tall and weighed 61.2 kg. His blood pressure was 172/102 mmHg. There was prominent edema extending bilaterally from the ankle to the mid-thigh. Apart from these findings, the examination results were normal.
The laboratory findings were as follows: white blood cell (WBC) count, 4,700 /μL; red blood cell count, 4.82×106 /μL; hemoglobin, 13.8 g/dL; platelet count, 28.7×104 /μL; total protein, 5.2 g/dL; albumin, 2.1 g/dL; serum urea nitrogen, 19 mg/dL; serum creatinine (Cre), 0.9 mg/dL; total cholesterol (TC), 473 mg/dL (normal range: 122-220); high-density lipoprotein cholesterol (HDL-C), 70 mg/dL (normal range: 35 to 70); low-density lipoprotein cholesterol (LDL-C), 360 mg/dL (normal range: <140); apo-B100, 212 mg/dL (normal range: 73 to 109), triglycerides (TG), 394 mg/dL (normal range: 122 to 220), sodium, 144 mmol/L; potassium, 3.6 mEq/L; chloride, 109 mmol/L; and C-reactive protein (CRP), 0.1 mg/dL.
Serological tests showed that antinuclear antibody (ANA), anti-double-stranded DNA antibody, and U1-nuclear ribonucleoprotein antibody (RNP) were all negative. Immunoglobulin (Ig) G was 1,669 mg/dL, IgA was 409 mg/dL, and IgM was 184 mg/dL. The serum level of C3 was 88 mg/dL (normal: >86 mg/dL), C4 was 23 mg/dL (normal: >18 mg/dL), and CH50 was 38 U/mL (normal: >30 U/mL). Both hepatitis B virus antibody and hepatitis C virus antibody were negative.
The urinary sediment contained <1 erythrocyte and <1 leukocyte per high-power field. In addition, the 24-h protein excretion was 13.1 g, creatinine clearance was 79.5 mL/min, and the estimated glomerular filtration rate (eGFR) was 66.9 mL/min. Urinary Bence-Jones protein was negative by electrophoresis.
No findings suggestive of malignancy were revealed by imaging studies, including computed tomography, ultrasonography, and gastrointestinal endoscopy.
Renal biopsy
The renal biopsy specimen contained 13 glomeruli, including 2 with global sclerosis. There was no definite spike formation or bubbling of the glomerular basement membrane (GBM) and no cellular proliferation or expansion of the mesangial matrix (Fig. 1a). Tubules and the interstitium were almost normal, while the arterioles displayed mild hyalinosis.
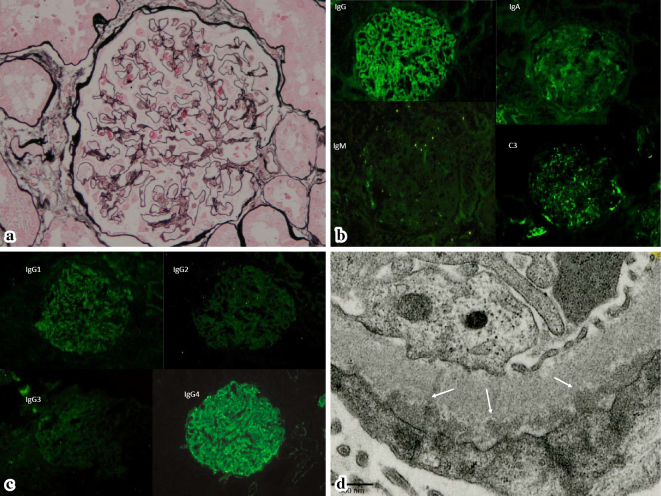
Figure 1.
(a) The glomeruli are almost normal. (b) Immunofluorescence microscopy reveals granular IgG staining along the GBM, as well as weak staining for IgA and C3. (c) IgG subclass staining predominantly reveals IgG4. (d) Electron microscopy confirms numerous electron-dense subepithelial deposits under the GBM.
Immunofluorescence microscopy revealed granular IgG staining along the GBM, as well as weak staining for IgA and C3 (Fig. 1b). Staining of IgG subclasses revealed that IgG4 was predominantly positive (Fig. 1c).
Electron microscopy confirmed the presence of numerous subepithelial electron-dense deposits under the epithelial cells, but definite spike-like protrusions arising from the GBM were not identified (Fig. 1d). There were no histological features of diabetic nephropathy, such as GBM thickening or an increase in the mesangial matrix. Accordingly, Stage I idiopathic MGN was diagnosed.
Clinical course
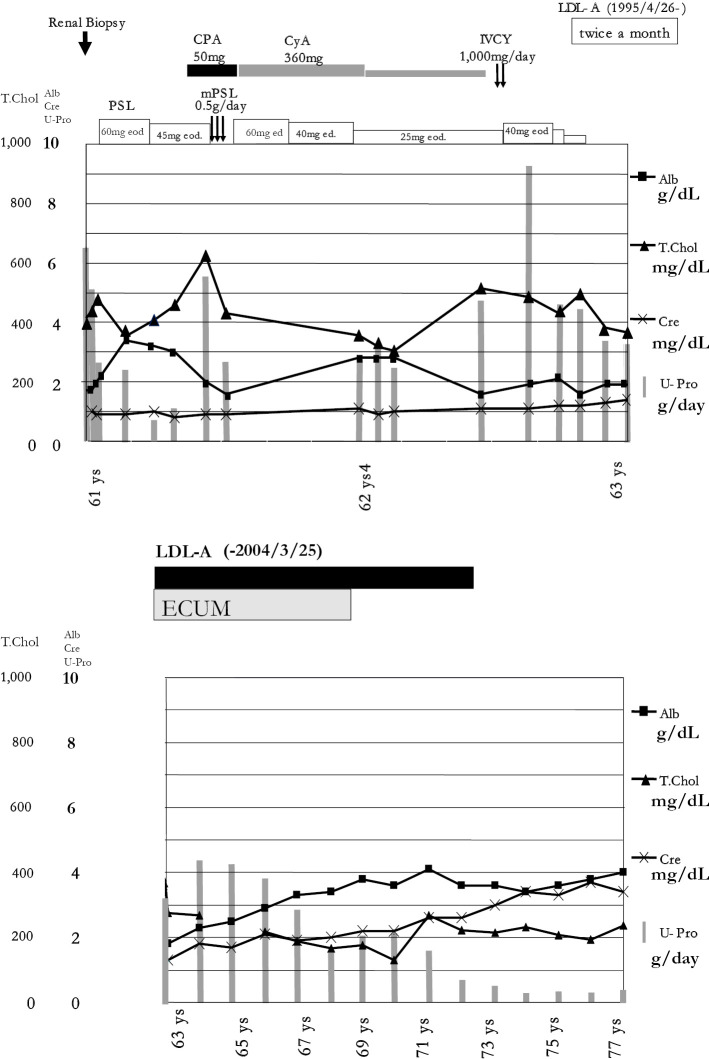
Treatment was initiated with 60 mg of PSL (1 mg/kg) every other day. An angiotensin-converting enzyme inhibitor (ACE-I, captopril at 25 mg daily) and a statin (pravastatin sodium at 10 mg daily) were added and continued, but this regimen was not effective (Fig. 2a). After 5 months, CPA (50 mg/day) was also added, and intravenous methylprednisolone (mPSL; 0.5 g daily) was administered for three consecutive days. CyA was also initiated at 360 mg/day. Insulin therapy was started for poor glycemic control. After 6 months, 2 courses of intravenous cyclophosphamide (IVCY) pulse therapy (1,000 mg per course) were administered, but proteinuria persisted in the nephrotic range. After 2 years (at 63 years of age), immunosuppressive therapy including PSL and ACE-I was discontinued, and subsequently, LDL-A was commenced twice a month using an LDL sorbent column containing dextran sulfate cellulose beads (Liposorba LA-15) (Fig. 2b) (1).
Figure 2.
(a) Clinical course: 1 (on immunosuppressive therapy). PSL50mg eod: PSL at 50 mg every other day. PSL40mg ed: PSL at 40 mg every day. (b) Clinical course: 2 (on LDL-A)
Discussion
Diabetic nephropathy (DN) is the most frequent cause of renal disease in patients with DM, but other primary glomerular diseases can also cause proteinuria in these patients (3). The following points suggest non-diabetic renal disease in patients with Type 2 DM: (a) NS associated with a normal renal function, (b) renal dysfunction without proteinuria, (c) absence of retinopathy, (d) acute deterioration of the renal function, (e) active urinary sediment, (f) gross or microscopic hematuria, and (g) short duration of DM (3). Biopsy studies have suggested that 25-50% of patients with Type 2 DM have glomerular lesions that are unrelated to DN (or in addition to DN), such as FSGS, MCNS, MGN, IgA nephropathy, or amyloidosis (3).
Das et al. reported that MCNS was present in 12.5% of Indian patients with DM, and MGN was found in 10.4% (4). In addition, Mazzucco et al. reported that a kidney biopsy detected MGN as the most common primary glomerular disease (affecting 28.4%) among patients with type 2 DM in Italy (5).
Idiopathic MGN is the most common type of primary glomerulonephritis causing NS in adults. It sometimes shows resistance to immunosuppressive therapy and progresses to end-stage renal disease (6), although spontaneous complete remission also occurs (7). A standard regimen for the treatment of idiopathic MGN has not been established.
Cattran et al. reported that the highest sustained six-month level of proteinuria was the most important factor determining the long-term outcome of idiopathic MGN (8). Combined administration of CyA and steroids may be useful for idiopathic MGN resistant to steroids alone (9).
LDL-A has been reported to be effective for refractory types of NS such as FSGS (1). Many case reports have shown that LDL-A can be successfully employed for the treatment of drug-resistant NS, and that reducing serum lipids can lead to a decrease in proteinuria. However, these studies have provided relatively low-level clinical evidence because their retrospective nature or small patient population. Recently, Muso et al. reported the results of a prospective multicenter Japanese study (Prospective Observational Survey on the Long-Term Effects of LDL Apheresis on Drug-Resistant Nephrotic Syndrome: POLARIS). Among 44 subjects followed for 2 years, 21 (47.7%) showed remission of NS defined as urinary protein <1.0 g/day. That study also showed that the favorable outcome rate was 50.0% in non-FSGS patients with diseases such as MCN, MGN, lupus nephritis, or DN, which was comparable to or better than the rate in FSGS patients. Sato also reported that LDL-A therapy showed similar efficacy for patients with idiopathic MGN or FSGS (10).
A rapid decrease in the LDL level after initiating LDL-A may contribute to a dramatic reduction in the urinary protein excretion after one month (1). In addition, the POLARIS study revealed that the change in the urinary protein after LDL-A may be a significant predictor of the outcome (2).
There is no standard LDL-A regimen and no consensus about the duration of treatment, but twice a week for less than six weeks is most commonly selected in Japan. This may be because the Japanese national health insurance scheme only covers a total of 12 LDL-A sessions within 3 months (11). In addition, Yokoyama et al. reported the efficacy of LDL-A for patients with focal glomerulosclerosis (FGS) using a total of 6 sessions (2 times a week for 3 weeks). Considering both of these reports, short-term intensive apheresis may contribute to an early response to refractory NS.
Some reports have suggested the long-term efficacy of long-term intermittent apheresis at two-week intervals. Nakao et al. reported that this regimen slows the progression of overt nephropathy of six Type 2 diabetic patients (12). Mabuchi et al. reported the efficacy against coronary events after 6 years of treatment by LDL-A using this regimen for 130 heterozygous familial hypercholesterolemia (FH) patients with coronary heart disease (CHD) (13). Matsuzaki et al. reported that this regimen may induce regression of coronary atherosclerotic plaque in FH patients. In their paper, several possible mechanisms for the efficacy of this treatment were summarized, including improvement in the vascular endothelial function, stabilization of plaques, prolongation of the oxidizability of low-density lipoprotein, reduction or suppression of the expression of adhesion molecules and suppression of platelet activation (14). They reported that long-term intermittent LDL-A is effective for patients with DM nephropathy and FH, but there have been no reports for MGN until now. The present case suggests that this regimen may be effective for MGN because of good persistent glycemic control for DM.
The decrease in the renal blood flow by extracorporeal ultrafil-traction method (ECUM) sometimes may bring about a reduction in proteinuria resulting in the improvement of NS. Nishi et al. reported that ECUM is effective in improving refractory edema and ascites in patients with NS (15).
In conclusion, we reviewed the long-term outcome of treatment in a Japanese man with idiopathic MGN and Type 2 DM. Initial immunosuppressive therapy (steroids, CyA, and CPA) was not effective. However, subsequent treatment with LDL-A decreased his proteinuria to less than 3 g/day after 4 years and to less than 1 g/day after 9 years. Subsequently, proteinuria has not relapsed for 5 years after the discontinuation of LDL-A. These findings suggest that long-term intermittent apheresis at two-week intervals may be a useful treatment for drug-resistant idiopathic MGN that is also unresponsive to short-term intensive LDL-A via the above-mentioned mechanisms, although the proteinuria may have decreased naturally and not due to LDL-A.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
This study was funded by the Okinaka Memorial Institute for Medical Research.
References
- 1.Muso E, Yashiro M, Matsushima M, Yoshida H, Sawanishi K, Sasayama S. Does LDL-apheresis in steroid-resistant nephrotic syndrome affect prognosis? Nephrol Dial Transplant 9: 257-264, 1994. [PubMed] [Google Scholar]
- 2.Muso E, Mune M, Hirano T, et al. . A prospective observational survey on the long-term effect of LDL apheresis on drug-resistant nephrotic syndrome. Nephron Extra 5: 58-66, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaqub S, Kashif W, Hussain SA. Non-diabetic renal disease in patients with type-2 diabetes mellitus. Saudi J Kidney Dis Transpl 23: 1000-1007, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Das U, Dakshinamurty KV, Prayaga A, Uppin MS. Nondiabetic kidney disease in type 2 diabetic patients: A single center experience. Indian J Nephrol 22: 358-362, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzucco G, Bertani T, Fortunato M, et al. . Different patterns of renal damage in type 2 diabetes mellitus: a multicentric study on 393 biopsies. Am J Kidney Dis 39: 713-720, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Fervenza FC, Sethi S, Specks U. Idiopathic membranous nephropathy: diagnosis and treatment. Clin J Am Soc Nephrol 3: 905-919, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Donadio JV Jr, Torres VE, Velosa JA, et al. . Idiopathic membranous nephropathy: the natural history of untreated patients. Kidney Int 33: 708-715, 1988. [DOI] [PubMed] [Google Scholar]
- 8.Cattran DC, Pei Y, Greenwood CM, Ponticelli C, Passerini P, Honkanen E. Validation of a predictive model of idiopathic membranous nephropathy: its clinical and research implications. Kidney Int 51: 901-907, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Cattran DC, Appel GB, Hebert LA, et al. ; North America Nephrotic Syndrome Study Group Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int 59: 1484-1490, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Sato Y, Tsunoda S, Nozue T, Pan Q, Wakasugi H, Yoshimura A. Low-density lipoprotein apheresis therapy for steroid- and cyclosporine-resistant idiopathic membranous nephropathy. Intern Med 51: 2597-2602, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama K, Sakai S, Sigematsu T, et al. . LDL adsorption improves the response of focal glomerulosclerosis to corticosteroid therapy. Clin Nephrol 50: 1-7, 1998. [PubMed] [Google Scholar]
- 12.Nakao T, Yoshino M, Matsumoto H, et al. . Low-density lipoprotein apheresis retards the progression of hyperlipidemic overt diabetic nephropathy. Kidney Int 71 (Suppl): S206-S209, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Mabuchi H, Koizumi J, Shimizu M, et al. ; Hokuriku-FH-LDL-Apheresis Study Group Long-term efficacy of low-density lipoprotein apheresis on coronary heart disease in familial hypercholesterolemia. Am J Cardiol 82: 1489-1495, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzaki M, Hiramori K, Imaizumi T, et al. . Intravascular ultrasound evaluation of coronary plaque regression by low density lipoprotein-apheresis in familial hypercholesterolemia: the Low Density Lipoprotein-Apheresis Coronary Morphology and Reserve Trial (LACMART). J Am Coll Cardiol 40: 220-227, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Nishi S, Ubara Y, Utsunomiya Y, et al. . Evidence-based clinical practice guidelines for nephrotic syndrome 2014. Clin Exp Nephrol 20: 342-370, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]