Abstract
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging disease caused by a novel Bunyavirus with a high mortality rate. We herein report a fatal case of an 86-year-old woman with SFTS complaining of a fever, fatigue, and bicytopenia. Her condition deteriorated with rapid progression of bleeding tendency, disturbance of consciousness, and multiple organ failure leading to death on Day 6 of her illness. The histopathological findings in the autopsy revealed marked infiltration of macrophages with hemophagocytosis in the bone marrow, liver, and spleen leading to a diagnosis of hemophagocytic lymphohistiocytosis (HLH). HLH might be a critical pathogenesis in fatal cases of SFTS.
Keywords: SFTS, hemophagocytic lymphohistiocytosis, Bunyavirus, tick-borne infectious disease
Introduction
Sever fever with thrombocytopenia syndrome (SFTS) is a tick-borne viral infectious disease that was first reported in China in 2009 (1). SFTS virus (SFTSV) is a novel Bunyavirus classified as a member of the genus Phlebovirus and identified as the causative agent of SFTS. SFTS is an emerging disease with a high mortality rate, ranging from approximately 12-30% (1-4). The major clinical features are a fever, muscle weakness, gastroenteric symptoms (nausea, vomiting, and diarrhea), thrombocytopenia (platelet count less than 100×109/L), leukopenia, and elevated levels of aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and creatine kinase (CK). Severe cases suffer from disturbance of consciousness, convulsion, bleeding tendency with disseminated intravascular coagulation (DIC), and multiple organ failure, leading to death (1,2,4,5).
In Japan, the first case of SFTS was reported in Yamaguchi Prefecture in 2012 (6). The phylogenetic analysis of SFTSV revealed that the SFTSV isolated from the Japanese patient was not identical to those reported in China and had been naturally circulating in Japan (6). The clinical manifestation was similar to the cases reported in China. Eleven cases of diagnosed SFTS were in patients aged over 50 years and living in western Japan. The onset of illness was between the month of April and December. Presently, there are no effective anti-viral drugs against SFTSV, and patients have received supportive treatment such as hydration, transfusion, and respiratory support. Although the pathogenesis of SFTS is not fully understood, it was recently reported that a cytokine storm might be correlated with the severity of the illness (3,7).
Hemophagocytic lymphohistiocytosis (HLH) is a rare disorder characterized by an uncontrolled immune response and cytokine storm following lymphoma, autoimmune disease, and infection (8,9). In cases of HLH, the patient's condition rapidly deteriorates in association with DIC and multiple organ failure, leading to death (8,9). Because viral replication and the host immune responses are reported to play an important role in determining the severity and clinical outcome in patients with SFTS (3,7), HLH may play a role in the pathogenesis of severe cases of SFTS.
We herein report a fatal case of SFTS that showed marked infiltration of activated macrophages and hemophagocytosis in the liver, spleen, and bone marrow.
Case Report
An 86-year-old woman with a fever, fatigue, and weakness was carried to the emergency room in our hospital one evening in August 2013. She had been lying on the ground for 3 hours before her family found her, because she could not get up by herself. She was engaged in agriculture and had been taking medicine for hypertension for several years. A physical examination revealed a temperature of 38.6 ℃, tachycardia, dehydration, and mild muscle weakness in all four extremities. The findings from cardiac, pulmonary, and abdominal examinations were normal.
Laboratory tests showed leukopenia (white blood cell [WBC] count 1.6×109/L), thrombocytopenia (platelet count 80×109/L) and increased levels of transaminase (AST 574 IU/L and alanine aminotransferase (ALT) 179 IU/L), LDH 706 IU/L, and CK 280 IU/L. A urinary test showed proteinuria and microhematuria. Plain computed tomography (CT) scans of her chest and abdomen revealed no lymphadenopathy or any remarkable changes in the organs. The initial diagnosis was heat stroke, because she had been lying on the ground for over 3 hours in the hot, humid evening, or an infectious disease, and she was treated with fluid replacement, non-steroidal anti-inflammatory drugs (NSAIDs), and antibiotics.
On Day 3, she was still feverish, and her blood tests results were WBC count 15×109/L (differential leukocyte count, stab neutrophils 60.0%, segmented neutrophils 12.0%, eosinophils 0.0%, basophils 1.0%, monocytes 1.0%, lymphocytes 24.0%, atypical lymphocytes 2.0%, and erythroblasts 2/100 WBC) and platelet count 65×109/L. The erythrocyte sedimentation rate and C-reactive protein (CRP) were normal. The levels of AST, LDH, and CK were further increased. The serum ferritin level was markedly increased to 7,066.4 ng/L, and soluble interleukin (IL)-2R was 1,640 IU/L. Blood and urine cultures were sterile. Bone marrow aspirate showed hypocellular marrow with increased numbers of monocytes and macrophages (13.4%), and hemophagocytosis was seen as shown in Fig. 1.
Figure 1.
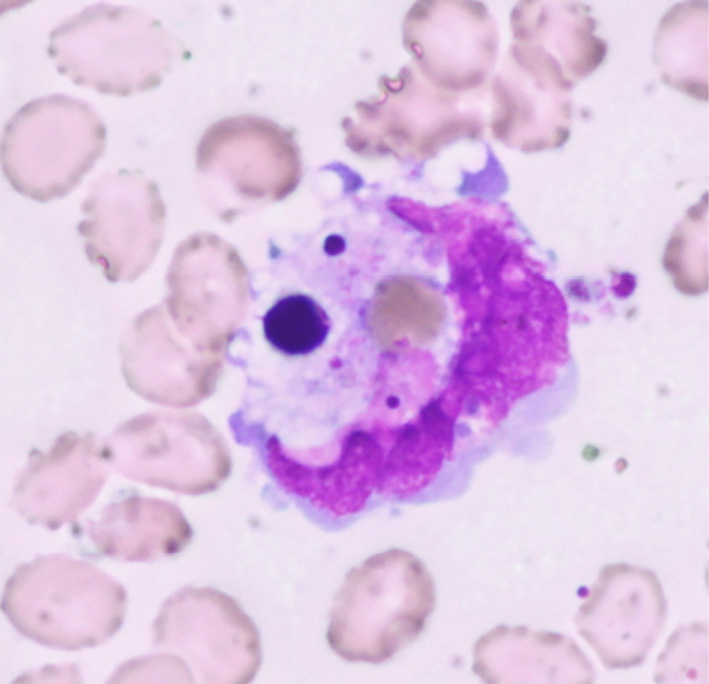
Bone marrow aspirate showed hypocellular marrow (absolute nuclear cell count 1.1×109 cells/L) with increased proportions of macrophages, up to 13.6%. The macrophages engulfed red blood cells, thrombocytes, and nuclear cells. Original magnification, 1,000×.
On Day 4, a tick was found attached to the left side of the patient's back (Fig. 2), and it was removed while taking care not to leave the hypostome in her skin. The tick was identified as a semi-engorged Amblyomma testudinarium nymph. Serum IgM antibodies against Rickettsia Japonica, R. typhi, and Orientia tsutsugamuchi were negative.
Figure 2.
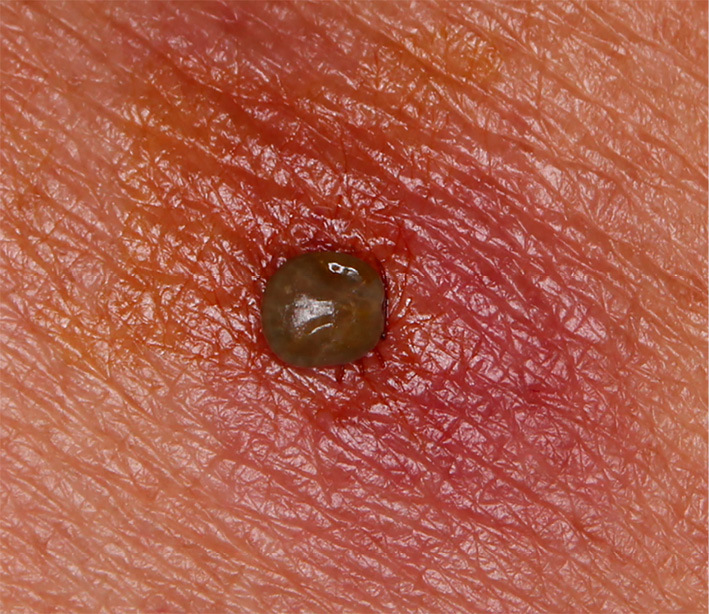
The tick that bit the patient on the back was identified as a semi-engorgedAmblyomma testudinarium nymph.
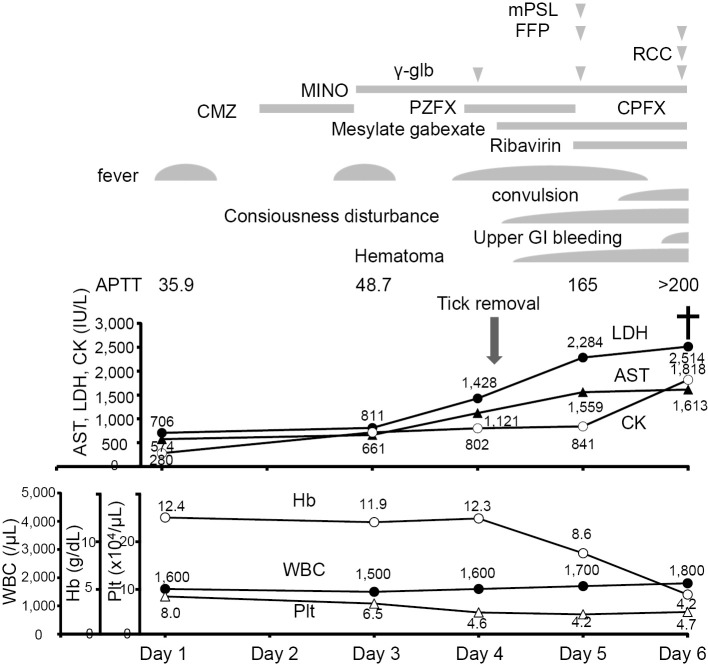
Her condition deteriorated rapidly with the appearance of consciousness disturbance, convulsion, and bleeding tendency at the site of the tick bite and the insertion sites of the peripheral and central venous catheters. A hemostatic test revealed coagulopathy, and transfusion of fresh frozen plasma was performed. Because her condition met the diagnostic criteria for HLH by the HLH Study Group of the Histiocyte Society (8), corticosteroids were administered. The definite diagnosis of SFTS was made based on a polymerase chain reaction assay for SFTSV in her blood and urine, and she was treated with ribavirin.
In the morning on Day 6, she was intubated because of sudden respiratory arrest associated with remarkable metabolic acidosis (pH 6.833, pCO2 33.2 torr, pO2 135.8 torr, HCO3 6.1 mmol/L, and BE -24.2 mmol/L). Upper gastrointestinal bleeding was also present, and she died shortly thereafter (Fig. 3).
Figure 3.
Clinical course in this case. The symptoms, WBC count, Hb level, platelet count, and the levels of AST, LDH, and CK are shown. The definite diagnosis of SFTS, accompanied by DIC and HLH, was made on Day 5, and she was treated with ribavirin, methylprednisolone (mPSL), and fresh frozen plasma (FFP). Her condition deteriorated rapidly, and she died on Day 6. APTT: activated partial thromboplastin time, CMZ: cefmetazole, CPFX: ciprofloxacin, GI: gastrointestinal, γ-glb: γ-globulin, MINO: minocycline, PZFX: pazufloxacin, RCC: red cell concentrate
Pathological findings on autopsy
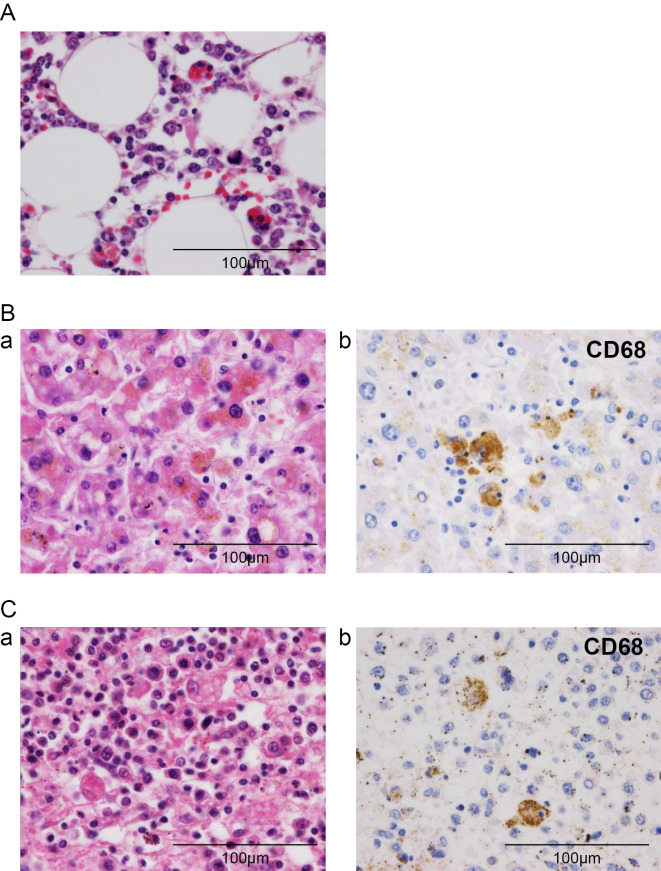
The autopsy revealed bilateral pleural effusion (right 300 mL, left 100 mL), ascites (300 mL), and esophageal erosion, which accounted for the bleeding. In the bone marrow, we noted a massive invasion of CD68-positive macrophages, and hemophagocytosis was seen in the activated macrophages (Fig. 4A). The liver showed single-cell necrosis and focal necrosis in hepatic lobules, and the invasion of lymphocytes was seen in Glisson's sheath, with increases in the numbers of macrophages in the sinusoids and around focal necrosis (Fig. 4B). In the spleen, the accumulation of CD68-positive macrophages and hemophagocytosis was also seen in the splenic red pulp (Fig. 4C). There was no lymphocyte invasion in the brain or spinal cord. The autopsy diagnosis was consistent with HLH.
Figure 4.
Histopathological findings in the autopsy. (A) Bone marrow: Increased numbers of macrophages with active hemophagocytosis were seen in the bone marrow (Hematoxylin and Eosin (H&E) staining). (B) Liver: CD68-positive macrophages with hemophagocytosis were seen in the sinusoids and around focal necrosis. (a) H&E staining and (b) CD68 stain. (C) Spleen: CD68-positive macrophages with hemophagocytosis were seen in the red pulp. (a) H&E staining and (b) CD68 stain. Original magnification, 400×.
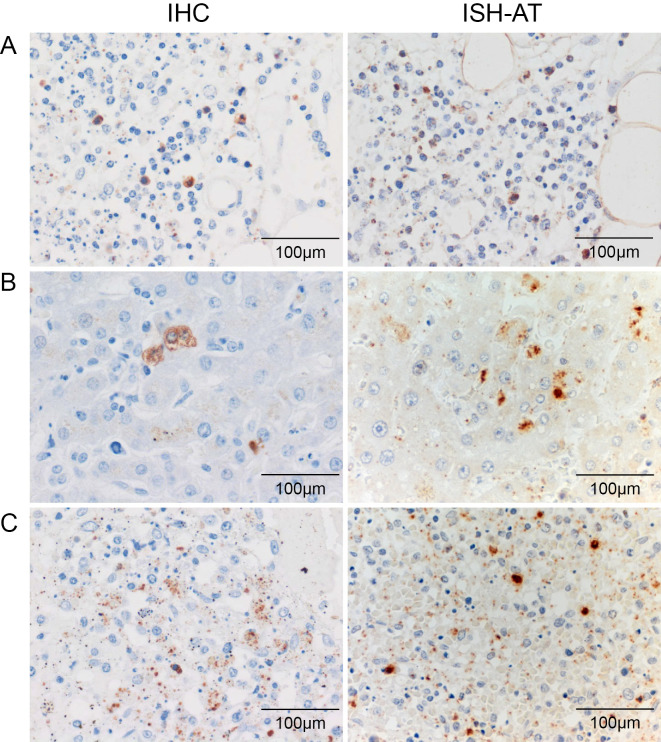
Immunohistochemistry (IHC) and in situ hybridization AT tailing (ISH-AT) (10) detected SFTSV in the cytoplasm of the activated macrophages that had infiltrated the bone marrow, liver, and spleen (Fig. 5).
Figure 5.
SFTSV was detected in the cytoplasm of the macrophages in the bone marrow (A), liver (B), and spleen (C). SFTSV protein was detected by immunohistochemistry (IHC) (left panel) and SFTSV RNA byin situ hybridization AT tailing (ISH-AT) (right panel). Original magnification, 400×.
Discussion
In the present case of SFTS, the patient's condition deteriorated rapidly with a fever, disturbances in consciousness, convulsion, bleeding tendency, severe metabolic acidosis, and multiple organ failure; the histopathological findings revealed infiltration of activated macrophages and hemophagocytosis in the bone marrow, liver, and spleen. Viral encephalitis was not seen in the autopsy findings. Therefore, the cause of death was deemed to be HLH triggered by SFTSV infection.
HLH is a life-threatening disease that is caused by excessive activation of the immune system and is associated with many underlying conditions (8,9). Primary HLH is an inherited disease that usually affects young children. Genetic abnormalities, which lead to defects in the cytolytic process of natural killer (NK) cells and cytotoxic T cells, have been reported in primary HLH. Secondary HLH occurs in association with lymphoma, autoimmune disease, and infection. In virus-associated HLH, Epstein-Barr virus infection is the most common cause. Although the pathogenesis of secondary HLH is unclear, some patients have recently been reported to have heterozygous changes or polymorphisms in the familial HLH genes (8).
The clinical manifestations of HLH are caused by the hyperactivation and proliferation of CD8+ T lymphocytes and macrophages and hypercytokinemia with persistently elevated levels of multiple proinflammatoy cytokines, leading to progressive organ dysfunction (8,9). Although few reports have mentioned the findings of bone marrow aspirates, hemophagocytosis was also seen in the first fatal case in Japan (6). A recent study of SFTS demonstrated that the viral load was significantly higher in fatal cases and correlated with reduced platelet counts, increased levels of serum enzymes, elevated pro-inflammatory and anti-inflammatory cytokines, and activated CD 69+ T cells (3,5,7,11). The blood viral load gradually decreased over 3-4 weeks after illness onset, accompanied by the resolution of the symptoms and laboratory abnormalities in the surviving cases (5). Since a host cytokine storm induced by SFTSV infection is suggested to be associated with the disease severity (3,5,7,11), HLH triggered by SFTSV infection may be responsible for the disease severity and may be a critical pathogenesis in fatal cases.
In a mouse model of SFTS, SFTSV adhered to platelets and facilitated the phagocytosis of SFTSV-adhered platelets by macrophages in the spleen, and virus replication occurred not in platelets but in macrophages (12). Interestingly, SFTSV RNA was detected in the cytoplasm of macrophages that had engulfed blood cells in the bone marrow, liver, and spleen in our case. Many viral hemorrhagic fever viruses in the Bunyaviridae family can also infect macrophages (13,14). Based on these findings and reports, SFTSV-infected macrophages may induce hemophagocytosis and cause a cytokine storm, which leads to multiple organ failure and death.
In the present case, the administration of steroids was not effective because of the rapid progression and severity of the disease. Because the viral load has been reported to be much higher in fatal cases (2,11) and two autopsy cases of SFTS, who had been treated with steroids and thereafter died of associated fungal infections (15); therefore, the administration of immunosuppressants may exacerbate the condition.
In summary, we treated a fatal case of SFTS that showed marked infiltration of activated macrophages and hemophagocytosis in the liver, spleen, and bone marrow in the autopsy. SFTSV was detected in the cytoplasm of activated macrophages. HLH might be a critical pathogenesis in fatal cases of SFTS. Because we did not examine the cytokine levels or T cell subsets in this case, and given the dearth of reports of autopsied cases, further studies are needed to clarify the pathogenesis of SFTS.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We would like to thank Dr. Tetsuya Nagae, Department of Dermatology, for removing the tick from the patient.
References
- 1.Yu XJ, Liang MF, Zhang SY, et al. . Fever with thrombocytopenia associated with a novel Bunyavirus in China. N Engl J Med 364: 1523-1532, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gai ZT, Zhang Y, Liang MF, et al. . Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patient. J Infect Dis 206: 1095-1102, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Deng B, Zhang S, Geng Y, et al. . Cytokine and chemokine levels in patients with severe fever with thrombocytopenia syndrome virus. PLoS One 7: e41365, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui N, Bao XL, Yang ZD, et al. . Clinical progression and predictors of death in patients with severe fever with thrombocytopenia syndrome in China. J Clin Virol 59: 12-17, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Zhang YZ, He YW, Dai YA, et al. . Hemorrhagic fever caused by a novel Bunyavirus in China: Pathogenesis and correlate of fatal outcome. Clin Infect Dis 54: 527-533, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T, Maeda K, Suzuki T, et al. . The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J Infect Dis 209: 816-827, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Jin C, Zhan F, et al. . Host cytokine storm in associated with disease severity of severe fever with thrombocytopenia syndrome. J Infec dis 206: 1085-1094, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Weitzman S. Approach to hemophagocytic syndrome. Hematology Am Soc Hematol Educ Program 178-183, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Janka G, Stadt U. Familial and acquired hemophagocytic lymphohistiocytosis. Hematology Am Soc Hematol Educ Program 82-88, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima N, Ionescu P, Sato Y, et al. . In situ hybridization AT-Tailing with catalyzed signal amplification for sensitive and specific in situ detection of human immunodeficiency virus-1 mRNA in formalin-fixed and paraffin-embedded tissues. Am J Pathol 162: 381-389, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Han Y, Xing Y, et al. . Concurrent measurement of dynamic changes in viral load, serum enzymes, T cell subsets, and cytokines in patients with severe fever with thrombocytopenia syndrome. PLoS One 9: e91679, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin C, Liang M, Ning J, et al. . Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model. Proc Natl Acad Sci 109: 10053-10058, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagai T, Tanishita O, Takahashi Y, et al. . Isolation of haemorrhagic fever with renal syndrome virus from leukocytes of rats and virus replication in cultures of rat and human macrophages. J Gen Virol 66: 1271-1278, 1985. [DOI] [PubMed] [Google Scholar]
- 14.Lewis RM, Morrill JC, Jahrling PB, Cosgriff TM. Replication of hemorrhagic fever viruses in monocytic cells. Rev Infect Dis 11: S736-S742, 1985. [DOI] [PubMed] [Google Scholar]
- 15.Hiraki T, Yoshimitsu M, Suzuki T, et al. . Two cases of severe fever with thrombocytopenia syndrome (SFTS) in Japan: A pathognomonic histological feature and unique complication of SFTS. Pathol Int 64: 569-575, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]