Abstract
As a treatment for superficial transitional cell carcinoma, Bacillus Calmette-Guerin (BCG) intravesical instillation can rarely cause unpredictable systemic side effects. We describe a patient admitted due to continuous pyrexia and general fatigue. He was previously treated with intravesical BCG. Laboratory data indicated a hepatic disorder, and chest computed tomography revealed extensive bilateral miliary nodules. Transbronchial lung biopsy specimens showed several small noncaseating granulomas. The diagnosis was unsolved on the basis of acid fast staining, polymerase chain reaction and microbiological cultures, so we considered the possibility of BCG side effect-induced granuloma. Two months after treatment with antituberculous agents and corticosteroids, his clinical symptoms were improved.
Keywords: miliary tuberculosis, Bacillus Calmette-Guerin, intravesical instillation, noncaseating granulomas
Introduction
Intravesical instillation of Bacillus Calmette-Guerin (BCG) is a well-established treatment for superficial transitional cell carcinoma of the bladder. The efficacy of BCG immunotherapy after transurethral resection of bladder tumor has been proven by increased disease-free survival periods. BCG treatment is usually limited to local side effects and/or mild systemic side effects (such as dysuria, pyrexia, hematuria, and general fatigue), which are self-limiting in most cases. Serious adverse events caused by intravesical BCG are rare. In this report, we describe a patient with miliary pulmonary shadows following initial BCG immunotherapy for bladder carcinoma.
Case Report
In April 2012, a 62-year-old man (also a former smoker) was admitted to our hospital for evaluation of a fever (39℃) and general fatigue. He had been well until two months prior, when he was found to have urothelial carcinoma of the pyelus. He was started on a regimen of weekly immunotherapy with BCG (Institute Armand Frappier, Lavel, Canada), which was initially administered at 81 mg BCG instillate into his pyelus every week and repeated 8 times. However, he discontinued the regimen after the initial instillation because of pyrexia. No other medical conditions and/or diseases were documented in his medical history. He had been vaccinated with BCG in early childhood.
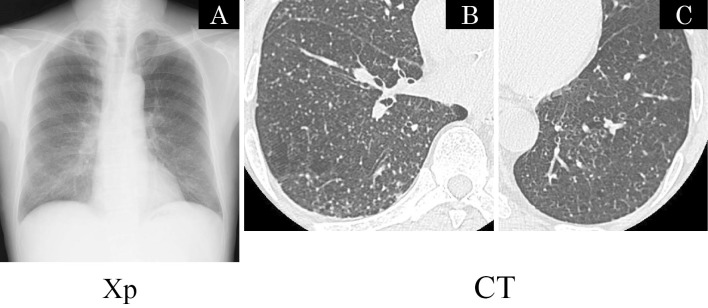
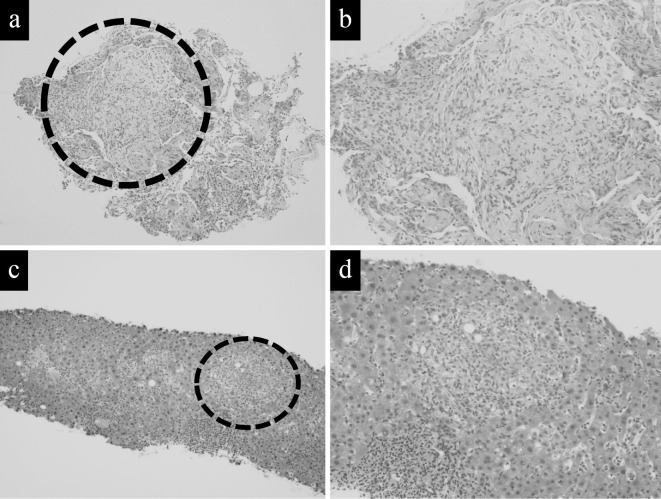
At admission, the patient was in fair condition overall with a fever (38.5℃); a physical examination revealed skeletal and skin jaundice and painful hepatomegaly on palpation. A chest examination showed no remarkable changes, and the patient had no peripheral lymphadenopathy. Complete blood counts revealed only mild normochromic normocytic anemia, a slightly low platelet count, and a normal white blood cell count. Blood chemistry showed abnormal liver function test results (alanine aminotransferase (ALT), 203 IU/L; aspartate aminotransferase (AST), 142 IU/L; and gamma-glutamyl-transferase, 113 IU/L) with elevated lactate dehydrogenase (LDH) (361 IU/L) and C-reactive protein (4.5 mg/L) levels. Blood and urine cultures did not show any common pathogens. The results of serological testing, including the QuantiFERON test, performed to assess the viral and bacterial exposures, were negative. A blood gas analysis on room air on the day of admission showed a PaCO2 of 41 mmHg and PaO2 of 51 mmHg (Table 1). Abdominal ultrasonography confirmed mild hepatomegaly (16×13 cm in size) in association with splenomegaly (14×11 cm in size). Chest X-ray showed diffuse miliary nodular lesions. High-resolution chest computed tomography (CT) confirmed the presence of multiple diffuse and bilateral micronodules with random distribution (Fig. 1). Transbronchial lung and liver biopsy specimens revealed tiny non-caseating granulomas (Fig. 2). Acid-fast bacilli were negative on both smear and polymerase chain reaction tests. Ten days after admission, his serum levels of AST and ALT values decreased without any treatment. Twenty-four days after admission, we diagnosed the patient with a systemic BCG infection. It appeared that BCG had been released into the bloodstream via the urothelial duct.
Table 1.
Laboratory Data on Admission.
| <Hematology> | <biochemistry> | <fast-acid test> | |||||
| RBC | 3.84×106 | /mm3 | CRP | 4.5 | mg/dL | Urine smear | negative |
| Hb | 12.6 | g/dL | Na | 141 | mM | PCR | negative |
| Hct | 32.3 | % | K | 2.7 | mM | blood smear | negative |
| MCV | 92.4 | fl | Cl | 103 | mM | PCR | negative |
| MCHC | 32.0 | % | TP | 6.3 | g/dL | Sputum smear | negative |
| Plt | 115,000 | /mm3 | Alb | 3.2 | g/dL | PCR | negative |
| T-bil | 0.5 | mg/dL | Gastric smear | negative | |||
| WBC | 3,300 | /mm3 | UN | 16 | mg/dL | PCR | negative |
| Baso | 0.0 | % | Cr | 0.73 | mg/dL | BALF | |
| Eos | 0.0 | % | AST | 203 | IU/L | smear | negative |
| Neutro | 57.8 | % | ALT | 142 | IU/L | PCR | negative |
| Lymph | 35.3 | % | LDH | 361 | IU/L | ||
| Mono | 6.6 | % | γ-GTP | 113 | IU/L | <others> | |
| T-cho | 141 | mg/dL | Tuberculin test | negative | |||
| <coaguration> | TG | 114 | mg/dL | Quantiferon test | negative | ||
| PT | 12.7 | s | BS | 104 | mg/dL | ||
| PT INR | 1.1 | ||||||
| APTT | 31.5 | s | |||||
| Fibrinogen | 266 | mg/dL | |||||
| D-dimer | 22.4 | μg/dL | |||||
| FDP | 36.7 | μg/dL | |||||
Figure 1.
(A) Chest radiograph with diffuse micronodules involving both lung fields. (B), (C) CT obtained 2 cm above of the diaphragm showing multiple micronodules randomly distributed with respect to lobular structures, consistent with a miliary pattern.
Figure 2.
The histopathological findings of this case. (a), (b) Transbronchial lung biopsied specimens. Resected nodule from the lower left lobe showing noncaseating granuloma. (Hematoxylin and Eosin (H&E) staining, scale bar=[a] 1 mm, [b] 100 μm). (c), (d) Liver biopsied specimens showing noncaseating granuloma (H&E staining, scale bar=[c] 1 mm, [d] 100 μm)
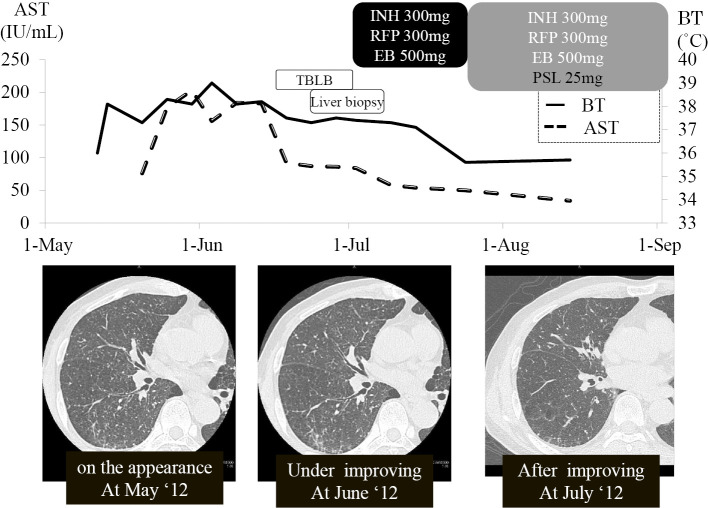
The patient was started on a regimen of isoniazid 300 mg per day plus rifampicin 300 mg per day for 9 months and ethambutol 500 mg per day for 2 months. Ten days after treatment, his dyspnea had disappeared and body temperature had declined to 37℃, but the fever had not completely subsided. Liver function tests showed improvement. Thirty-eight days after admission, prednisolone was added to the antituberculous regimen due to a prolonged fever, which was considered an allergic reaction to BCG. After receiving prednisolone 25 mg/day for the initial dosage and then having the dose tapered to 5 mg per day after 2 weeks, his fever subsided, and the C-reactive protein (CRP) titer declined.
Forty-five days after admission, he was discharged with normalization of inflammation signs and liver function tests and disappearance of pulmonary diffuse nodules. The corticosteroid dosages were tapered over six months. Ethambutol was discontinued after two months, and isoniazid and rifampicin were discontinued after nine months. Mycobacterial cultures were negative after 12 weeks of incubation (Fig. 3).
Figure 3.
The clinical course. AST: aspartate aminotransferase, INH: isoniazid, RFP: rifampicin, EB: ethambutol, PSL: prednisolone, TBLB: transbronchial lung biopsy, BT: body temperature
Discussion
Since the publication of the original report by Morales et al. describing the use of BCG for the treatment of recurrent superficial bladder tumors, BCG therapy has been used extensively for the treatment and secondary prophylaxis of this disease (1,2). Local BCG immunotherapy for early stages of urotherial carcinoma is a well-established treatment option. Systemic complications (dysuria, pyrexia, hematuria, and general fatigue) are less common than local side effects, which include traumatic catheterization and concurrent cystitis. Severe systemic complications such as hepatitis, pneumonitis, or septic presentation tend to rarely occur (<1%) (3,4). Factors increasing the risk of systemic side effects include a bladder biopsy or difficult and traumatic catheterizations of the bladder. The incidence rate of systemic complications ranged from approximately 2.9% to 4.3% according to previous reports (4,5).
Both of these situations allow large inoculates of BCG to gain easy access to the bloodstream (6). In our patient, even without any supporting microbiological evidence of BCG dissemination, we first considered the diffuse military nodules as an infection based on the CT pattern. We then empirically started anti-tuberculous therapy. Table 2 shows the findings from previous reports of patients with pulmonary miliary nodular patterns on chest CT after intravesical instillation of BCG (7-14). Of the nine total cases, including our own, all were resolved. Steroids been added to the anti-tuberculosis medications in only four cases, and the other two cases were resolved without any medications. The pathogenesis of such systemic involvement of intravesical BCG therapy is still controversial. Some authors consider this to be a systemic infection due to hematogenous spread from the pyelus, whereas others believe it is a type IV hypersensitivity mechanism to BCG because the Ziehl-Neelsen staining and cultures were negative. Gonzalez et al. reported a case that revealed a diffuse miliary pattern; they regarded the case as infection-based because of co-existing granulomatous hepatitis (6). Other similar cases with co-existing granulomatous hepatitis have been reported.
Table 2.
Schematic Presentation of Patients with Pulmonary Miliary Nodular Pattern on Chest CT.
| Reference | Age, Sex |
No. of BCG instillations |
Symptoms | Time to symptoms |
Pathological findings |
AFB on microscopic examination |
Myco- bacterial culture |
Treatment | Adding steroids |
Treatment duration |
Outcome |
| 7) | 67, M |
16 | fever, dyspnea, dysuria |
14-16 wks | ND | - | - | RFP+ EM+ LVFX |
+ | 12 | resolved |
| 8) | 73, M |
9 | fever, night sweat anorexia weight loss |
2-3 wks | NCG (TBLB) |
- | - | INH+ RFP+ PZA+ EB |
- | 6 | resolved |
| 9) | 79, M |
8 | fever, nausea, anorexia, weight loss |
10-11 wks | Granuloma (TBLB) |
ND | - | INH+ RFB+ PZA+ EB |
- | ND | resolved |
| 10) | 62, M |
3 | fever, malaise |
7 days | CG (TBLB) |
- | - | INH+RFP+ EB+AMK+ LVFX |
+ | 6 | resolved |
| 11) | 56, M |
3 | dry cough, shortness of breath |
few days | ND | ND (PCR:+) |
- | INH+ RFP |
+ | 9 | resolved |
| 12) | 51, M |
5 | weakness, vomiting, anosmia, weight loss |
few days | NCG | - | - | INH+ RFP |
- | 6 | resolved |
| 13) | 74, M |
7 | fever, night sweat, shivering, anorexia, weight loss |
1 month | ND | ND | ND | Nothing | - | 12 | resolved |
| 14) | 71, M |
3 | soreness, fatigue, hematuria |
6 months | ND | ND | ND | Nothing | - | 1 | resolved |
In our case, granulomatous changes in the lung and liver were the main findings for the disseminated BCG infection. The patient did not allow us to extract his bone marrow by aspiration or to use his bronchoalveolar lavage to measure total alveolar cell counts, alveolar lymphocytes, neutrophils, eosinophils, and the CD4/CD8 ratio. Bone marrow involvement during disseminated disease with BCG has been reported only in a few cases, and they showed involvement of the lungs and/or the liver in addition to bone marrow infiltration as part of the septic process. Our patient's fever had largely improved, but a slight fever remained. Therefore, corticosteroids were added, leading to the rapid amelioration of his symptoms. Some cases have reported using steroids for these symptoms (Table 2). If the pathogenesis had been due to mycobacterial infections, any acid-fast staining would have given positive results or shown a clinical response to antituberculosis medications. In our case, the hypersensitivity appeared to be an additional mechanism, as suggested by the negative stains and cultures, as well as a rapid response to the steroids. The findings of non-caseating granulomas and the success of the anti-tuberculosis plus steroid regimen support the theory of the dissemination of BCG and a subsequent systemic reaction.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol 116: 180-183, 1976. [DOI] [PubMed] [Google Scholar]
- 2.Sylvester RJ, Van der Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol 168: 1964-1970, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Brausi M, Oddens J, Sylvester R, et al. . Side effects of Bacillus Calmette-Guerin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC Cenito-Urinary Cancers Group randomized phase 3 years of comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol 65: 69-76, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Lamm DL, van der Meijden PM, Morales A, et al. . Incidence and treatment of complications of Bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol 147: 596-600, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Pérez-Jacoiste Asín MA, Fernández-Ruiz M, López-Medrano F, et al. . Bacillus Calmette-Guerin (BCG) infection following intaravesical BCG administration as adjunctive therapy for bladder cancer. Incidence, risk factors, and outcome in a single-institution series and review of the literature. Medicine 93: 236-254, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez OY, Musher DM, Brar I, et al. . Spectrum of Bacille Calmette-Guerin (BCG) infection after intravesical BCG immunotherapy. CID 36: 140-148, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Elkabani M, Greene JN, Vincent AL. Disseminated Mycobacterium bovis after intravesicular bacillus Calmette-Guerin treatments for bladder cancer. Cancer Control 7: 476-481, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Jasmer RM, McCowin MJ, Webb WR, et al. . Miliary lung disease after intravesical bacillus Calmette-Guerin immunotherapy. Radiology 201: 43-46, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Iantorno R, Nicolai M, Storto LM, et al. . Miliary tuberculosis of the lung in a patient treated with bacillus calmette-guerin for superficial bladder cancer. J Urol 159: 1639-1640. [DOI] [PubMed] [Google Scholar]
- 10.Nadasy KA, Patel RS, Emmett M, et al. . Four cases of disseminated Mycobacterium bovis infection following intravesical BCG instillation for treatment of bladder carcinoma. South Med J 101: 91-95, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Colmenero JD, Sanjuan-Jimenez R, Ramos B, et al. . Miliary pulmonary tuberculosis following intravesical BCG therapy: case report and literature review. Diagn Microbiol Infect Dis 74: 70-72, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Choi C-HR, Lee SO, Smith G. Subclinical military mycobacterium bovis following BCG immunotherapy for transitional cell carcinoma of the bladder. BMJ Case Rep, 2014. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venn RM, Sharma N. Resolution without treatment of granulomatous pneumonitis due to intravesical BCG for bladder carcinoma. BMJ Case Rep, 2014. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang H, Klein JS, Norotsky M, et al. . Granulomatous chest disease following intravesical Bacillus Calmette-Guerin immunotherapy. J Thorac Imaging 19: 60-62, 2004. [DOI] [PubMed] [Google Scholar]