Abstract
Many of the physiological, cellular, and molecular rhythms that are present within the eye are under the control of circadian clocks. Experimental evidence suggests that the retinal circadian clock, or its output signals (e.g., dopamine and melatonin), may contribute to eye disease and pathology. We recently developed a retinal pigment ephithelium (RPE)-choroid preparation to monitor the circadian clock using PERIOD2 (PER2)::LUC knock-in mouse. In this study we report that dopamine, but not melatonin, is responsible for entrainment of the PER2::LUC bioluminescence rhythm in mouse RPE-choroid. Dopamine induced phase-advances of the PER2::LUC bioluminescence rhythm during the subjective day and phase-delays in the late subjective night. We found that dopamine acts exclusively through Dopamine 2 Receptors to entrain the circadian rhythm in PER2::LUC bioluminescence. Finallly, we found that DA-induced expression of core circadian clock genes Period1 and Period2 accompanied both phase advances and phase delays of the RPE-choroid clock, thus suggesting that – as in other tissues – the rapid induction of these circadian clock genes drives the resetting process. Since the RPE cells persist for the entire lifespan of an organism, we believe that RPE-choroid preparation may represent a new and unique tool to study the effects of circadian disruption during aging.
Introduction
The presence of a retinal circadian clock in mammals was first reported in the late 90s1, 2 and several studies have now demonstrated that many of the physiological, cellular and molecular rhythms within the retina are under the control of cell-autonomous intrinsic circadian clocks3, 4. Emerging experimental evidence also indicates the presence of circadian clocks in other ocular structures such as cornea and retinal pigment epithelium (RPE) that are involved in the control of many ocular circadian rhythms (e.g., intraocular pressure, photoreceptor disk shedding and phagocytosis, axial chamber length, choroidal volume, corneal curvature and cornea thickness)3, 4.
The current understanding of the circadian system within the mouse eye has been greatly advanced by the use of the PERIOD2::Luciferase (PER2::LUC) mouse that allows real-time monitoring of clock functions5. Using this mouse, several laboratories have been able to demonstrate that circadian clocks are present not only in the retina6, 7, but also the cornea5, 8 and that the (RPE)9. Hence the mouse eye is a bona fide circadian system containing circadian oscillators in several different cell types and cell types, all of which must be synchronized for the system to function correctly. Although we do not know which of these clocks function as the ‘master’ circadian oscillator within the eye, we know that only the retina responds directly to light1, 2, 7, 9, and the circadian rhythm in PER2::LUC bioluminescence in the retina is likely to be entrained via OPN 510. Therefore, the retina must communicate photo-entrainment signals within the retina and to the rest of the ocular circadian system in order to maintain the clocks and synchrony in different non-photosensitive ocular structures. Among the several neurotransmitters/hormones present in the retina, melatonin (MLT) and dopamine (DA) have emerged as two likely candidates to transmit this signal throughout the eye. DA functions as a rhythmic humoral signal for light, producing light-adaptive physiology7, 11, and MLT is a rhythmic signal of darkness and has dark-adaptive effects12, 13. Consistent with this hypothesis, we have recently shown that the PER2::LUC bioluminescence rhythm in the mouse cornea can be entrained by melatonin via activation of Melatonin Receptor 28.
The aim of the present study was to investigate whether MLT or/and DA play a role in the entrainment of the PER2::LUC bioluminescence rhythm in RPE. Our data indicate that DA - via Dopamine 2 Receptors (D2R) present in RPE - entrains circadian PER2::LUC bioluminescence rhythms in the mouse RPE.
Results
Dopamine, but not melatonin, phase-shifts the circadian rhythm in PER2::LUC bioluminescence
To determine whether DA and/or MLT can entrain RPE rhythms, we applied either compound to PER2::LUC RPE-choroid cultures at various times and measured the resultant shift in rhythm phase. We found that, in contrast to the cornea clock8, the RPE clock was not reset by 100 nM MLT (Fig. 1A–C) when applied at any time of day. In contrast, 100 μM DA shifts the clock forward (advance) or backward (delay) by up to 8–12 hours, depending on the time it was administered (Fig. 1D–F). To aid in analysis, we binned individual phase-shifts into 4-hour phase intervals for statistical analysis. Two-way ANOVA revealed a significant interaction between treatment (vehicle or drug) and time of treatment in the DA treated cultures (p < 0.001; Fig. 1F), but not in the MLT treated cultures (p > 0.05; Fig. 1C). Overall, DA phase-delayed the RPE clocks by ~6.4 hours when given in an 8-hour window centered on a circadian “dawn” (CT0), phase advanced the RPE clock by approximately 5 hours when given throughout the rest of the circadian “day” (CT 4–12), and produced little effect on phase when given during the first 8 hours of the circadian “night” (CT 12–20) (Fig. 1F). Overall, these results suggest that DA, and not MLT, may be a circadian entraining signal for RPE in the retinal circadian system.
Figure 1.
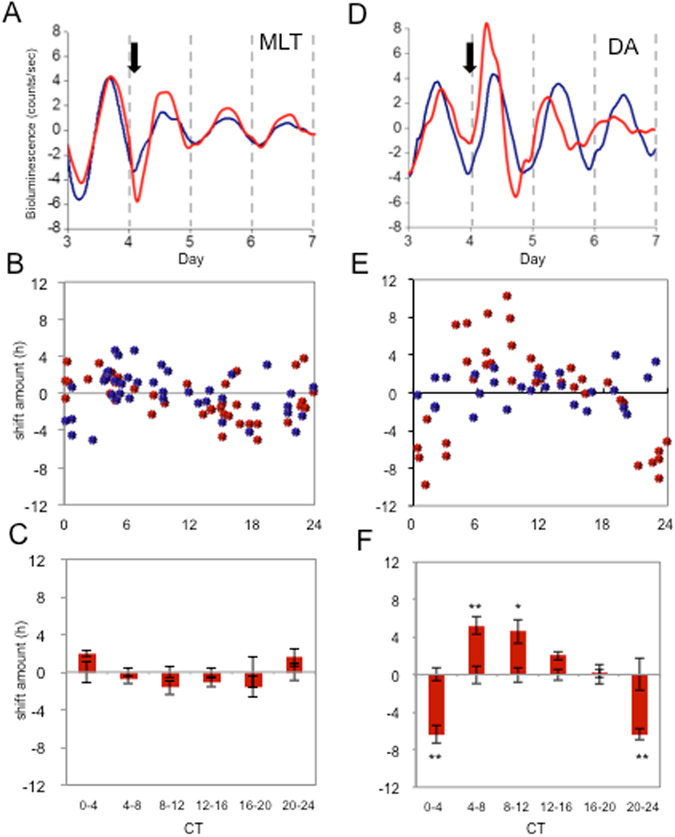
Dopamine phase-shifts PER2::LUC bioluminescence rhythms in mouse RPE-choroid. DA or MLT were added to the culturing media after the third peak of the RPE-choroid PER2::LUC bioluminescence rhythm. The representative data shows application of MLT at CT 14 did not phase-shift the RPE-choroid bioluminescence PER2::LUC rhythm (A). On the other hand, DA application at CT 8 phase-shifted the RPE-choroid bioluminescence rhythm (D). The blue traces indicate controls (vehicle treated) while red traces indicate MLT (A) or DA (D) treated RPE-choroid cultures. The black arrows indicate time of the drug or vehicle (Veh) treatments (A and D). The amount of phase-shift for each individual RPE-choroid rhythm was plotted to create a PRC (B and E). Blue circles indicate cultures treated with Veh and red circles indicate culture treated with either MLT (B) or DA (E). Data were divided into 6 bins at 4-hour intervals for statistical analysis. Data were then used to calculate the phase change of MLT or DA versus their vehicle controls. Bars show the mean amount of phase change from controls and error bars show ±SEM for experimental groups. Error bars from x axis show ±SEM for control groups. MLT did not phase-shift the RPE-choroid PER2::LUC bioluminescence rhythm (n = 4–14 for each bin, Two way ANOVA, p > 0.1, (C) whereas DA significantly phase-shifted PER2::LUC rhythm (Two way ANOVA following Tukey tests, *p < 0.05, **p < 0.01, n = 6–8 for each bin, (F).
Activation of D2R Signaling in the RPE Phase-shifts the Circadian Rhythm in PER2::LUC Bioluminescence
We then investigated which of the different DA receptors were responsible for phase-shift of the PER2::LUC circadian rhythm. There are five DA receptors in mammals, which are classified into D1-like (D1R, D5R) or D2-like (D2R, D3R, D4R) based on similar pharmacological profiles and coupling to second-messenger cascades14, 15. We found that all but the D3R s are detectably expressed in RPE (Fig. 2). We therefore investigated whether general D1-like or D2-like receptor agonists (SKF38393 or quinpirole, respectively) produced phase-response curves comparable to DA (Fig. 1F). We found that SKF38393 could not induce a phase-shift, regardless of when it was given (Fig. 3A and B). However, quinpirole administration phase-shifted the rhythm in a manner that was similar to that of DA (Fig. 3C). Quinpirole significantly phase-delayed PER2::LUC rhythms by 2.05 ± 0.65 hrs. when applied at CT 20–24 h (p < 0.05) and significantly phase-advanced PER2::LUC rhythms by 4.60 ± 0.54 hrs. and 3.16 ± 0.55 hrs when applied at CT 0–4 h and CT 4–8 h, respectively (p < 0.05; Fig. 3C,D). Overall, these data suggest that DA acting on D2Rs is sufficient to reset RPE rhythms.
Figure 2.
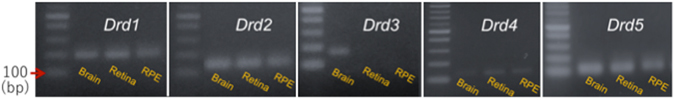
Expression of dopamine receptors in the brain, retina, and RPE. Agarose gel electrophoresis of PCR amplicons specific to D1R, D2R, D3R, D4R or D5R transcripts in brain, retina and RPE. D3R mRNA was present in the brain (positive control), but was not amplified in the retina (negative control) or RPE. The electrophoresis bands matched the expected amplicon size.
Figure 3.
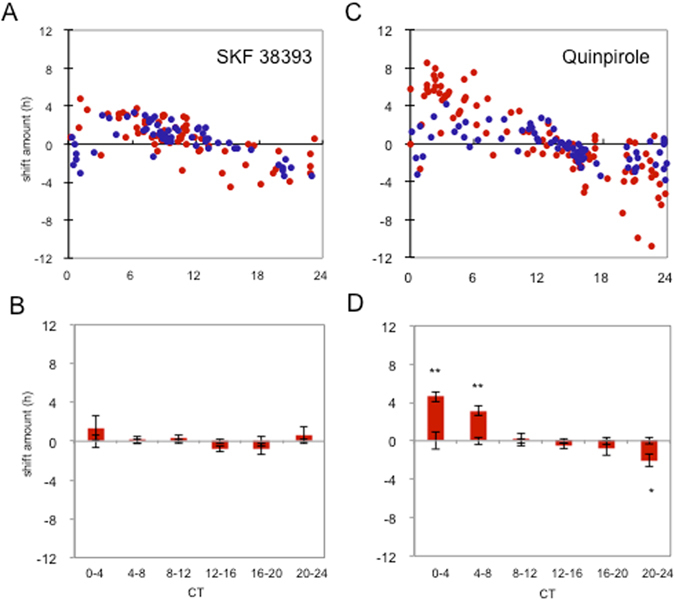
Effects of D1-like agonist (SKF38393) and D2-like agonist (Quinpirole) on PER2::LUC bioluminescence rhythm. 50 µM of SKF38393 did not phase-shift the PER2::LUC bioluminescence rhythm (A and B), whereas quinpirole significantly phase-advanced PER2::LUC bioluminescence rhythm at CT 0–4 and CT 4–8 hrs and phase-delayed when applied at CT 20–24 (C and D). Blue circles indicate cultures treated with vehicle and red circles indicate culture treated with active compounds. Data were divided to 6 bins at 4-hour intervals for statistical analysis (Two-way ANOVA following Tukey tests, *p < 0.05). n = 3–21 for each bin (B and D). Data were then used to calculate the phase change of drug treated versus their vehicle controls. Bars show the mean amount of phase change from controls and error bars show ±SEM for experimental groups. Error bars from x axis show ±SEM for control groups.
We next investigated which of the D2-like receptor subtypes where responsible for the observed phenomenon. Since D3R mRNA was undetectable in the RPE (Fig. 2), we focused on discriminating between D2R and D4R. Administration of Sumanirole, a D2R specific agonist, at various times of day shifted the clock in a manner similar to DA and quinpirole (Fig. 4A and B), whereas administering PD168077, D4R receptor specific agonist, did not phase-shift PER2::LUC rhythms in RPE-choroid (Fig. 4C and D). Thus, it appears that activation of D2Rs is sufficient to reset the RPE clock.
Figure 4.
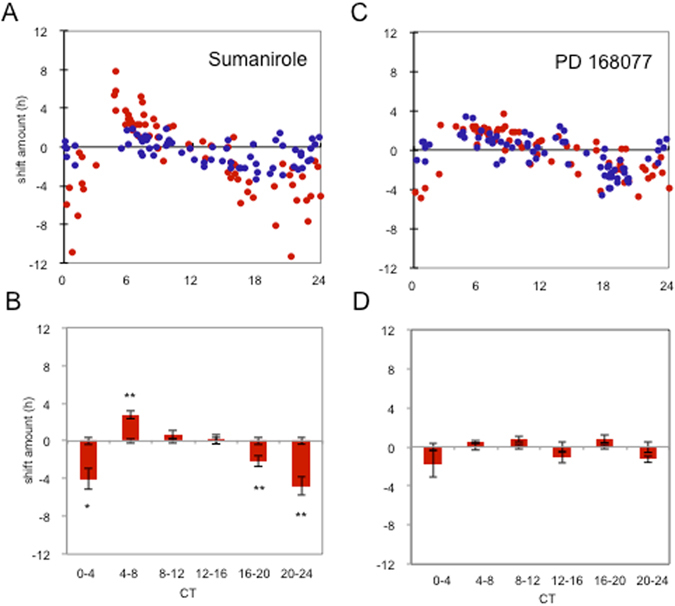
Effects of D2R (Sumanirole) and D4R (PD168077) agonists on the PER2::LUC bioluminescence rhythm. Sumanirole of 1 µM significantly phase-delayed PER2::LUC bioluminescence rhythm at CT 0–4, CT 16–20, and CT 20–24 h. Sumanirole significantly phase-advanced the bioluminescence rhythm at CT 4–8 (A and B). PD166077 did not phase-shift the PER2::LUC bioluminescence rhythms (C and D). Blue circles indicate cultures treated with vehicle and red circles indicate culture treated with active compounds (A and C). Data were divided to 6 bins at 4-hour intervals for statistical analysis (Two-way ANOVA following Tukey tests, *p < 0.05) n = 6–21 for each bin (B and D). Data were then used to calculate the phase change of drug treated versus their vehicle controls. Bars show the mean amount of phase change from controls and error bars show ±SEM for experimental groups. Error bars from x axis show ±SEM for control groups.
Removal of D2R Signaling Prevents DA-induced Phase-shift of PER2::LUC Bioluminescence Rhythm in RPE
We next tested if D2R was required for DA’s action on the RPE-clock by determining if DA could reset the clock of RPE-choroid from D2R-deficient PER2::LUC (Drd2 −/−; PER2::LUC) mice. The RPE-choroid tissues obtained from D2R−/−; PER2::LUC mice showed robust circadian rhythms (Fig. 5A) that were comparable in phase and periods to wild-type PER2::LUC controls (phases: 16.54 ± 0.20 hrs vs. 15.67 ± 0.53 hrs, periods: 23.88 ± 0.12 hrs vs. 23.68 ± 0.11 hrs p > 0.05, t-test, control vs. D2R−/− respectively). We then treated RPE-choroid with 100 μM of DA at circadian times when DA induces phase advances or delays. As expected, D2R-deficiency eliminated DA-induced phase-shifts of RPE-choroid bioluminescence rhythms (Fig. 5A and B), confirming that DA is signaling RPE clocks exclusively via the D2R.
Figure 5.
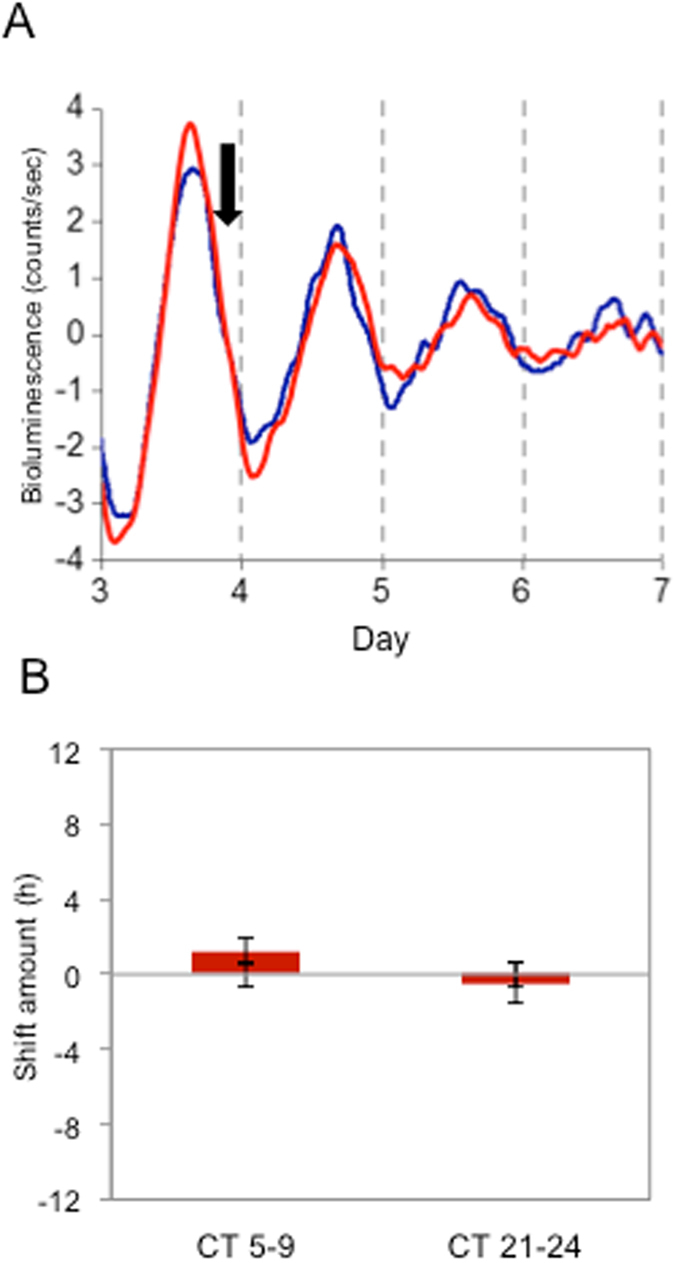
DA did not induce phase-shifts in the RPE-choroid of PER2::LUC bioluminescence rhythms in D2R−/−PER2::LUC mice. Representative example shows that DA treatment at CT 24 did not phase-shift D2R−/−PER2::LUC bioluminescence rhythm (A). The blue trace indicates a control (Veh treated) and red trace indicates a DA treated RPE-choroid culture. The black arrow indicates time of the drug or vehicle treatment (A). DA treated RPE-choroid cultures at CT 5–9 and at CT 21–24 were averaged for statistical analysis. Data were used to calculate the phase change of D2R−/− versus controls. Bars show the mean amount of phase change from controls and error bars show ±SEM for experimental groups. Error bars from x axis show ±SEM for control groups. No phase-shifts were observed in the D2R−/−PER2::LUC RPE-choroid rhythms at either time points (B) t-test, n = 6 for DA and Veh for each time point).
DA Induces Period1 and Period2 Gene Expression in the RPE
Previous studies have shown that acute induction of Period1 (Per1) and Period2 (Per2) gene expression mediates phase-shifting of the circadian clock16–18. Hence, we decided to measure acute induction of Per1 and Per2 expression in cultured RPE-choroids after either one hour or three hours of DA treatment. DA applied at CT 6 to cause a phase advance (Fig. 1) significantly induced Per1 expression (Fig. 6A, t-test, p < 0.05). Interestingly, Per1 expression was only elevated at 1 hour after the DA treatment, returning to baseline 3 hours after the DA treatment (Fig. 6B). DA applied at CT 6 did not significantly alter Per2 or Bmal1 mRNA levels (Fig. 6B, t-test, p > 0.05 for both cases). DA administered at CT23, when it induces phase-delays (Fig. 1), did not significantly change levels of Per1, Per2 and Bmal1 mRNAs at 1-hr (Fig. 6C, t-test, p > 0.05), but significantly induced Per1 and Per2 mRNA 3-hrs after the DA pulse (Fig. 6D; t-test, p < 0.05). No changes were observed in Bmal1 mRNA levels (Fig. 6C,D; t-test, p > 0.05). Thus, taken together, our results suggest that D2R activation mediates clock reset by acutely inducing expression of Per1 and Per2.
Figure 6.
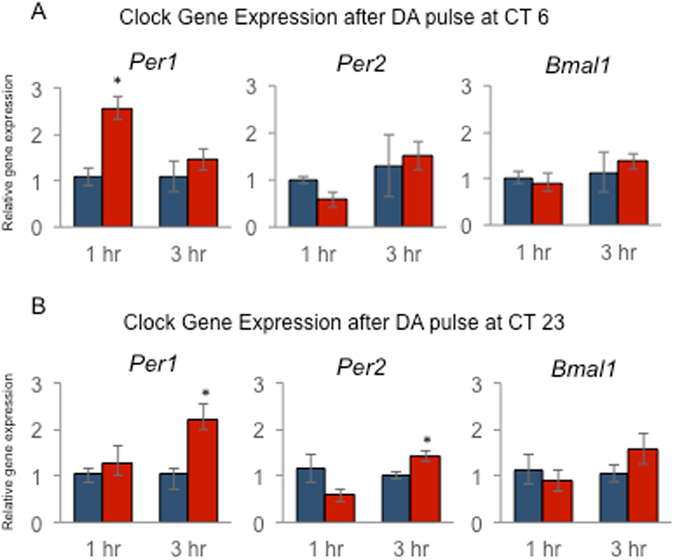
DA treatment increases Per1 and Per2 mRNA in RPE-choroid. RPE-choroid cultures were prepared and treated with DA or vehicle at ZT6 (A, advance) or ZT 23 (B, delay) as indicated above, followed by collection for Q-PCR analysis of the indicated mRNAs at 1 or 3 hour intervals. Expression data were normalized using 18S, and are plotted relative to vehicle controls. Blue bars indicate mean ± SEM of vehicle control. Red bars indicate mean ± SEM of DA treated. *Indicates p < 0.05 (t-test) compared to vehicle controls (n = 3–6 in each group).
Discussion
Accumulating evidence indicates that disruption of circadian rhythms due to genetic mutations or environmental factors contributes to the development of many diseases and premature aging. Indeed circadian disruption has been associated with numerous immune, inflammatory, and metabolic disorders19–21. Experimental evidence also suggests that the retinal circadian clock, or its output signals (e.g., DA and MLT), may contribute to eye disease and pathology. For example, diabetes is associated with reduced clock gene expression in the retina22, and circadian disruption recapitulates diabetic retinopathy in mice23. Removal of Bmal1 from the neural retina alters inner retinal function24 and a recent paper reported that mice lacking Per1 and Per2 show significant alteration in the distribution of cone photoreceptors25. Finally a series of studies have implicated the clock genes Rev-erbα and Rora in retinal functioning26, 27 and age-related macular degeneration28. Similarly, disruption of DA or MLT signaling in the mouse retina greatly affects retinal physiology and retinal cell viability11–13, 29–32.
Disruption of the daily rhythm of RPE phagocytosis impairs retinal and/or RPE functions. RPE of mice lacking αvβ5 integrin (β5−/− mice) fail to show a circadian burst of phagocytic activity one of two hours after light onset. Also, during the aging process, β5−/− mice lose both cone and rod photoreceptors faster than control mice33. The mechanism controlling the daily rhythm in RPE phagocytosis appears to be located in the RPE34 and is likely to be directly controlled by the circadian clock located in this tissue9.
Previous studies have also reported that DA receptors are involved in the regulation of rhythmic RPE functions. For example, inhibition of DA synthesis during the early part of the light phase induced a significant reduction of disk shedding and phagocytosis35. In addition, mice whose dopaminergic neurons have been destroyed by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) accumulate a large number of residual bodies in the RPE36. It has also been reported that dopamine and a D1-like activation decrease phagocytosis by RPE cells37. However, the presence of dopamine receptors in the mammalian RPE is still controversial38.
Our data indicated that DA can phase-shift in a time dependent manner the circadian rhythm in PER2::LUC bioluminescence (Fig. 1), and that D1R, D2R, D4R and D5R mRNAs are present in the mouse RPE (Fig. 2), albeit only in pharmacological activation of D2R induced a phase-shift in the PER2::LUC bioluminescence rhythm (Figs 3 and 4). The timing of quinpirole induced phase shifts was advanced compared to DA or Sumanirole. However, this is probably due to a difference in affinity of these ligands for D2R39–41 and not due to involvement of other DA receptors as removing the D2R receptor completely abolished any resetting responses of the RPE clock in response to DA (Fig. 5).
A number of studies support a model in which the rapid induction of the circadian clock genes Per1 and Per2 drives the resetting process42, 43. The induction of Per1 mRNA in the suprachiasmatic nucleus of the hypothalamus following a photic stimulus is thought to be driven by activation of cAMP response element-binding protein (CREB) located in the promoter region of the Per1 gene44, 45. D2-like receptors are negatively coupled to adenylyl cyclase and thereby lead to a decrease of cAMP levels. Thus, it is unlikely that D2R activation induces Per1 mRNA via the cAMP signaling pathway. Surprisingly our data (Fig. 6) indicates that Per1 mRNA levels were significantly increased 1-hr after DA treatment at CT 6 when DA phase-advanced the PER2::LUC rhythm. In comparison, Per1 and Per2 expression was induced 3 hrs after the treatment at CT 23 when DA phase-delayed the PER2::LUC rhythm. These data agree well with previous studies in the mouse in which it was reported that mice lacking Per1 did not show any phase-advance after a pulse of light, whereas mice lacking Per2 did not show any delays after a pulse of light43. Our results are also consistent with the findings of another investigation reporting that a light pulse during the delay zone of the PRC induces the expression of Per1 and Per2 genes, whereas a light pulse during the advance zone of PRC increases only Per1 46. Thus experimental evidence agrees well with our results and supports the hypothesis that DA – via D2R activation – can induce Per1 and Per2 expression. The molecular mechanism by which DA via D2R activation induces Per1 and Per2 is unknown and further studies will be required to address this important issue.
Finally, it is worth mentioning that the RPE is composed of a single cell type and persists for the entire lifespan of an organism. Thus, a RPE-choroid preparation may represent a new and unique tool to study the impact of circadian clock function and disruption on cellular biology and longevity over a full lifespan.
Methods
Animals
This study used PER2::LUC mice (C57Bl/6) of 3 to 6 months in age. PER2::LUC mice were crossed with Dopamine 2 Receptor knock- out (D2R−/−) to produce D2R−/− PER2::LUC mice. The original D2R−/− were purchased from Jackson Laboratory (strain Drd2 tm1Low/J). All mice used in this study were raised at Morehouse School of Medicine in 12 h light and 12 h dark with lights-on (Zeitgeber Time; ZT 0) at 06:00 and lights off (ZT 12) at 18:00 h local time. Water and food were available ad libitum. Light was supplied with fluorescent tubes and the light intensity ranged from 200 to 400 lux at cage level. Animal experimentation was carried out in accordance with the National Institutes of Health Guide on the Care and Use of Laboratory Animals and the ARVO Statement for the Use of Animals in Ophthalmic Vision Research, and was approved by the Institutional Animal Care and Use Committees of Morehouse School of Medicine.
Tissue Culture Preparation and Measurement of Bioluminescence
Mice were anesthetized with CO2 and then sacrificed by cervical dislocation. The eyes were removed and the retina and RPE-choroid cup were carefully separated under a dissecting microscope. The eye cup containing the RPE-choroid was flattened by four radical incisions, and then placed on the culture membrane (Millicell-CM, PICM030-50, Millipore, Billerica, MA) in a 35 mm Petri dish with 1.2 ml of 199 medium (Cambrex, Walkersville, MD) containing 0.1 mM D-Luciferin K salt (Molecular Imaging Products, Bend, OR). Dishes were sealed and kept at 37 °C. The cultures were prepared under fluorescent tubes between Zeitgeber Time (ZT, lights on at ZT0) 8–10. The bioluminescence emitted from the RPE was measured with photomultiplier tubes (Lumicycle®; Actimetrics, Wilmette, IL).
Quantitative Real Time RT-PCR (Q-PCR)
RPE-choroid cup culture dishes were prepared as described above and kept in the incubator at 37 °C. After 3 days of culture, dishes were taken from the incubator and either dopamine (final concentration of 100 µM) or vehicle was added to the culture medium at either CT 6 or CT23 and culture dishes were returned to the incubator. At 1 hour or 3 hours after the dopamine treatment, culture tissues were collected from dish and subjected for RNA extraction using Trizol (Thermo fisher scientific, MA). Q-PCR was performed using the CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) using iQ SYBR Green Supermix (Bio-Rad Laboratories). All data for individual genes were normalized to 18S, and are plotted relative to average levels in vehicle treated control samples collected in parallel (n = 6 cultures for DA groups, n = 3 cultures for vehicle controls).
The details of procedures and primer sequences of Per1, Per2, Bmal1 and 18S are reported in Hiragaki et al.47. The primers used to amplify dopamine receptors expression are as follows: Drd1 (193 bp) forward 5′-cagccttcatcctgattagcgtag-3′ reverse 5′-cttatgagggaggatgaaatggcg-3′, Drd2 (148 bp) forward 5′-tgccttcgtggtctactcct-3′ reverse 5′-tgccttcgtggtctactcct-3′, Drd3 (116 bp) forward 5′-ccagacacatggagagctga-3′ reverse 5′-aggagttccgagtcctctcc-3′, Drd4 (131 bp) forward 5′-cgtctctgtgacacgctcat-3′ reverse 5′-cactgaccctgctggttgta-3′, and Drd5 (109 bp) forward 5′-catccatcaagaaggagaccaagg-3′ reverse 5′-cagaagggaaccatacagttcagg-3′.
Drug treatments
Dopamine (Sigma) was dissolved (100 µM final concentration) in PBS and ascorbic acid (50 µM final concentration) was added to prevent oxidation. Melatonin (Sigma) was first dissolved in ethanol (8 mg/ml) and then was diluted with PBS (100 nM final concentration). SKF 38393 (Sigma) was dissolved in PBS (50 µM final concentration), and Quinpirole (Tocris) was dissolved in PBS (50 µM final concentration). Sumanirole (Tocris) was first dissolved in distilled water (32 mg/ml) and then diluted to 1 mM (1 µM final concentration) with PBS. PD168077 was first dissolved in DMSO (45 mg/ml) then diluted with PBS to 1 mM (1 µM final concentration). These concentrations were selected on the basis on the compound affinity for each of receptors. After 3 to 4 days of bioluminescence recording, the culture dishes containing a RPE-choroid cup were gently removed from the Lumicycle® and either 1.2 μl (DA, Sumanirole and PD168077) or 6 μl (SKF and Quinpirole) volumes of drug solutions or vehicles were added to the culture dishes. They were then re-sealed, returned to the Lumicycle® and placed in the same positions that they were occupying before the treatment. The culture dishes were kept in the Lumicycle® until the end of the experiment without a drug washout.
Analysis of Phase-shifts
Bioluminescence recordings emitted from RPE-choroid cultures were detrended by a 24-hour moving average subtraction method and smoothed by a 2-hour moving average. The circadian time (CT) of bioluminescence recordings were determined by the projection of the light cycle to which the mice were exposed (CT 12 = lights off). The circadian peak phase was determined as the highest point of the curve picked by Origin® (Origin Lab, Northampton, MA) software. The amount of phase-shift (in hours.) was calculated by comparing the regression lines fitted to the circadian peaks before and after treatment. Phase-shifts in an individual culture dish were plotted as the phase response curve. Data were then averaged in 4 hr bins: CT 0–4, CT 4–8, CT 8–12, CT 12–16, CT 16–20, CT 20–24 and normalized with respective vehicle control groups6. Two-way ANOVA with a post hoc Tukey test was performed to compare the difference between experimental groups and time points.
Acknowledgements
The National Institutes of Health Grants GM116760 to K.B., EY022216 EY026291and EY020821 to G.T., GM109861 to J.P.D and by 5U54NS083932, S21MD000101, G12-RR03034, U54-NS083932 to Morehouse School of Medicine.
Author Contributions
K.B. designed, performed experiments, analyzed the data, wrote the manuscript and prepared Figures 1–6; J.P.D. designed experiments and wrote the manuscript; G.T. designed, analyzed and wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 2.Tosini G, Menaker M. The clock in the mouse retina: melatonin synthesis and photoreceptor degeneration. Brain Res. 1998;789:221–228. doi: 10.1016/S0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- 3.McMahon DG, Iuvone PM, Tosini G. Circadian organization of the mammalian retina: from gene regulation to physiology and diseases. Prog Retin Eye Res. 2014;39:58–76. doi: 10.1016/j.preteyeres.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besharse JC, McMahon DG. The Retina and Other Light-sensitive Ocular Clocks. J. Biol. Rhythms. 2016;31:223–243. doi: 10.1177/0748730416642657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoo SH, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan GX, Allen GC, Yamazaki S, McMahon DG. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008;6(10):e249. doi: 10.1371/journal.pbio.0060249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruan GX, et al. Circadian organization of the mammalian retina. Proc. Natl. Acad. Sci. USA. 2006;103:9703–9708. doi: 10.1073/pnas.0601940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baba K, Davidson AJ, Tosini G. Melatonin Entrains PER2::LUC Bioluminescence Circadian Rhythm in the Mouse Cornea. Invest. Ophthalmol. Vis. Sci. 2015;56:4753–4758. doi: 10.1167/iovs.15-17124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baba K, et al. Circadian regulation of the PERIOD 2::LUCIFERASE bioluminescence rhythm in the mouse retinal pigment epithelium-choroid. Mol. Vis. 2010;16:2605–2611. [PMC free article] [PubMed] [Google Scholar]
- 10.Buhr ED, et al. Neuropsin (OPN5)-medicated photoentrainment of lacal circadian oscillators in mammalian retina and cornea. Proc. Natl. Acad. Sci. USA. 2015;112:13093–13098. doi: 10.1073/pnas.1516259112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pozdeyev N, et al. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. Eur. J. Neurosci. 2008;27:2691–2700. doi: 10.1111/j.1460-9568.2008.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baba K, et al. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc. Natl. Acad. Sci. USA. 2009;106:15043–15048. doi: 10.1073/pnas.0904400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baba K, et al. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci. Signal. 2013;6:ra89. doi: 10.1126/scisignal.2004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Missale S, et al. Dopamine receptors: From structure to function. Physiol. Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 15.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 16.Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. doi: 10.1016/S0092-8674(00)80495-X. [DOI] [PubMed] [Google Scholar]
- 17.Yagita K, Okamura H. Forskolin induces circadian gene expression of rPer1, rPer2 and dbp in mammalian rat-1 fibroblasts. FEBS Lett. 2000;465:79–82. doi: 10.1016/S0014-5793(99)01724-X. [DOI] [PubMed] [Google Scholar]
- 18.Fukuhara C, Dirden JC, Tosini G. Regulation of period 1 expression in cultured rat pineal. Neurosignals. 2002;11:103–114. doi: 10.1159/000058547. [DOI] [PubMed] [Google Scholar]
- 19.Castanon-Cervantes O, et al. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ. Res. 2010;106:447–462. doi: 10.1161/CIRCRESAHA.109.208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musiek ES, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Invest. 2013;123:5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busik JV, et al. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J. Exp. Med. 2009;206:2897–2906. doi: 10.1084/jem.20090889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatwadekar AD, et al. Per2 mutation recapitulates the vascular phenotype of diabetes in the retina and bone marrow. Diabetes. 2013;62:273–282. doi: 10.2337/db12-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storch KF, et al. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ait-Hmyed O, et al. Mice lacking Period 1 and Period 2 circadian clock genes exhibit blue cone photoreceptor defects. Eur. J. Neurosci. 2013;37:1048–1060. doi: 10.1111/ejn.12103. [DOI] [PubMed] [Google Scholar]
- 26.Mollema NJ, et al. Nuclear receptor Rev-erb alpha (Nr1d1) functions in concert with Nr2e3 to regulate transcriptional networks in the retina. PLoS One. 2011;6:e17494. doi: 10.1371/journal.pone.0017494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ait-Hmyed Hakkari, O. et al. Rev-Erbalpha modulates retinal visual processing and behavioral responses to light. FASEB J. 30, pii: fj.201600414R (2016). [DOI] [PubMed]
- 28.Jun G, et al. Influence of ROBO1 and RORA on risk of age-related macular degeneration reveals genetically distinct phenotypes in disease pathophysiology. PLoS One. 2011;6:e25775. doi: 10.1371/journal.pone.0025775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alcantara-Contreras S, Bab K, Tosini G. Removal of melatonin receptor type 1 increases intraocular pressure and retinal ganglion cells death in the mouse. Neurosci. Lett. 2011;494:61–4. doi: 10.1016/j.neulet.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson CR, Chaurasia SS, Hwang CK, Iuvone PM. Dopamine D(4) receptor activation controls circadian timing of the adenylyl cyclase 1/cyclic AMP signaling system in mouse retina. Eur. J. Neurosci. 2011;34:57–64. doi: 10.1111/j.1460-9568.2011.07734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sengupta A, et al. Localization of melatonin receptor 1 in mouse retina and its role in the circadian regulation of the electroretinogram and dopamine levels. PLoS One. 2011;6:e24483. doi: 10.1371/journal.pone.0024483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang CK, et al. Circadian rhythm of contrast sensitivity is regulated by a dopamine-neuronal PAS-domain protein 2-adenylyl cyclase 1 signaling pathway in retinal ganglion cells. J. Neurosci. 2013;33:14989–14997. doi: 10.1523/JNEUROSCI.2039-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nandrot EF, et al. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J. Exp. Med. 2004;200:1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruggiero L, et al. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5−/− or Mfge8−/− mouse retina. Proc. Natl. Acad. Sci. USA. 2012;109:8145–8148. doi: 10.1073/pnas.1121101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reme C, Wirz-Justice A, Rhyner A, Hofmann S. Circadian rhythm in the light response of rat retinal disk-shedding and autophagy. Brain Res. 1986;369:356–360. doi: 10.1016/0006-8993(86)90550-0. [DOI] [PubMed] [Google Scholar]
- 36.Mariani AP, Neff NH, Hadjiconstantinou M. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment decreases dopamine and increases lipofuscin in mouse retina. Neurosci. Lett. 1986;72:221–226. doi: 10.1016/0304-3940(86)90084-4. [DOI] [PubMed] [Google Scholar]
- 37.Masri H, et al. Dopamine slows phagocytosis of rods from bovine pigment epithelium in vitro trough D1 receptor. C. R. Acad. Sci. III. 1996;319:687–691. [PubMed] [Google Scholar]
- 38.Nguyen-Legros J, Versaux-Botteri C, Vernier P. Dopamine receptor localization in the mammalian retina. Mol. Neurobiol. 1999;19:181–204. doi: 10.1007/BF02821713. [DOI] [PubMed] [Google Scholar]
- 39.Levant B, Grigoriadis DE, DeSouza EB. Characterization of [3H]quinpirole binding to D2-like dopamine receptors in rat brain. J. Pharmacol. Exp. Ther. 1999;262:929–35. [PubMed] [Google Scholar]
- 40.Bowery B, Rothwell LA, Seabrook GR. Comparison between the pharmacology of dopamine receptors mediating the inhibition of cell firing in rat brain slices through the substantia nigra pars compacta and ventral tegmental area. Br. J. Pharmacol. 1994;112:873–80. doi: 10.1111/j.1476-5381.1994.tb13161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo N, et al. Impact of D2 receptor internalization on binding affinity of neuroimaging radiotracers. Neuropsychopharmacology. 2010;35:806–17. doi: 10.1038/npp.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akiyama M, et al. Modulation of mPer1 gene expression by anxiolytic drugs in mouse cerebellum. Br. J. Pharmacol. 1999;128:1616–1622. doi: 10.1038/sj.bjp.0702957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albrecht U, et al. MPer1 and mper2 are essential for normal resetting of the circadian clock. J. Biol. Rhythms. 2001;16:100–104. doi: 10.1177/074873001129001791. [DOI] [PubMed] [Google Scholar]
- 44.Obrietan K, Impey S, Storm DR. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nat. Neurosci. 1998;1:693–700. doi: 10.1038/3695. [DOI] [PubMed] [Google Scholar]
- 45.Obrietan K, et al. Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. J. Biol. Chem. 1999;274:17748–17756. doi: 10.1074/jbc.274.25.17748. [DOI] [PubMed] [Google Scholar]
- 46.Yan L, Silver R. Resetting the brain clock: time course and localization of mPER1 and mPER2 protein expression in suprachiasmatic nuclei during phase shifts. Eur. J. Neurosci. 2004;19:1105–1109. doi: 10.1111/j.1460-9568.2004.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hiragaki S, et al. Melatonin signaling modulates clock genes expression in the mouse retina. PLoS One. 2014;9:e106819. doi: 10.1371/journal.pone.0106819. [DOI] [PMC free article] [PubMed] [Google Scholar]


