Abstract
Campylobacter infection is the most commonly notified bacterial enteritis in Germany. We performed a large combined case-control and source attribution study (Nov 2011-Feb 2014) to identify risk factors for sporadic intestinal Campylobacter infections and to determine the relative importance of various animal sources for human infections in Germany. We conducted multivariable logistic regression analysis to identify risk factors. Source attribution analysis was performed using the asymmetric island model based on MLST data of human and animal/food isolates. As animal sources we considered chicken, pig, pet dog or cat, cattle, and poultry other than chicken. Consumption of chicken meat and eating out were the most important risk factors for Campylobacter infections. Additional risk factors were preparation of poultry meat in the household; preparation of uncooked food and raw meat at the same time; contact with poultry animals; and the use of gastric acid inhibitors. The mean probability of human C. jejuni isolates to originate from chickens was highest (74%), whereas pigs were a negligible source for C. jejuni infections. Human C. coli isolates were likely to originate from chickens (56%) or from pigs (32%). Efforts need to be intensified along the food chain to reduce Campylobacter load, especially on chicken meat.
Introduction
Intestinal Campylobacter infections are the most frequently reported bacterial infections in Germany and in other European countries1, 2. An overall increasing trend has been observed in Germany, from 55,000 laboratory-diagnosed Campylobacter infections reported in 2001 to 70,190 reported in 2015 (87 infections/100,000 population)1. Most intestinal Campylobacter infections are caused by Campylobacter jejuni (90%) and Campylobacter coli (7%), and are acquired in Germany (92%)3. Typical symptoms are diarrhoea, abdominal pain and fever. Sequelae such as reactive arthritis, irritable bowel syndrome, and neurological complications such as Guillain Barré syndrome can also occur, albeit with lower incidence4–6. The vast majority of cases (97%) are reported as sporadic, that is not as part of an outbreak3. Campylobacteriosis is a zoonotic disease. Important animal reservoirs for the organism are poultry, in particular chicken, and cattle7–14. Humans typically become infected via consumption of meat. Chicken meat plays an important role, because it is often contaminated with Campylobacter 15–17. Epidemiological studies conducted in several European and non-European countries have identified the consumption of poultry or chicken meat as an important risk factor for campylobacteriosis14, 18–23, and in source attribution studies outside of Germany about 50–90% of human infections were attributed to chicken7, 8, 10, 11, 13, 14, 24. Few studies have combined epidemiological and source attribution data10, 14, 25. The aim of our study was to identify risk factors for sporadic Campylobacter infections in Germany and combine epidemiological, molecular typing and source attribution data to determine the relative importance of potential sources for human infections.
Methods
The study was approved by the German data protection authority, Bonn, Germany (Number III-401/008#0045; 28 July 2011), and by the ethical committee of the Charité University Medicine Berlin, Germany (Number EA2/012/11; 14 March 2011). The study was conducted in accordance with relevant guidelines and regulations. Informed consent was obtained from all subjects.
Data availability
Data are available in Supplementary Information. Additional data of the case-control study will be made available upon request to the corresponding first or the last author.
Study design
The study was conducted between 1 November 2011 and 28 February 2014 in rural county districts of the federal state of Brandenburg and in Berlin (urban region). To increase the number of study participants under the age of 15 years, the study region for this age group was expanded in 2013 to select urban and rural county districts in North Rhine-Westphalia and Saxony. Regions were classified as rural or urban as described previously3. According to the German Protection against Infection Act of 2001, laboratory diagnosed Campylobacter infections in patients have to be reported to the local health authority by the primary diagnostic laboratories. Local health authorities contacted patients to obtain informed consent for study participation. Patients willing to participate were sent a self-administered questionnaire by the local health authority, which was mailed free of charge directly to the Robert Koch Institute (RKI) by the patients after completion. Primary diagnostic laboratories that participated in the study forwarded Campylobacter isolates of patients to Hannover Medical School (MHH) for further characterisation. If patients agreed to the analysis of their bacterial isolate in writing, multilocus sequence typing (MLST) analysis of the Campylobacter isolate was performed at MHH. Results were uploaded to a SeqSphere (Ridom Bioinformatics GmbH; Münster, Germany) database. Questionnaire and respective Campylobacter isolate could be matched using the sample number given by the primary diagnostic laboratory.
Controls were frequency-matched to cases by age group and federal state. Intended ratios were 1 case: 1 control in persons ≥15 years of age, and 1: 4 in persons younger than 15 years of age, based on sample size calculations and expected low number of cases among children. Control persons were selected in a two-step randomised procedure from address lists provided by regional population registries26. The self-administered questionnaires were sent out to potential control persons every month during the study period proportional to the number of expected cases, which was estimated based on surveillance data of previous years. Participating control persons returned the completed questionnaire free of charge to the RKI by mail.
Data Collection
Cases and controls were queried about potential risk factors with a focus on consumption of certain food items. Questions on eating habits, kitchen hygiene, eating out, contact with animals, leisure activities, occupational exposure (e.g., to raw meat or young children), medication, certain chronic illnesses, travel abroad, and basic demographics (e.g., sex, month and year of birth, postal code, level of professional education, migrant background, household size) were also included. Questions about possible exposures referred to the 7 days before disease onset (case patients) or before completion of the questionnaire (controls), unless stated otherwise (see Supplementary Information: Questionnaire). Parents/caregivers were asked to complete the questionnaire for, or when appropriate, with their children.
Data Analysis
Data was entered into an EpiData database (version 3.1, The EpiData Association, Denmark) and validated by double data entry. Missing data on sex, age, and date of disease onset of case patients was supplemented with data obtained from the national surveillance database of notified cases hosted at the Robert Koch Institute, if possible. Missing answers in item lists of the questionnaire were converted to “No” answers as described before26. According to the definition of the German Federal Statistical Office27, persons who were borne with a non-German citizenship or with at least one parent that was borne with a non-German citizenship were considered as persons with a migrant background. Seasons were categorised as follows: spring (March-May), summer (June-August), autumn (September-November), winter (December-February).
Data was analysed with Stata 14 (Stata Corporation, USA). For risk factor analyses cases were defined as patients with a laboratory diagnosed, notified Campylobacter enteritis. Cases were excluded if they had travelled abroad in the 7 days before the onset of illness, or if the time period between onset of illness and completion of the questionnaire was 60 days or longer. Controls were excluded from data analysis if they had travelled abroad in the 7 days before completing the questionnaire. We conducted unconditional logistic regression analyses based on single exposure variables adjusted for sex and the two matching variables age group (0–4, 5–14, ≥15 years) and federal state (“univariable analyses”) to determine adjusted odds ratios (aOR) with 95% confidence intervals (CI). Statistical significance was assessed using Wald tests. Variables were considered for multivariable analysis (MVA) if the P-value was 0.1 or lower in univariable analysis. To reduce the overall number of variables in the starting set for MVA, correlating variables measuring related exposures were combined to one composite variable, when plausible, or only one of the correlating variables was chosen for model building. MVA was conducted as described before26. Matching variables and the variable sex were forced into the model. The age group variable was modified (0–2, 3–4 years) in the multivariable model for identification of risk factors in children under 5 years of age. When building models for identifying risk factors for Campylobacter infections at the species level (C. jejuni or C. coli), we only included cases with isolates confirmed as either C. jejuni or C. coli by detailed molecular and biochemical analysis at MHH. The model for identifying risk factors for C. coli infections was limited to the age group ≥15 years because only 2 confirmed C. coli infections occurred in younger age groups. A modified age group variable (15–29, 30–59, 60 + years) was included in this model. We compared multivariable logistic regression models with and without exclusion of cases and controls that had travelled abroad.
We also determined risk factors for Campylobacter infections attributed to the source chicken by our source attribution model described below. In this approach, we conducted univariable and multivariable logistic regression analyses, comparing cases that were attributed to chicken with a relative posterior probability (Pr) of 0.5 or higher (n = 486; mean Pr 0.76, range 0.50–0.90) with controls (n = 3,983). The number of human isolates attributed to other putative sources based on Pr ≥0.5 was too small to allow meaningful logistic regression analysis for the identification of source-specific risk factors (pig: n = 24; mean Pr 0.81, range 0.55–1.00; pet: n = 19; mean Pr 0.68, range 0.50–0.79; poultry other than chicken: n = 4; mean Pr 0.65, range 0.53–0.83); cattle: n = 0).
Population attributable fractions (PAF) of each statistically significant risk factor in the final models were determined as described by Bruzzi et al.28. Confidence intervals of population attributable fractions were calculated in R, version 3.2.329, based on the percentile method for samples obtained by an age-group and federal-state stratified bootstrap30.
Campylobacter isolates from animal, food, and environmental samples
Various animal and environmental samples were obtained and food items for sampling were purchased in stores in the study region (Berlin and Brandenburg) within the study time period (total number of samples: 1,471). Campylobacter was isolated from a total of 183 samples. A selection of the C. jejuni (n = 77) and the C. coli isolates (n = 34) were collected at MHH and further analysed using MLST31, 32. Additional C. jejuni and C. coli isolates from chicken meat samples (n = 67) from the study region were provided by the National Reference Laboratory for Campylobacter at the Federal Institute for Risk Assessment, Berlin, Germany, and also analysed using MLST at MHH.
Multilocus Sequence Typing (MLST)
MLST of Campylobacter isolates was performed using the C. jejuni/C. coli typing system developed by Dingle et al.31 and primer sequences available from http://pubmlst.org/campylobacter/info/primers.shtml. Briefly, fragments from 7 housekeeping genes, aspA, glnA, gltA, glyA, pgmA, tkt, and uncA, were PCR-amplified from purified genomic DNA of C. jejuni or C. coli isolates, and sequenced from both strands on an ABI 3130xl capillary sequencer. Sequence reads were imported into a SeqSphere+ (Ridom Bioinformatics GmbH, Münster, Germany) database for further processing and assignment to known sequence types (STs). Novel allele sequences and isolates with novel combinations of alleles were submitted to the PubMLST Campylobacter database (http://pubmlst.org/campylobacter/) sited at the University of Oxford33, to obtain allele and ST numbers. All MLST profiles for isolates newly described in this study have been deposited at the PubMLST database (www.pubmlst.org). Minimal spanning trees were generated using BioNumerics 7.1 (Applied Maths, Sint-Martens-Latem, Belgium).
Source attribution analysis based on MLST data
MLST-typed human isolates (n = 613) were compared to 504 MLST-typed isolates from animal and food samples that were obtained in the study region during the study period or were obtained in Germany in the time period 2006–2010 in a previous study performed within the FBI-Zoo network32. As sources relevant for Germany we considered chicken, pig, cattle, pet (dog or cat), and poultry other than chicken (“other poultry”: duck, goose, turkey, or quail). We increased the number of animal and food isolates for our source attribution analyses by supplementing sequence type data from isolates of the same 5 sources obtained in Germany (outside the FBI-Zoo network) and neighbouring European countries (Switzerland, The Netherlands, Luxembourg, France, Belgium) in 2003 or later that were available in the PubMLST Campylobacter database. A large proportion of these isolates had been used for source attribution analysis in studies conducted in Luxembourg14 and Switzerland11. A total of 2,549 animal and food isolates were included (766 from Germany, 1,783 from neighbouring countries) (Table 1). Source attribution was performed using Bayesian inference on an asymmetric island model7 as implemented in the iSource program available from http://www.danielwilson.me.uk/iSource.html. This analysis estimates relative posterior probabilities for each human isolate to originate from the different sources. In one approach, we excluded the source pet and restricted the sources that were considered for attribution to chicken, pig, cattle, and poultry other than chicken (“other poultry”).
Table 1.
Origin of Campylobacter isolates (typed with MLST) used for source attribution analysis.
| Source | Germany (FBI-Zoo network: 2011–2014) | Germany (FBI-Zoo network 2006–201032) | Germany (PubMLST, 2003 or later) | Luxembourg (PubMLST14; includes pig isolates from Belgium, France) | Switzerland (PubMLST11) | The Netherlands (PubMLST; 2003 or later) | Total |
|---|---|---|---|---|---|---|---|
| Human | 613 | — | — | — | — | — | 613 |
| Pet (Dog or Cat) | 21 | 4 | 2 | — | 140 | — | 167 |
| Chicken | 90 | 136 | 138 | 252 | 540 | 350 | 1,506 |
| Pig | 47 | 106 | 9 | 45 | 257 | 17 | 481 |
| Cattle | 1 | 40 | 61 | 101 | — | 5 | 208 |
| Other Poultry | 29 | 30 | 52 | 62 | — | 14 | 187 |
The source “other poultry” includes isolates from ducks (n = 47), turkeys (n = 102), geese (n = 11), and quails (n = 27).
We validated our model with 32 down-sampled datasets in each of which a random subset of 10% of animal/food isolates were excluded to attribute the origin of the human isolates. Mean posterior source probability of the 5 sources was calculated. Results remained relatively stable, which means that the output did not depend much on exactly which animal/food isolates were used for analysis (Supplementary Fig. S5).
Results
Study population
We received 2,073 questionnaires from case patients, corresponding to 22% of all cases notified to the local health authorities in the study region, and to 68% of patients that had received a questionnaire from the local health authority. Participation of parents of children <5 years of age was slightly lower (20%) than participation of parents of older children (22%) and of persons ≥15 years of age (23%). Comparing participating campylobacteriosis cases to all non-participating cases that were notified to local health authorities in the study region, we found that they were similar in age, but a slightly higher proportion of participants was female (participants: median age 40 years; interquartile range (IQR) 24–54 years; 52% female; non-participants: median age 35 years; IQR 22–54 years; 47% female). For risk factor analyses, we excluded case patients that had travelled abroad in the 7 days before disease onset (n = 246, 12%), or had completed the questionnaire ≥60 days after onset of disease (n = 15, 0.7%), which resulted in a total of 1,812 case patients included in data analysis (Table 2). Most cases (84%) were in the age group 15 years and older, mainly because the number of notified Campylobacter infections was substantially lower in the younger age groups. The median time interval between disease onset and completing the questionnaire was 16 days (IQR: 12–22 days).
Table 2.
Characteristics of the study population.
| Characteristics | Case patients n (%) | Control persons n (%) |
|---|---|---|
| Total | 1,812 (100) | 3,983 (100) |
| Age group | ||
| 0–4 years | 119 (7) | 878 (22) |
| 5–14 years | 161 (9) | 1,010 (26) |
| 15 years and older | 1,532 (84) | 2,027 (52) |
| Sex | ||
| Male | 875 (48) | 1,825 (47) |
| Female | 937 (52) | 2,093 (53) |
| Federal state | ||
| Berlin | 1,030 (57) | 2,021 (51) |
| Brandenburg | 669 (37) | 1,234 (31) |
| Saxony | 87 (5) | 495 (12) |
| North Rhine-Westphalia | 26 (1) | 232 (6) |
Case-control study, Germany, 2011–2014.
Of the 16,287 potential control persons that were mailed a questionnaire, 4,196 (26%) completed it. Of those, 213 questionnaires (5%) were excluded because the person had travelled abroad. The resulting control group comprised 3,983 persons. In the study population, the proportion of female and male persons was similar among cases (52% female) and controls (53% female). Controls were younger than cases because the control to case ratio was higher in the age groups <15 years (Table 2).
Clinical aspects
Symptoms of Campylobacter infection in case patients were diarrhoea (95%), abdominal pain (81%), fever (53%), nausea (48%) and vomiting (19%). 25% of case patients reported bloody stools. Additional symptoms, such as headaches, chills, body aches, and weakness were reported by 62% of patients. Median duration of symptoms was 6 days (IQR 5–9 days). 18% of case patients were hospitalised because of their Campylobacter infection; median duration of hospital stay was 4 days (IQR 3–6 days). The proportion of case patients that reported bloody stools, fever, nausea or vomiting was higher in the hospitalised than in the non-hospitalised group. About one third of case patients (31%) reported treatment of their Campylobacter infection with antibiotics. The most frequently named antibiotics were ciprofloxacin (45%) and erythromycin (21%). In total, 79% of patients at working age (15–64 years) or working parents of children reported absence from work due to the illness or the illness of their child, respectively, for a median of 6 days (IQR 4–9 days).
Risk factor analyses
Exposures related to consumption of poultry, in particular consumption of chicken meat, and to preparation of poultry in the household were positively associated with Campylobacter infection in univariable analyses. Other variables that were positively associated included, e.g., contact with chickens or ducks and geese; eating out; contact with sand in a sandbox; and use of gastric acid inhibitors. An exposure implying inadequate kitchen hygiene (simultaneous preparation of raw meat and food items eaten uncooked, e.g., raw vegetables, fruit, lettuce), but also exposures implying good kitchen hygiene (frequently or always using separate utensils for raw meat and other food items; frequently or always using a dishwasher for utensils that came in contact with raw meat) were positively associated with disease. A variety of variables were negatively associated with Campylobacter infection in univariable analyses, e.g., mostly vegetarian lifestyle; consumption of fresh fruit or herbs, raw milk, or food items purchased directly at a farm; mostly or always cleaning kitchen utensils with hot water after preparing raw meat; swimming in a pool; attending day care; medium or high professional education.
In the multivariable logistic regression model, consumption of chicken meat (aOR 1.6; population attributable fraction (PAF) 31%) and eating out (aOR 1.6; PAF 30%) were the most important risk factors for Campylobacter infections according to PAF, which corresponds to the proportion of cases that could be avoided in the population if this risk factor was eliminated (Table 3). Preparation of packaged poultry meat in the household (PAF 14%), simultaneous preparation of raw meat and food items consumed uncooked (PAF 12%), and contact with poultry animals (PAF 3%) were risk factors as well (Table 3). The use of gastric acid inhibitors in the past 4 weeks was also positively associated with Campylobacter infection (aOR 1.9; PAF 10%). We found a negative association with disease in the final model for a mostly vegetarian lifestyle (aOR 0.5); consumption of beef (aOR 0.7); consumption of lamb/mutton (aOR 0.6); consumption of fruit (aOR 0.6); contact with a dog (aOR 0.8); and recreational swimming (aOR 0.7) (Table 3). Results of multivariable analysis were similar when the model was restricted to C. jejuni infections, except that the association of contact with poultry animals and disease was no longer statistically significant (data not shown).
Table 3.
Factors positively associated (risk factors) and factors negatively associated with Campylobacter infections.
| Exposure | Cases Exposed % (n) | Controls Exposed % (n) | aORa (95% CIb) | Population Attributable Fraction % (95% CIb) |
|---|---|---|---|---|
| Consumed any chicken meat*** | 87.0 (1,445/1,661) | 79.1 (2,967/3,753) | 1.6 (1.2–2.0) | 31 (17–42) |
| Ate out (at food stand, restaurant, canteen, etc.)*** | 81.9 (1,437/1,755) | 78.6 (3,089/3,929) | 1.6 (1.3–2.0) | 30 (18–40) |
| Prepared poultry meat (packaged) in household*** | 53.9 (860/1,597) | 43.8 (1,617/3,692) | 1.4 (1.1–1.6) | 14 (8–20) |
| Prepared uncooked food and raw meat in household at the same time** | 52.0 (856/1,646) | 45.8 (1,684/3,677) | 1.3 (1.1–1.5) | 12 (4–18) |
| Used anti-acidic drug (PPI)*** | 21.1 (371/1,755) | 8.1 (315/3,869) | 1.9 (1.5–2.3) | 10 (7–12) |
| Had contact with poultry (animal)*** | 5.3 (92/1,725) | 4.4 (170/3,856) | 2.1 (1.4–3.0) | 3 (2–4) |
| Consumed mostly vegetarian food* | 1.5 (25/1,646) | 4.1 (151/3,669) | 0.5 (0.3–1.0) | — |
| Consumed (unpeeled) fruit*** | 62.8 (1,055/1,679) | 72.7 (2,757/3,794) | 0.6 (0.5–0.7) | — |
| Consumed lamb/mutton** | 8.0 (129/1,615) | 8.5 (321/3,767) | 0.6 (0.5–0.9) | — |
| Consumed beef*** | 51.1 (793/1,551) | 52.6 (1,923/3,654) | 0.7 (0.6–0.8) | — |
| Had contact with dog** | 29.0 (498/1,716) | 32.8 (1,256/3,828) | 0.7 (0.6–0.9) | — |
| Went swimming (in pool, lake, ocean, etc.)** | 14.6 (257/1,755) | 23.4 (913/3,910) | 0.7 (0.6–0.9) | — |
Case-control study, Germany, 2011–2014. The proportion of exposed cases and controls was based on the number of cases and controls with complete answers in univariable analysis (without adjustment for age group, sex, federal state). Adjusted odds ratios (aOR) were determined in multivariable logistic regression analysis (adjusted for age group, sex, federal state; 1,003 cases and 2,569 controls with complete answers for all variables in the final model). aAdjusted odds ratio. bConfidence interval. *Indicates P < 0.05. **Indicates P < 0.01. ***Indicates P < 0.001.
In an analysis restricted to children under 5 years of age, contact with sand in a sandbox (PAF 39%), preparation of poultry meat (fresh or packaged) in the household (PAF 38%), contact with poultry animals (PAF 22%), and a migrant background (PAF 10%) were positively associated with a Campylobacter infection. Consumption of chicken meat was not a statistically significant risk factor for this age group. No exposure variables were negatively associated with disease in this analysis (Table 4).
Table 4.
Risk factors for Campylobacter infections in children <5 years of age.
| Exposure | Cases Exposed % (n) | Controls Exposed % (n) | aORa (95% CIb) | Population Attributable Fraction % (95% CIb) |
|---|---|---|---|---|
| Had contact with sand (in a sandbox or similar)c | 85.5 (94/110) | 76.5 (643/841) | 1.9 (1.0–3.5) | 39 (0–63) |
| Prepared poultry meat (fresh or packaged) in household* | 78.0 (85/109) | 61.8 (525/850) | 2.0 (1.2–3.4) | 38 (11–56) |
| Had contact with poultry (animal)*** | 24.1 (26/108) | 6.2 (52/835) | 5.2 (2.9–9.5) | 22 (17–24) |
| Migrant background** | 13.7 (16/117) | 11.6 (100/862) | 2.7 (1.4–5.5) | 10 (4–13) |
Case-control study, Germany, 2011–2014. The proportion of exposed cases and controls is based on the number of cases and controls with complete answers in univariable analysis (without adjustment for age group, sex, federal state). Adjusted odds ratios (aOR) were determined in multivariable logistic regression analysis (adjusted for age group (0–2 years, 3–4 years), sex; federal state; 90 cases and 762 controls with complete answers for all variables in the final model). No factors were negatively associated with disease in the final model. aAdjusted odds ratio. bConfidence interval. c P = 0.054. *Indicates P < 0.05. **Indicates P < 0.01. ***Indicates P < 0.001.
In an analysis to identify risk factors for C. coli infections, consumption of pork (PAF 66%) and the use of gastric acid inhibitors in the past 4 weeks (PAF 20%) were positively associated with disease, whereas consumption of beef and consumption of fruit were negatively associated (Table 5).
Table 5.
Factors positively associated (risk factors) and factors negatively associated with Campylobacter coli infections in age group ≥15 years.
| Exposure | Cases Exposed % (n) | Controls Exposed % (n) | aORa (95% CIb) | Population Attributable Fraction % (95% CIb) |
|---|---|---|---|---|
| Consumed pork* | 95.1 (58/61) | 83.8 (1,690/2,016) | 3.3 (1.0–11.0) | 66 (18–94) |
| Used anti-acidic drug (PPI)** | 31.8 (20/63) | 15.1 (305/2,023) | 3.1 (1.6–5.9) | 20 (11–25) |
| Consumed (unpeeled) fruit** | 54.2 (32/59) | 71.7 (1,461/2,037) | 0.4 (0.2–0.8) | — |
| Consumed beef* | 46.3 (25/54) | 56.2 (1,106/1,967) | 0.5 (0.3–0.9) | — |
Case-control study, Germany, 2011–2014. The proportion of exposed cases and controls is based on the number of cases and controls with complete answers in univariable analysis (without adjustment for age group, sex, federal state). Adjusted odds ratios (aOR) were determined in multivariable logistic regression analysis (adjusted for age group (15–29 years, 30–59 years, ≥60 years), federal state, sex; 50 cases and 1,786 controls with complete answers for all variables in the final model). aAdjusted odds ratio. bConfidence interval. *Indicates P < 0.05. **Indicates P < 0.01.
When we performed the same multivariable analysis including cases and controls that had travelled abroad and with an additional exposure variable “travelled abroad”, the results did not change substantially. Travelling abroad was positively associated with Campylobacter infection in the model for all age groups (P < 0.001; Supplementary Table S1), and in the models for C. jejuni (P < 0.001) and C. coli infections (P < 0.05). Travelling abroad was not statistically significantly associated with Campylobacter infection in the model for children <5 years of age (data not shown).
We also performed multivariable logistic regression analysis with cases whose Campylobacter isolate had been attributed to the source chicken with a probability of 50% or higher in the source attribution model (n = 486) and the controls (n = 3,983). Risk factors and strengths of associations were similar to the model with all 1,812 cases: consumption of chicken meat (aOR 1.9); eating out (aOR 1.4); preparation of packaged poultry meat in the household (aOR 1.6); simultaneous preparation of raw meat and food items consumed uncooked (aOR 1.4); use of gastric acid inhibitors (aOR 1.8). In the final model, contact with poultry animals and having gone swimming were not statistically significantly associated with Campylobacter infections attributed to chicken (Supplementary Table S2). Compared to the model with all cases (Table 3), population attributable fractions calculated for consumption of chicken meat (41% vs. 30%), preparation of poultry meat in the household (21% vs. 14%) and simultaneously preparing uncooked food and raw meat in the household (17% vs. 12%) were higher, whereas the PAF for eating out was lower (25% vs. 30%), and the PAF for use of gastric acid inhibitors was about the same (11% vs. 10%) (Supplementary Table S2).
Due to the small number of human isolates that were attributed to sources other than chicken considered in our source attribution model (pigs, pets, cattle, poultry other than chicken), multivariable analyses similar to the ones we performed for chicken could not be conducted. In univariable analyses (not adjusted for age group, sex, federal state) we found statistically significant associations of Campylobacter infections attributed to pig (n = 24) and consumption of pork (100% of cases (with complete answers) had consumed pork (23/23; OR could not be calculated) vs. 85% of controls (3,220/3,787); P < 0.001), but not a statistically significant association with consumption of chicken (OR 0.95; P = 1.0). The majority of case patients with isolates assigned to the source pig reported both consumption of pork and chicken (77%; 17/22). None of the case patients with Campylobacter isolates assigned to the source pet (dog or cat; n = 10) reported contact with a dog (versus 33% of controls) and 3/10 reported contact with a cat (versus 33% of controls; OR 0.9; P = 1.0).
Multilocus Sequence Typing
In total, 613 human Campylobacter isolates that could be matched to a questionnaire completed by the respective case patient and an additional 203 isolates from animal and food samples were characterised by MLST. The human isolates were assigned to 186 different sequence types (STs); 28 of those STs were more prevalent (ranging from 4 to 91 isolates each), whereas 158 STs were detected only 1–3 times (34% of isolates). 550 of the 613 isolates could be assigned to 28 different clonal complexes (CCs). Campylobacter sequence types (STs) that occurred most frequently among human isolates were ST-50 (15%), ST-572 (7%), ST-122 (5%), ST-257 (5%), ST-354 (4%), and ST-464 (4%). The most frequent clonal complexes were CC-21 (25%), CC-206 (13%), and CC-828 (10%). Notably, some STs occurred at high frequency in the dataset that had not been part of the 10 most common STs in the previous FBI-Zoo MLST study32. Examples were ST-122, which occurred in 29 samples, ST-354 (27 samples) and ST-464 (23 samples). The most prevalent STs of human isolates were detected in animal/food samples as well (Figs 1 and 2). The number of isolates from patients with a history of travel outside of Germany within 7 days before disease onset was too low for detailed analysis, but it was apparent that all abundant STs were detected both in patients with and without travel history (Supplementary Figs S3 and S4).
Figure 1.
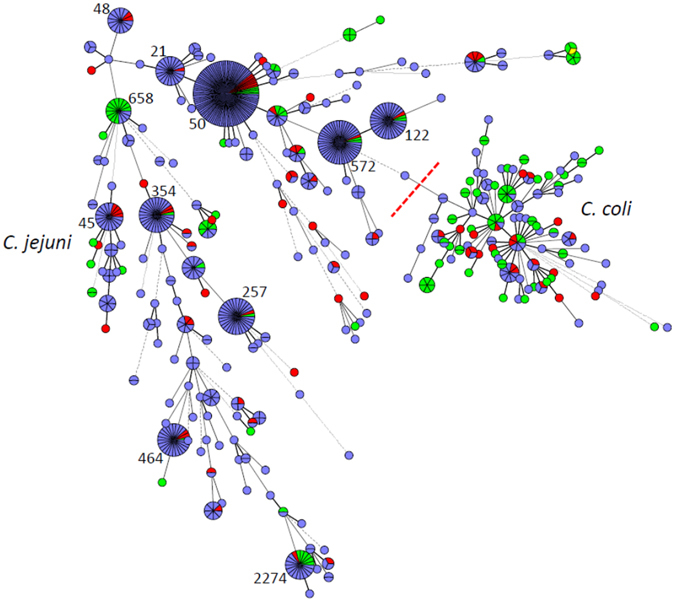
Minimal Spanning Tree generated from MLST comparisons of 816 C. jejuni and C. coli isolates from human study participants and from animals and food samples from the study region. Colouring according to isolate source: blue, isolates from patients; green, isolates from animals; red, isolates from food samples. Only abundant sequence types (STs) are labelled. A version of the figure with full labelling is available as Supplementary Figure S1.
Figure 2.
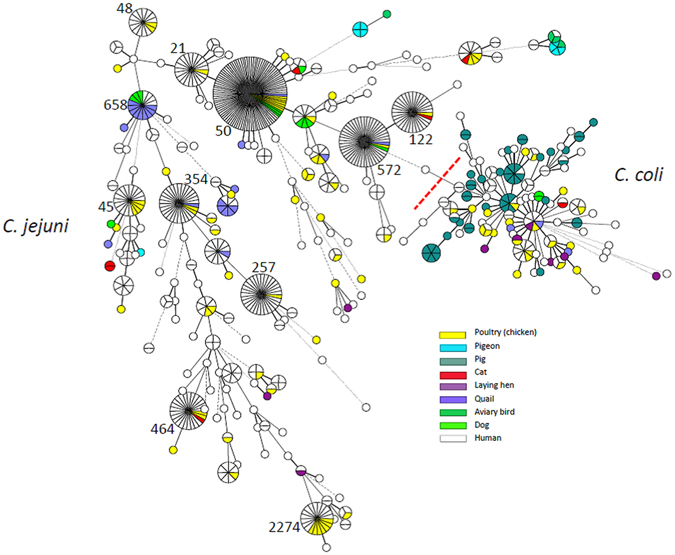
Minimal Spanning Tree generated from MLST comparisons of 816 C. jejuni and C. coli isolates from human study participants and from animals and food samples from the study region. Colouring according to host species as specified in colour legend. Only abundant sequence types (STs) are labelled. A version of the figure with full labelling is available as Supplementary Figure S2.
Molecular source attribution on the basis of MLST
The MLST typing data of human isolates from the case patients of the case-control study and time-matched animal and food isolates was used to perform source attribution of each human isolate. This analysis was based on an asymmetric island model for which Bayesian inference is implemented in the software iSource7. The output is a matrix of posterior probabilities of each human isolate originating from each of the putative sources (Fig. 3). We considered 5 putative sources: chicken, pig, pet dog or cat, cattle, other poultry (turkey, duck, goose, quail). To ensure that the population of each source was well characterised, we supplemented the German MLST data with publicly available MLST data obtained in neighbouring European countries. When using a 50% cut-off on the posterior probability of origin, 91% (555/613) of human isolates were attributed to chicken, 4% (27/613) to pig, 1% (6/613) to pet, and 0.8% (5/613) to other poultry. None of the isolates were attributed to cattle. The proportion of isolates that could not be attributed to any of the 5 sources was 3% (20/613). The mean probability of human isolates to originate from chicken was 71%, from pig 4%, from pet 14%, from cattle 1%, and from other poultry 9% (Table 6). The probability to originate from chicken was high for the most frequent STs of human isolates: 74% for ST-50, 77% for ST-572, 74% for ST-122, 85% for ST-257, 83% for ST-354, and 85% for ST-464. For C. coli isolates, the mean probability to originate from pig was higher, whereas the probability to originate from chicken was lower relative to C. jejuni isolates (Table 6). The probability to originate from pigs was almost zero for C. jejuni isolates (Table 6). We stratified human isolates according to sex, age group, region of living (urban or rural) of case patients, and by season of disease onset in case patients but did not find substantial differences between the strata (Table 6). When we excluded the source pet (dog or cat) from our analysis, the mean posterior probability of the human isolates to originate from the remaining 4 sources was as follows: chicken 0.82; pig 0.04; cattle 0.03; poultry other than chicken 0.11.
Figure 3.
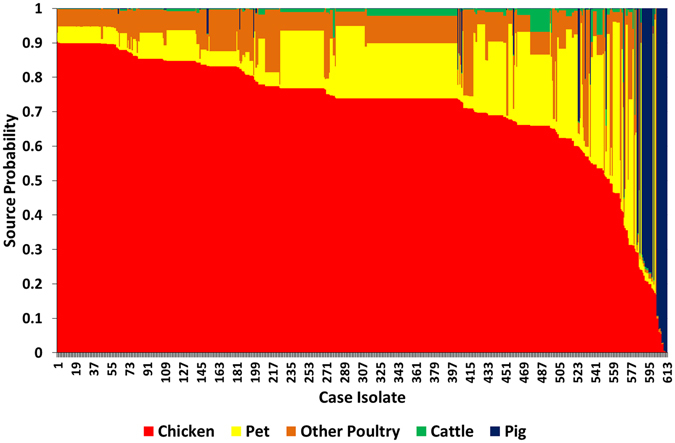
Source probabilities for human isolates (n = 613) to originate from each of the five sources (chicken, pet, pig, cattle, poultry other than chicken) as determined by source attribution analysis. MLST data from animal and food isolates obtained in Germany and in neighbouring European countries was used for source attribution analysis (Table 1).
Table 6.
Mean posterior probabilities of human isolates of originating from one of the putative sources (chickens, pigs, pet dogs or cats, cattle, poultry other than chicken (duck, goose, turkey, quail: “other poultry”)) as determined by asymmetric island source attribution modelling based on MLST data.
| Human isolates | Mean posterior source probability | ||||
|---|---|---|---|---|---|
| Chicken | Pig | Pet | Cattle | Other poultry | |
| All human isolates (n = 613) | 0.71 | 0.04 | 0.14 | 0.01 | 0.09 |
| Campylobacter species | |||||
| C. jejuni (n = 537) | 0.74 | 0.001 | 0.16 | 0.01 | 0.09 |
| C. coli (n = 76) | 0.56 | 0.32 | 0.04 | 0.004 | 0.08 |
| Sex of case patient | |||||
| Female (n = 334) | 0.72 | 0.04 | 0.14 | 0.01 | 0.09 |
| Male (n = 279) | 0.71 | 0.05 | 0.14 | 0.01 | 0.09 |
| Age group of case patient | |||||
| 0–4 years (n = 17) | 0.70 | <0.001 | 0.16 | 0.02 | 0.12 |
| 5–14 years (n = 32) | 0.71 | 0.02 | 0.15 | 0.01 | 0.09 |
| ≥15years (n = 564) | 0.72 | 0.04 | 0.14 | 0.01 | 0.09 |
| Region of living of case patient | |||||
| Urban (n = 445) | 0.72 | 0.03 | 0.14 | 0.01 | 0.10 |
| Rural (n = 168) | 0.69 | 0.08 | 0.14 | 0.01 | 0.08 |
| Season of disease onset in case patient | |||||
| Spring (n = 123) | 0.71 | 0.05 | 0.13 | 0.01 | 0.08 |
| Summer (n = 247) | 0.73 | 0.03 | 0.14 | 0.01 | 0.09 |
| Autumn (n = 108) | 0.70 | 0.06 | 0.13 | 0.01 | 0.09 |
| Winter (n = 135) | 0.69 | 0.04 | 0.15 | 0.01 | 0.10 |
Stratification according to Campylobacter species or characteristics of the case patients. Due to rounding of numbers the sum of probabilities may not add up to 1.00.
Discussion
We describe the first combined case-control and source attribution study for Campylobacter infections in Germany. We identified consumption of chicken meat, eating out, preparation of packaged chicken meat in the household and contact with poultry animals, as significant risk factors for Campylobacter infections. The use of gastric acid inhibitors was also positively associated with Campylobacter infection. Consumption of chicken meat was the most important risk factor for Campylobacter infections with a population attributable fraction (PAF) of 31%, confirming results from studies conducted in other countries10, 14, 18–21, 23. Chicken meat is frequently contaminated with Campylobacter. 25% of caecum samples and 52% of neck skin samples taken from broilers at abattoirs tested positive for Campylobacter in a zoonosis monitoring by veterinary authorities in Germany34. The higher prevalence on skin samples indicated that the carcasses were contaminated during slaughtering. A high proportion of samples (38–54%) taken from fresh broiler meat at retail also tested positive for Campylobacter 2, 34, 35.
Eating out, especially eating chicken at a restaurant, has been described as a risk factor in other studies14, 21, 22, 36–38. Exposure to Campylobacter while eating out may have occurred through consumption of insufficiently heated meat, e.g., from chicken, or through cross-contamination of food items in the restaurant kitchen. Cross-contamination due to inadequate kitchen hygiene in the private household may also underlie the positive association of Campylobacter infection and preparation of packaged chicken meat in this study. Use of gastric acid inhibitors, such as omeprazole and pantoprazole, for other therapeutically indicated reasons not related to Campylobacter infection was identified as another risk factor for Campylobacter infections, which confirms results from previous studies on Campylobacter and other bacterial gastrointestinal infections20, 21, 39–44. The association appears plausible, because an increase in the stomach pH may result in the survival of higher bacterial loads of incoming intestinal pathogens, such as Campylobacter, in the stomach45. Patients using gastric acid inhibitors should be informed by their physicians or pharmacists about the association with Campylobacter or other bacterial gastrointestinal infections, so that the patients can make an informed choice to avoid eating risk food items while on this medication.
In our analysis of young children we found an association of contact with sand in a sandbox and Campylobacter infection, which has been demonstrated as a risk factor for infection with other gastrointestinal pathogens as well26, 42, 46. It remains to be elucidated whether sandboxes are the actual source of infection with these pathogens, e.g., because of contamination of the sand with animal faeces (e.g., dogs, wild birds47), or if infectious gastrointestinal pathogens can survive well in sandboxes and can be readily transferred from child to child, or if playing in a sandbox is a proxy for a still unidentified environmental exposure. Interestingly, consumption of chicken meat was not a statistically significant risk factor for Campylobacter infections in young children48, 49, but preparation of chicken meat (fresh or packaged) in the household was, again indicating that infections may have occurred via cross-contamination of other food items. A migrant background was associated with Campylobacter infections among young children. Cases and controls in this age group that reported a migrant background came from a wide variety of European and non-European countries and it is unclear why the odds of cases having a migrant background would be higher than for controls. One explanation may be that parents of healthy children with a migrant background more frequently decided against participating in our study, compared to parents of children with a migrant background that had been ill.
Besides the use of gastric acid inhibitors we identified consumption of pork as a risk factor for C. coli infections in persons 15 years and older. In line with this result, the mean probability of human C. coli isolates to originate from pig in our source attribution model was relatively high (32%). Consumption or preparation of chicken was not a risk factor for C. coli infections, which was unexpected because 28% of chicken meat samples taken at retail as part of the zoonosis monitoring program in Germany tested positive for C. coli in 201450. One possible explanation is the small number of confirmed C. coli cases in our dataset (age group >= 15 years: n = 65), which resulted in insufficient analytical power for detection of a presumably weak association between consumption of chicken and C. coli infection. Interestingly, the mean probability of C. coli isolates to originate from chicken was higher than the mean probability to originate from pigs (56% vs. 32%). The mean probability of the C. jejuni isolates in our study to originate from pigs was close to zero and consumption of pork was not identified as a risk factor for C. jejuni infections. This finding is in accordance with results from the zoonosis monitoring program in Germany, where C. jejuni is rarely isolated from pig matrices51.
According to our source attribution model, about 90% of human isolates were attributable to chicken, albeit using a 50% cut-off. This is in line with other studies, where about 50–90% of human infections could be attributed to the chicken reservoir7, 8, 10, 11, 13, 14, 24, 52. Contrary to results from other studies7, 10–14, 24, 25, 53, none of the human isolates in our study was attributed to cattle. Campylobacteriosis outbreaks are frequently caused by consumption of unpasteurised (“raw”) milk, especially among children, implying cattle as the source for human Campylobacter infections in these outbreaks54–56. However, consumption of raw milk and other raw milk products was not a risk factor in our study or other studies on sporadic Campylobacter infections22. In univariable analyses, consumption of raw milk or raw milk products was even negatively associated with disease.
It was puzzling that some variables indicating good kitchen hygiene at home were identified as risk factors for disease in univariable analyses (frequently or always using separate utensils for raw meat and other food items; frequently or always using a dishwasher for utensils that came in contact with raw meat). Unexpected associations related to kitchen hygiene have been observed in a recent salmonellosis case-control study as well, and one interpretation was that case patients may overemphasise their hygienic behaviour in hindsight or may give answers that they think are socially desirable39.
Like any case-control study, ours is not without limitations. The proportion of female persons among case participants was slightly higher than among non-participants, therefore, participants may not be representative of all notified campylobacteriosis cases. Eating habits of women likely differ from those of men, which may bias the results of our study regarding the association with consumption of certain food items. However, the proportion of female persons was also higher in the control group, thus minimising this potential bias. In all case-control studies, including ours, recall bias may be an inherent problem. Cases may not have remembered consumption of particular food items as well as controls because the time period they were queried about was farther in the past than that of controls. The median time period between disease onset and completing the questionnaire was 16 days. Differential recall may result in an underestimation of the strength of the association. We supplemented our German MLST data with animal and food isolates from neighbouring countries to obtain a sufficiently large number of isolates for our source attribution model. This may have introduced a bias, because animal and food isolates from other countries and from other years may differ from those obtained in Germany in recent years57, especially if they are not a representative sample. We tried to minimise this bias by supplementing our MLST data with animal and food isolates only from neighbouring countries. Many of these isolates had been used in recent source attribution studies (Switzerland and Luxembourg). It is plausible to assume that consumption pattern and exposures pathways would be comparable between Germany and the directly neighbouring countries57.
Our source attribution analysis comes with certain limitations. We included pet animals (dogs and cats) as one of the possible sources of human isolates. However, since pets and their owners share the environment and possibly some of the food, it is possible that both human and pet Campylobacter isolates originate from another common reservoir, e.g., chicken, cattle, or pig. Pets could be viewed as a separate population in source attribution modelling because the asymmetric island model takes migration between source populations into account. The mean posterior probability of human isolates to originate from pets was rather small in our study (0.14), and, therefore, pets appeared not to be a relevant source. Only 10 human isolates (of 533) could be attributed to pets (using a 50% cut-off) and only 3 of the corresponding case-patients reported contact with a cat. When we excluded pets as a putative source from our attribution analysis, the mean posterior probability for human isolates to originate from chicken increased from 0.71 to 0.82, mean posterior probabilities to originate from other sources (cattle; poultry other than chicken) increased only slightly, or remained the same (pig). We did not consider sheep as a possible source of human isolates in our model because in Germany, in contrast to some other countries, e.g., New Zealand or Scotland, contact of people with sheep is limited and consumption of lamb/mutton does not play a major role as was confirmed by results from our case-control study (only about 9% of control persons had consumed lamb/mutton; about 2% of control persons had contact with sheep). Sheep were also a minor source of human infections in a study from the Netherlands10. Any source attribution modelling is limited by the putative animal or environmental sources that are included in the modelling, and, therefore, there is always the possibility to overlook sources of human infections.
This study was the first to investigate risk factors for sporadic Campylobacter infections in Germany and combine the results with source attribution analysis. We confirm that consumption and handling of chicken meat are important risk factors for campylobacteriosis. Tens of thousands of Campylobacter infections are notified in Germany every year. The true number of Campylobacter enteritis cases in the population is estimated to be about five to ten times higher58–60, and the burden of disease is considered as substantial61, 62. Therefore, efforts should be strengthened to minimise contamination of chicken meat with Campylobacter and to educate consumers about health risks associated with preparation and consumption of chicken meat. Examples from other countries have shown that improvements are possible if multidisciplinary approaches are undertaken63, 64.
Conclusions
To reduce the risk of Campylobacter infections among consumers, control measures and intervention strategies should be adopted to reduce prevalence of Campylobacter in poultry and poultry meat along the food chain65. Until such measures are initiated and effective, consumers and food handlers should be better educated about risks associated with consumption and preparation of poultry and personal protection measures, such as sufficient heating of poultry meat before consumption and kitchen hygiene when handling raw poultry or other types of raw meat to avoid cross-contamination.
Electronic supplementary material
Acknowledgements
This study was supported by grant 01KI1012F (Foodborne Zoonotic Infections of Humans; FBI-Zoo) from the German Federal Ministry of Education and Research (BMBF). The authors thank all study participants, the local and state health authorities in Berlin, Brandenburg, North Rhine-Westphalia and Saxony, and the participating diagnostic laboratories for their support. We thank Sally Bütgenbach, Mariya Chubrieva, Christiane Cyberski, Mandy Hoffmann, Benjamin Jentzsch, Marlene Kretschmer, Caterina Lindig, and Hannes Ulrich for their help with mailing of questionnaires and data entry. Birgit Brenneke and Kerstin Ellrott are acknowledged for expert technical support.
Author Contributions
B.M.R., A.S., C.J., S.S. and K.Sta. designed and coordinated the study, performed data analysis, and wrote the manuscript. X.D. performed molecular source attribution analyses. F.K., C.J. and S.S. cultured and archived all Campylobacter isolates, performed multilocus sequence typing and analysed the MLST data. J.B. contributed to study coordination. N.W. performed source attribution analyses and provided statistical expertise. G.G. and T.A. collected food and animal samples, performed laboratory analyses, and provided Campylobacter isolates. K.Sti. performed laboratory analyses and provided Campylobacter isolates. All authors have contributed substantially to data interpretation, have critically reviewed the manuscript, and approved the final version as submitted. B.M.R. and A.S. contributed equally to this study. S.S. and K.Sta. contributed equally to this study.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Bettina M. Rosner, Anika Schielke, Sebastian Suerbaum and Klaus Stark contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-05227-x
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bettina M. Rosner, Email: RosnerB@rki.de
Sebastian Suerbaum, Email: suerbaum@mvp.uni-muenchen.de.
Klaus Stark, Email: StarkK@rki.de.
References
- 1.Robert Koch-Institut, Berlin. Infektionsepidemiologisches Jahrbuch für 2015 (2016).
- 2.EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA Journal13, 4329 (2015).
- 3.Schielke A, Rosner BM, Stark K. Epidemiology of campylobacteriosis in Germany - insights from 10 years of surveillance. BMC Infect Dis. 2014;14:30. doi: 10.1186/1471-2334-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nachamkin I, Allos BM, Ho T. Campylobacter species and Guillain-Barre syndrome. Clin Microbiol Rev. 1998;11:555–567. doi: 10.1128/cmr.11.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butzler JP. Campylobacter, from obscurity to celebrity. Clin Microbiol Infect. 2004;10:868–876. doi: 10.1111/j.1469-0691.2004.00983.x. [DOI] [PubMed] [Google Scholar]
- 6.Keithlin J, Sargeant J, Thomas MK, Fazil A. Systematic review and meta-analysis of the proportion of Campylobacter cases that develop chronic sequelae. BMC Public Health. 2014;14:1203. doi: 10.1186/1471-2458-14-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson DJ, et al. Tracing the source of campylobacteriosis. PLoS Genetics. 2008;4:e1000203. doi: 10.1371/journal.pgen.1000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheppard SK, et al. Campylobacter genotyping to determine the source of human infection. Clin Infect Dis. 2009;48:1072–1078. doi: 10.1086/597402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullner P, et al. Source attribution of food-borne zoonoses in New Zealand: a modified Hald model. Risk Analysis. 2009;29:970–984. doi: 10.1111/j.1539-6924.2009.01224.x. [DOI] [PubMed] [Google Scholar]
- 10.Mughini Gras L, et al. Risk factors for campylobacteriosis of chicken, ruminant, and environmental origin: a combined case-control and source attribution analysis. PLoS One. 2012;7:e42599. doi: 10.1371/journal.pone.0042599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kittl S, Heckel G, Korczak BM, Kuhnert P. Source attribution of human Campylobacter isolates by MLST and fla-typing and association of genotypes with quinolone resistance. PLoS One. 2013;8:e81796. doi: 10.1371/journal.pone.0081796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boysen L, et al. Source attribution of human campylobacteriosis in Denmark. Epidemiol Infect. 2014;142:1599–1608. doi: 10.1017/S0950268813002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dearlove BL, et al. Rapid host switching in generalist Campylobacter strains erodes the signal for tracing human infections. ISME J. 2016;10:721–729. doi: 10.1038/ismej.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mossong J, et al. Human Campylobacteriosis in Luxembourg, 2010–2013: a case-control study combinedwith multilocus sequence typing for source attribution and risk factor analysis. Sci Rep. 2016;6:20939. doi: 10.1038/srep20939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullner P, et al. Molecular epidemiology of Campylobacter jejuni in a geographically isolated country with a uniquely structured poultry industry. Appl Environ Microbiol. 2010;76:2145–2154. doi: 10.1128/AEM.00862-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Food Safety Authority (EFSA). Scientific Opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA Journal9, 2105 (2011).
- 17.Skarp CP, Hanninen ML, Rautelin HI. Campylobacteriosis: the role of poultry meat. Clin Microbiol Infect. 2016;22:103–109. doi: 10.1016/j.cmi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Wingstrand A, et al. Fresh chicken as main risk factor for campylobacteriosis, Denmark. Emerg Infect Dis. 2006;12:280–285. doi: 10.3201/eid1202.050936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danis K, et al. Risk factors for sporadic Campylobacter infection: an all-Ireland case-control study. Euro Surveill. 2009;14:19123. [PubMed] [Google Scholar]
- 20.Tam CC. Chicken consumption and use of acid-suppressing medications as risk factors for Campylobacter enteritis, England. Emerg Infect Dis. 2009;15:1402–1408. doi: 10.3201/eid1509.080773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doorduyn Y, et al. Risk factors for indigenous Campylobacter jejuni and Campylobacter coli infections in The Netherlands: a case-control study. Epidemiol Infect. 2010;138:1391–1404. doi: 10.1017/S095026881000052X. [DOI] [PubMed] [Google Scholar]
- 22.Domingues AR, Pires SM, Halasa T, Hald T. Source attribution of human campylobacteriosis using a meta-analysis of case-control studies of sporadic infections. Epidemiol Infect. 2012;140:970–981. doi: 10.1017/S0950268811002676. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald E, et al. Risk Factors for sporadic domestically acquired Campylobacter infections in Norway 2010-2011: a national prospective case-control study. PLoS One. 2015;10:e0139636. doi: 10.1371/journal.pone.0139636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullner P, et al. Assigning the source of human campylobacteriosis in New Zealand: a comparative genetic and epidemiological approach. Infect Genet Evol. 2009;9:1311–1319. doi: 10.1016/j.meegid.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Levesque S, et al. Campylobacteriosis in urban versus rural areas: a case-case study integrated with molecular typing to validate risk factors and to attribute sources of infection. PLoS One. 2013;8:e83731. doi: 10.1371/journal.pone.0083731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosner BM, Stark K, Höhle M, Werber D. Risk factors for sporadic Yersinia enterocolitica infections, Germany 2009-2010. Epidemiol Infect. 2012;140:1738–1747. doi: 10.1017/S0950268811002664. [DOI] [PubMed] [Google Scholar]
- 27.Federal Statistical Office, Germany. https://www.destatis.de (2016)
- 28.Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C. Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol. 1985;122:904–914. doi: 10.1093/oxfordjournals.aje.a114174. [DOI] [PubMed] [Google Scholar]
- 29.R, Core Team. R: a language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org (2014).
- 30.Llorca J, Delgado-Rodriguez M. A comparison of several procedures to estimate the confidence interval for attributable risk in case-control studies. Stat Med. 2000;19:1089–1099. doi: 10.1002/(SICI)1097-0258(20000430)19:8<1089::AID-SIM411>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Dingle KE, Colles FM, Falush D, Maiden MC. Sequence typing and comparison of population biology of Campylobacter coli and Campylobacter jejuni. J Clin Microbiol. 2005;43:340–347. doi: 10.1128/JCM.43.1.340-347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gripp E, et al. Closely related Campylobacter jejuni strains from different sources reveal a generalist rather than a specialist lifestyle. BMC Genomics. 2011;12:584. doi: 10.1186/1471-2164-12-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jolley KA, Maiden MC. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics. 2010;11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL). Reports on food safety 2013-zoonosis monitoring. Berichte zur Lebensmittelsicherheit 2013- Zoonosen Monitoring. [In German]. doi:10.1007/978-3-319-15380-3 (2015).
- 35.Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL). Reports on food safety 2014-zoonosis monitoring. Berichte zur Lebensmittelsicherheit 2014- Zoonosen Monitoring. [In German]. doi:10.1007/978-3-319-30151-8 (2016).
- 36.Friedman CR, et al. Risk factors for sporadic Campylobacter infection in the United States: a case-control study in FoodNet sites. Clin Infect Dis. 2004;38(Suppl 3):S285–296. doi: 10.1086/381598. [DOI] [PubMed] [Google Scholar]
- 37.Gallay A, et al. Risk factors for acquiring sporadic Campylobacter infection in France: results from a national case-control study. J Infect Dis. 2008;197:1477–1484. doi: 10.1086/587644. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues LC, et al. The study of infectious intestinal disease in England: risk factors for cases of infectious intestinal disease with Campylobacter jejuni infection. Epidemiol Infect. 2000;127:185–193. doi: 10.1017/s0950268801006057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rettenbacher-Riefler S, et al. Sporadic salmonellosis in Lower Saxony, Germany, 2011–2013: raw ground pork consumption is associated with Salmonella Typhimurium infections and foreign travel with Salmonella Enteritidis infections. Epidemiol Infect. 2015;143:2777–2785. doi: 10.1017/S0950268814003768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neal KR, Scott HM, Slack RC, Logan RF. Omeprazole as a risk factor for campylobacter gastroenteritis: case-control study. BMJ. 1996;312:414–415. doi: 10.1136/bmj.312.7028.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neal KR, Slack RC. Diabetes mellitus, anti-secretory drugs and other risk factors for campylobacter gastro-enteritis in adults: a case-control study. Epidemiol Infect. 1997;119:307–311. doi: 10.1017/S0950268897008224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doorduyn Y, Van Den Brandhof WE, Van Duynhoven YT, Wannet WJ, Van Pelt W. Risk factors for Salmonella Enteritidis and Typhimurium (DT104 and non-DT104) infections in The Netherlands: predominant roles for raw eggs in Enteritidis and sandboxes in Typhimurium infections. Epidemiol Infect. 2006;134:617–626. doi: 10.1017/S0950268805005406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freeman R, et al. Association between use of proton pump inhibitors and non-typhoidal salmonellosis identified following investigation into an outbreak of Salmonella Mikawasima in the UK, 2013. Epidemiol Infect. 2016;144:968–975. doi: 10.1017/S0950268815002332. [DOI] [PubMed] [Google Scholar]
- 44.Bouwknegt M, van Pelt W, Kubbinga ME, Weda M, Havelaar AH. Potential association between the recent increase in campylobacteriosis incidence in the Netherlands and proton-pump inhibitor use - an ecological study. Euro Surveill. 2014;19:pii: 20873. doi: 10.2807/1560-7917.ES2014.19.32.20873. [DOI] [PubMed] [Google Scholar]
- 45.Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34:1269–1281. doi: 10.1111/j.1365-2036.2011.04874.x. [DOI] [PubMed] [Google Scholar]
- 46.Werber D, et al. Shiga toxin-producing Escherichia coli infection in Germany: different risk factors for different age groups. Am J Epidemiol. 2007;165:425–434. doi: 10.1093/aje/kwk023. [DOI] [PubMed] [Google Scholar]
- 47.French NP, et al. Molecular epidemiology of Campylobacter jejuni isolates from wild-bird fecal material in children’s playgrounds. Appl Environ Microbiol. 2009;75:779–783. doi: 10.1128/AEM.01979-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carrique-Mas J, et al. Risk factors for domestic sporadic campylobacteriosis among young children in Sweden. Scand J Infect Dis. 2005;37:101–110. doi: 10.1080/00365540510027165. [DOI] [PubMed] [Google Scholar]
- 49.Tenkate TD, Stafford RJ. Risk factors for campylobacter infection in infants and young children: a matched case-control study. Epidemiol Infect. 2001;127:399–404. doi: 10.1017/S0950268801006306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bundesinstitut für Risikobewertung (BfR) Pathogens and zoonoses in the year 2014. Erreger und Zoonosen im Jahr 2014 [In German] http://www.bfr.bund.de/cm/350/erreger-von-zoonosen-in-deutschland-im-jahr-2014.pdf (2016).
- 51.Bundesamt für Verbraucherschutz und Lebenmittelsicherheit (BVL) Reports on food safety 2015. Berichte zur Lebensmittelsicherheit 2015 [In German] http://www.bvl.bund.de/SharedDocs/Downloads/01_Lebensmittel/04_Zoonosen_Monitoring/Zoonosen_Monitoring_Bericht_2015.pdf?__blob=publicationFile&v=6 (2016).
- 52.European Food Safety Authority (EFSA). Scientific Opinion on quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA Journal8, 1437 (2010).
- 53.Bessell PR, et al. Using sequence data to identify alternative routes and risk of infection: a case-study of campylobacter in Scotland. BMC Infect Dis. 2012;12:80. doi: 10.1186/1471-2334-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hauri AM, et al. [Campylobacteriosis outbreaks in the state of Hesse, Germany, 2005–2011: raw milk yet again] Dtsch Med Wochenschr. 2013;138:357–361. doi: 10.1055/s-0032-1332884. [DOI] [PubMed] [Google Scholar]
- 55.Heuvelink AE, et al. Two outbreaks of campylobacteriosis associated with the consumption of raw cows’ milk. Int J Food Microbiol. 2009;134:70–74. doi: 10.1016/j.ijfoodmicro.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention (CDC) Recurrent outbreak of Campylobacter jejuni infections associated with a raw milk dairy–Pennsylvania, April-May 2013. MMWR Morb Mortal Wkly Rep. 2013;62:702. [PMC free article] [PubMed] [Google Scholar]
- 57.Smid JH, et al. Practicalities of using non-local or non-recent multilocus sequence typing data for source attribution in space and time of human campylobacteriosis. PLoS One. 2013;8:e55029. doi: 10.1371/journal.pone.0055029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Havelaar AH, Ivarsson S, Lofdahl M, Nauta MJ. Estimating the true incidence of campylobacteriosis and salmonellosis in the European Union, 2009. Epidemiol Infect. 2013;141:293–302. doi: 10.1017/S0950268812000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haagsma JA, et al. Community incidence of pathogen-specific gastroenteritis: reconstructing the surveillance pyramid for seven pathogens in seven European Union member states. Epidemiol Infect. 2013;141:1625–1639. doi: 10.1017/S0950268812002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tam CC, et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut. 2012;61:69–77. doi: 10.1136/gut.2011.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mangen MJ, et al. Cost-of-illness and disease burden of food-related pathogens in the Netherlands, 2011. Int J Food Microbiol. 2015;196:84–93. doi: 10.1016/j.ijfoodmicro.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 62.Tam CC, O’Brien SJ. Economic Cost of Campylobacter, Norovirus and Rotavirus Disease in the United Kingdom. PLoS One. 2016;11:e0138526. doi: 10.1371/journal.pone.0138526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sears A, et al. Marked campylobacteriosis decline after interventions aimed at poultry, New Zealand. Emerg Infect Dis. 2011;17:1007–1015. doi: 10.3201/eid/1706.101272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tustin J, et al. A national epidemic of campylobacteriosis in Iceland, lessons learned. Zoonoses Public Health. 2011;58:440–447. doi: 10.1111/j.1863-2378.2010.01387.x. [DOI] [PubMed] [Google Scholar]
- 65.Gölz G, et al. Relevance of Campylobacter to public health–the need for a One Health approach. Int J Med Microbiol. 2014;304:817–823. doi: 10.1016/j.ijmm.2014.08.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in Supplementary Information. Additional data of the case-control study will be made available upon request to the corresponding first or the last author.


