Abstract
Obstructive sleep apnea and hypopnea syndrome (OSAHS) is a clinical syndrome characterized by recurrent episodes of obstruction of the upper airway during sleep that leads to a hypoxic condition. Genioglossus, an important pharyngeal muscle, plays an important role in maintaining an open upper airway for effective breathing. Our previous study found that genistein (a kind of phytoestrogen) protects genioglossus muscle from hypoxia-induced oxidative injury. However, the underlying mechanism is still unknown. In the present study, we examined the effects of hypoxia on genioglossus myoblast proliferation, viability and apoptosis, and the protective effect of genistein and its relationship with the PI3K/Akt and ERK MAPK pathways. Cell viability and Bcl-2 were reduced under hypoxic condition, while ROS generation, caspase-3, MDA, and DNA damage were increased following a hypoxia exposure. However, the effects of hypoxia were partially reversed by genistein in an Akt- and ERK- (but not estrogen receptor) dependent manner. In conclusion, genistein protects genioglossus myoblasts against hypoxia-induced oxidative injury and apoptosis independent of estrogen receptor. The PI3K-Akt and ERK1/2 MAPK signaling pathways are involved in the antioxidant and anti-apoptosis effect of genistein on genioglossus myoblasts.
Introduction
Obstructive sleep apnea (OSAS) is a result of repeated episodes of upper airway (UA) obstruction during sleep, and it is considered to be a risk factor for cardiovascular disorders with life-threatening consequences1. OSAS is categorized as a hypoxic condition1, 2, which can impair UA muscle function4. UA dilator muscles of individuals with OSAS demonstrate an increased level of activity during wakefulness, which induces muscle fatigue5, 6. Furthermore, this hyperactivity not only leads to changes in contractile protein composition, but also muscle fiber damage7, 8.
Genioglossus is an important pharyngeal muscle that plays a crucial role in maintaining an open UA for effective breathing9, 10. Genioglossus muscle fatigue is increased in individuals with OSAS, which increases the likelihood of UA collapse especially during frequent and prolonged apnea, leading to a vicious cycle of further airway obstruction and muscle dysfunction4, 11. Genioglossus is under a hypoxia environment in OSAS patients, and degenerative morphological changes of genioglossus takes place such as myofibril discontinuities, dilation of mitochondria and disruption of cristae that parallel the changes of muscle fatigue12. In addition, oxidative stress and decreased cellular antioxidant capacity have been demonstrated in patients with OSAS13–15. Therefore, it is hypothesized that genioglossus muscle dysfunction is associated with a hypoxia-induced oxidative injury.
Genistein, a soy isoflavone, is a plant-derived polyphenolic non-steroidal compound with estrogen-like biological activity without side effect for long-term use16, 17. We previously found that genistein attenuates genioglossus muscle fatigue in vivo 4, 12. However, the detailed molecular mechanisms that underlie the effects of genistein on genioglossus remain to be elucidated. Furthermore, the PI3K/Akt and MAPK pathways regulate a variety of cellular activities including proliferation, differentiation, survival, oxidative stress, and death18–20. Genistein has been found to exert its protective effects in relation to the PI3K/Akt and MAPK pathways21–24. Therefore, the purpose of this study is to determine the effects of hypoxia on genioglossus myoblast viability, proliferation and apoptosis, and to identify the muscle protective effect of genistein and its underlying relationship with the PI3K/Akt and MAPK pathways.
Materials and Methods
Materials
Sprague–Dawley (SD) rats were obtained from the Experimental Animal Center of Second Military Medical University (Shanghai, China). All animal care and use were conducted according to the National Research Council guidelines and approved by the Animal Care and Use Committee of Zhejiang University. The cell culture medium, class II collagenase, trypsin and fetal bovine serum (FBS) were from Life Technologies (Grand Island, NY, USA). The 2′,7′-dichlorofluorescein diacetate (DCF-DA) was obtained from Molecular Probes (Eugene, Oregon, USA). The genistein, ICI 182780, SB 203580, and SP 600125 were obtained from Sigma Chemical Company (St Louis, MO, USA). The wortmannin, U0126, and antibodies of phospho-Akt, phospho-ERK1/2, and Bcl-2 were obtained from Cell Signaling Technology (Danvers, MA, USA). The anti-rabbit I horseradish peroxidase (HRP)-conjugated goat antibodies were obtained from Santa Cruz Biotechnologies (Heidelberg, Germany). The MTT, DAPI, MDA and caspase-3 detecting kit were obtained from Beyotime Institute of Biotechnology (Jiangsu, China). The BrdU detection kit was from obtained Boehringer Mannheim Biochemica (Mannheim, Germany).
Primary culture of genioglossus myoblast and hypoxia exposure
The genioglossus muscle tissues were obtained from newborn (3–5 d) SD rats. They were trimmed of excessive connective and fat tissues, hand-minced with a sterile scissor into approximately 1 mm3, and washed with PBS for three times. Enzymatic dissociation was performed by two-step digestion of minced muscles at 37 °C in 0.5% collagenase type II for 20 min and 0.05% trypsin for 10 min. The digestion was terminated by growing medium (GM, high glucose DMEM containing 10% FBS and 1% penicillin-streptomycin), and cells were separated from muscle fiber fragments and tissue debris by 120 mesh screen cloth. The cells were centrifuged, and plated in tissue culture dishes coated with poly-lysine in GM. Cells were incubated in normoxic condition with 37 °C and 5% CO2. However, hypoxic conditions were created in the hypoxia chamber with 0.5% O2 and 5% CO2.
Detection of cell proliferation
The cells were seeded into 96-well cell culture plates at a density of 2 × 104 cells/well. After 24 h culture at 37 °C and 5% CO2, 10 μM of genistein (the concentration was decided by our previous study) were added to the plates. After pretreated with genistien for 1 h, the cells were incubated under normoxic or hypoxic conditions for another 24 h, 48 h, and 72 h. MTT assay was performed following the well-described procedure25. Briefly, the wells were washed three times with DMEM. Then, 180 μl aliquots of DMEM and 20 μl aliquots of MTT solution (5 mg/ml of PBS) were added to each well at the established time. After 4 h of incubation at 37 °C and 5% CO2, the media were removed and formazan crystals were solubilized with 150 μl DMSO. The plates were read on ELx800 absorbance microplate reader (Bio-Tec Instruments INC, USA) at 550 nm wavelength. The results of MTT assay were verified by BrdU DNA incorporation assay via detection of DNA synthesis26. The incorporated BrdU was measured using a BrdU detection kit according to the manufacturer’s instructions. The absorbance of the extract was read at a wavelength of 450 nm with a reference wavelength of 630 nm.
DAPI staining
Cells were seeded onto 6-well tissue culture plates at a concentration of 1 × 105 cells/well and incubated under normoxia or hypoxia conditions at the presence or absence of genistein for 48 h. At the end of incubation, cells were fixed with ice-cold 4% paraformaldehyde for 20 min, permeabilized with 0.1% Triton x-100 for 25 min and washed with ice-cold PBS three times. At last the cells were stained with 4′, 6-diamidino-2-phenylindole (DAPI, 5 μg/ml) for 25 min and observed under a fluorescence microscope with a peak excitation wave length of 340 nm. Nuclei were identified as normal, fragmented, or condensed. Fragmented or condensed nuclei were classified as apoptotic. The results were expressed as a percentage of apoptotic cells. A minimum of 500 cells were counted for each treatment from at least three independent experiments.
Flow cytometry analysis
Cells were seeded onto 6-well tissue culture plates at a concentration of 1 × 105 cells/well and incubated under normoxia or hypoxia conditions at the presence or absence of genistein for 48 h. At the end of incubation, trypsin digestion was performed to detach the cells from culture plates. We resuspended the cells in 1x binding buffer and adjusted the concentration to 1 × 106 cells/mL. Cell suspensions were added to 5 mL Falcon tubes containing 5 μL of FITC-Annexin V and 5 μL of PI dye and then gently mixed. Following incubation in the dark at room temperature (~25 °C) for 15 min, 400 μL of 1x binding buffer was added to each tube, and the samples were analyzed within 1 h using a flow cytometer (BD Biosciences).
Measurements of ROS generation
Changes in intracellular ROS levels were determined by measuring the oxidative conversion of cell permeable 2′,7′-dichlorofluorescein diacetate (DCF-DA) to fluorescent dichlorofluorescein (DCF). Cells in 12 well culture dishes were incubated at normoxia or hypoxia conditions at the presence or absence of genistein for 24 h, 48 h, and 72 h. The cells were washed with PBS and incubated with DCF-DA at 37 °C for 20 min. Then, DCF fluorescence distribution of 20,000 cells was detected by a fluorescence microplate reader at an excitation and emission wavelength of 488 nm and 525 nm, respectively.
Determination of MDA production
Concentration of malondialdehyde (MDA), an index of lipid peroxidation, was determined spectrophotometrically according to the Draper and Hadley method27. A 0.1 mL aliquot of genioglossus myoblast extract supernatant was mixed with 0.2 mL of 0.37% trichloroacetic acid (TBA) solution, and the mixture were incubated for 10 min at 100 °C and cooled. Then, the mixture was centrifuged at 1,000 g for 10 min, and 0.2 mL aliquot of the mixture was added to 96 well. Absorbance of TBA-MDA complex was determined at 532 nm using a microplate reader.
Caspase fluorometric assay
Measurement of caspase-3 activity was performed as previously described28. Briefly, cytosolic extract (100 μg protein) was incubated for 1 h at 37 °C with the reaction buffer (25 mM HEPES, pH 7.5, 10% sucrose, 0.1% CHAPS, 5 mM DTT and 5 mM EDTA) in a total volume of 150 μl containing 25 μM acetyl-Asp-Glu-Val-Asp-p-nitroanilide (Ac-DEVD-pNA). Enzyme-catalyzed release of p-nitroanilide was measured at 405 nm by a microplate reader.
Western blot analysis
Cells were harvested into a lysis buffer consisting of 2.5 mM HEPES, pH 7.5, 10% glycerol, 5 mM EDTA, 5 mM EGTA, 100 mM NaCl, 100 mM Na pyrophosphate, 50 mM NaF, 0.1 mM NaVO4, 1% Triton X-100, 1 mM benzamidine, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 2 μg/ml pepstatin. Equal protein amounts (50 μg) of genioglossus homogenates were electrophoresed through 12% SDS-polyacrylamide gel and electroblotted onto polyvinylidene fluoride (PVDF) membranes (Millipore, USA). The blots were then washed in PBST (PBS with 0.05% Tween-20), blocked with 5% fat-free milk in PBST for 1 h, and incubated overnight with an appropriate primary antibody at the dilutions recommended by the supplier (1:1000). The detection was made with HRP-conjugated secondary antibodies (1:10000) and ECL System by Kodak IMAGE STATION 2000 MM (Kodak, Rochester, NY, USA).
Statistical analysis
Statistical analyses were performed using the SPSS Statistical Analysis Software, version 13.0. All data are expressed as the means ± the standard deviation from three independent experiments. Statistical significance of differences was calculated using ANOVA followed by Tukey’s HSD post hoc test. Statistical significance was established at P < 0.05.
Results
Oxidative stress and genioglossus myoblast injury can be induced by Hypoxia
Genioglossus myoblast were incubated in hypoxic conditions of 0.5% O2 and 5% CO2 for 24 h, 48 h, and 72 h. Cell viability unchanged following 24 h hypoxic exposure, while it was significantly decreased after 48 and 72 h (Fig. 1A). The levels of H2O2 was measured using DCF-DA to determine whether hypoxia stimulates the generation of ROS in genioglossus myoblast. The data showed that the intracellular H2O2 was significantly increased in a time-dependent manner (0–72 h) (Fig. 1B). Caspase 3 plays an important role in apoptosis and is responsible for chromatin condensation and DNA fragmentation29. Our data showed that the level of caspase 3 expression was increased as the extension of the time under the hypoxic conditions (0–72 h) (Fig. 1C). Besides, the index of lipid peroxidation, MDA, was increased following 72 h hypoxia exposure (Fig. 1D). B-cell lymphoma protein-2 (Bcl-2), as a regulator of permeabilization of the mitochondrial outer membrane and the consequent release of cytochrome c, prevents apoptosis and hypoxia-induced injury30, 31. The level of Bcl-2 was reduced at 48 h, but it was increased at 72 h. However, the level of Bcl-2 remained less than normal value (Fig. 1E,F).
Figure 1.

The time-dependent effects of hypoxia exposure on cell viability and cell injury. (A) The effects of hypoxia on cell viability. Cell viability was significantly reduced by 48 h and 72 h of hypoxia. (B) The effects of hypoxia on H2O2 generation. H2O2 generation was increased by hypoxia in a time-dependent manner. (C) The effects of hypoxia on caspase-3 activity. Caspase-3 activity was increased by hypoxia in a time-dependent manner. (D) The effects of hypoxia on MDA generation. MDA was unchanged after 24 h and 48 h hypoxia exposure, while it was significantly increased at 72 h. (E,F) The effects of hypoxia on the Bcl-2 protein expression. The level of Bcl-2 was reduced at 48 h, and it was partially increased at 72 h. *P < 0.05 versus control group; #P < 0.05 versus hypoxia group.
Effects of genistein on hypoxia-induced apoptosis of genioglossus myoblast
DAPI staining and Annexin V-FITC/PI flow cytometry analysis were used to detect the effects of hypoxia on the nuclear changes and apoptosis of genioglossus myoblast. As shown in Fig. 2A and B, nuclei had the normal phenotype demonstrating bright and homogenously at normoxic or 24 h hypoxic condition. But we found that this process was inhibited by genistein (Fig. 2C). Nuclei that emitted bright fluorescence, fragmented, or condensed were classified as apoptotic. The results from flow cytometry analyses indicated that the early-stage and late-stage apoptosis rate were significantly increased after 48 h hypoxia exposure. Genistein significantly decreased the early-stage apoptosis of genioglossus myoblasts (Fig. 2D,E).
Figure 2.
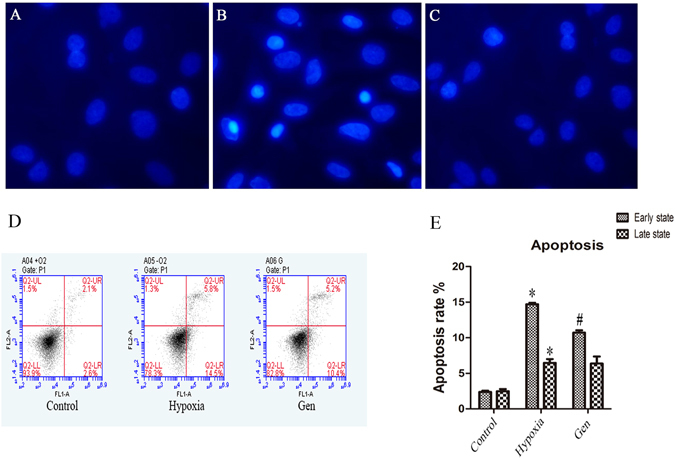
The effects of genistein on hypoxia-induced apoptosis. (A,B) Nuclei have the normal phenotype demonstrating bright colors and homogeneity at normoxic and 24 h hypoxic conditions. Nuclei that emitted bright fluorescence and condensed were classified as apoptotic. The apoptotic nuclei were increased after 48 h hypoxia conditions. (C) The number of apoptotic nuclei was reduced by genistein treatment. (D,E) The effects of genistein on hypoxia-induced apoptosis by Annexin V-FITC/PI flow cytometry analyses. Early-stage and late-stage apoptosis rates were significantly increased after 48 h hypoxia exposure. The apoptotic nuclei were increased after 48 h hypoxia exposure. Genistein significantly decreased the early-stage apoptosis of genioglossus myoblasts. *P < 0.05 versus control group; #P < 0.05 versus hypoxia group.
Relationship between hypoxia-induced cell injury, genistein and estrogen receptor (ER)
In order to observe the relationship between ER and genistein on hypoxia-induced genioglossus myoblast, cells were treated with genistein and ICI 182780 (ER antagonist, 100 nM). The inhibition of hypoxia on cell viability was attenuated after cells were treated by genistein with or without ICI 182780. Results showed genistein attenuated hypoxia-induced H2O2 generation and its effect on cell viability was not influenced by ICI 182780 (Fig. 3A,B). Exogenous H2O2 (100 μM) was used as a positive control for assessing the effect of hypoxia-induced H2O2 generation on the cell viability (Fig. 3C).
Figure 3.

The relationship between hypoxia-induced cell injury, genistein and estrogen receptor (ER). (A) The effects of genistein on cell viability. Cell viability was significantly reduced by hypoxia. Genistein partially reversed the hypoxia-induced cell injury independent of ER. (B) The effects of genistein on hypoxia-induced H2O2 generation. Genistein reduced the hypoxia-induced H2O2 generation at 48 h independent of ER. (C) Exogenous H2O2 (100 μM) was used as a positive control for assessing the effect of hypoxia-induced H2O2 on cell viability. *P < 0.05 versus control group; #P < 0.05 versus hypoxia group.
Relationship among the ERK1/2-MAPK and PI3K-Akt pathways, cell injury, and apoptosis
The signaling molecules associated with hypoxia-induced cell injury and apoptosis, p38, ERK1/2, JNK MAPK, and PI3K-Akt were evaluated. No changes were detected in p38 and JNK MAPK proteins under hypoxic conditions (data not shown). Meanwhile, we found phospho-Akt and phospho-ERK1/2 were remarkably reduced after 24 h and 48 h of hypoxia, but they were recovered at 72 h (Fig. 4A–D).
Figure 4.

The effects of hypoxia on p-Akt and p-ERK1/2 proteins. (A,B) The effects of hypoxia on p-Akt protein. P-Akt was remarkably reduced after 24 h and 48 h hypoxia exposure, while it was considerately recovered at 72 h. (C,D) The effects of hypoxia on p-ERK1/2 protein. P-ERK1/2 was significantly reduced after 24 h and 48 h hypoxia conditions, while it was returned to normal at 72 h. *P < 0.05 versus control group; #P < 0.05 versus hypoxia group.
To further validate the interaction between genistein and pathways, we treated cells with or without genistein, ICI 182780, wortmannin (1 μM, Akt inhibitor), U0126 (1 μM, MEK inhibitor which inhibits ERK1/2). Hypoxia exposure induced apoptosis of genioglossus myoblasts through up-regulation of caspase 3 and down-regulation of Bcl-2. Genistein treatment reduced the expression of caspase-3 and MDA, while wortmannin and U0126 increased it (Fig. 5A,B). The expression of Bcl-2 was increased by genistein, unaffected by ICI 182780, but reduced by wortmannin and U0126 (Fig. 5C,D). U0126 and wortmannin partially reversed the effects of genistein on hypoxia-induced injury through inhibiting ERK and Akt phosphorylation (Fig. 6A–D). In summary, genistein exerted an anti-apoptotic effect on genioglossus myoblasts under hypoxic conditions by activating PI3K-Akt and ERK1/2-MAPK pathway, but independent of ER.
Figure 5.
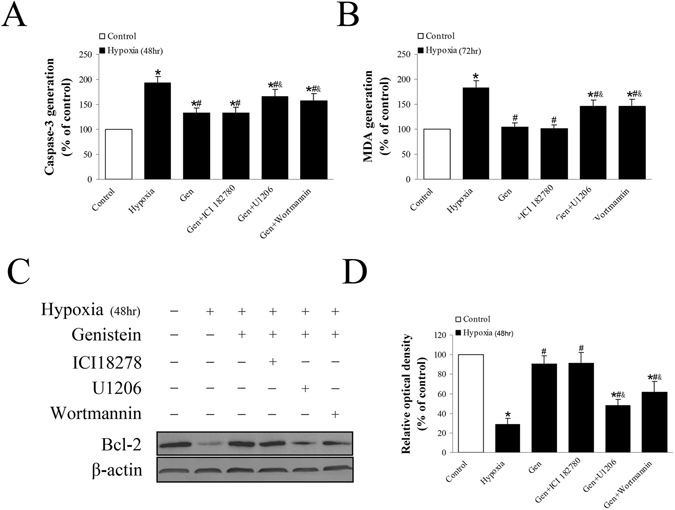
The relationship between apoptosis-related proteins, genistein and ER, PI3K-Akt, and ERK1/2 MAPK pathways. (A) The inhibitory effect of genistein on caspase-3 activity was partially inhibited by wortmannin and U0126. This effect was unaffected by ICI 182780. (B) The inhibitory effect of genistein on MDA generation was partially reversed by wortmannin and U0126. This effect was unaffected by ICI 182780. (C,D) Genistein increased the expression of Bcl-2 protein under hypoxic condition. However, this effect was reversed by wortmannin and U0126 but unaffected by ICI 182780. *P < 0.05 versus control group; #P < 0.05 versus hypoxia group; &P < 0.05 versus Gen group.
Figure 6.
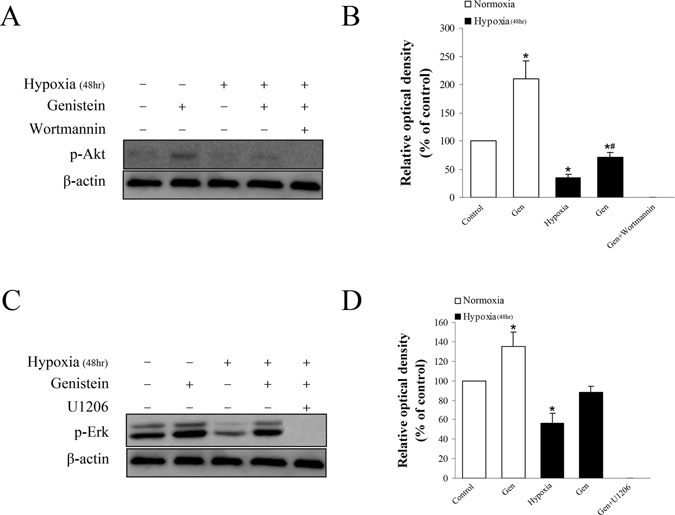
The effects of genistein on the PI3K-Akt and ERK1/2 MAPK pathways under normoxic or hypoxic condition. (A,B) P-Akt was remarkably increased by genistein under 48 h normoxic condition. It was partially reversed by genistein under 48 h hypoxic condition. Wortmannin completely inhibited the expression of p-Akt (C,D) P-ERK1/2 was increased by genistein under 48 h normoxic condition. It was reversed by genistein under 48 h hypoxic condition. U1206 completely inhibited the expression of p-ERK1/2. *P < 0.05 versus control group; #P < 0.05 versus hypoxia group.
Discussion
OSAS is associated with oxidative stress and UA muscle fatigue12. Furthermore, it is reported that oxidative-stress contributes to hypoxia-induced respiratory muscle impairment in an animal model of OSAS32. However, the underlying mechanism is still unknown. Genioglossus is a very important UA dilating muscle, which protrudes the tongue, increases the oropharyngeal size, and decreases airway collapsibility. Therefore, genioglossus plays a significant role in maintaining an open UA for effective breathing9. We established a genioglossus myoblast culture model under hypoxic conditions to detect the underlying mechanism of oxidative stress and muscle injury. The results of this study demonstrate that genistein partially protects genioglossus myoblast against hypoxia-induced oxidative stress injury. This protective effect is mediated by the regulation of ROS, lipid peroxidation, Bcl-2, and caspase-3 through the PI3K-Akt and ERK1/2 MAPK signaling pathways, which suggests that genistein is effective against oxidative stress through multiple ways in genioglossus myoblast.
This study found that hypoxic exposure stimulates the generation of ROS, which is coincident with OSAS and the animal model of the disorder33, 34. ROS includes superoxide anion, hydrogen peroxide and hydroxyl radical, and plays a physiological role in the function of a number of cellular signaling pathways35. However, excessive ROS induces cell injury including DNA damage, lipid peroxidation, and apoptosis. In this study, it was detected that hypoxic exposure significantly reduces the viability of genioglossus myoblasts. Apoptotic nuclei were increased by hypoxic exposure, indicating that hypoxia induces apoptosis of genioglossus myoblasts. In addition, caspase-3, an apoptosis marker, was also increased under hypoxic conditions. Bcl-2, an anti-apoptosis protein, was reduced under a hypoxic condition though it recovered partially with a longer exposure. MDA, the index of lipid peroxidation, was increased after 72 h of hypoxia. Therefore, we conclude that hypoxia induces oxidative cell injury in a ROS-dependent manner, leading to apoptosis through down-regulation of Bcl-2 and up-regulation of caspase-3, consistent with previous studies36.
Genistein, an isoflavone phytoestrogen, is a biologically active plant substance with a chemical structure similar to that of endogenous estrogen. In this study, we found that genistein attenuates the deleterious effects of hypoxia on cell viability, which is ROS-dependent but not ER-dependent. This discovery is different from the finding of a previous study which determined that genistien exerts protective effect on oxidative stress-induced human endothelial cells (HUVECs) injury through ERs37. The reason might be that genistein has a relatively high binding affinity for ER β. ER β is highly expressed in HUVECs, but lowly expressed in skeletal muscles38–40. In addition, genistein increased Bcl-2 levels and attenuated caspase-3 activity and lipid peroxidation independent of ERs. This suggests that anti-apoptotic and antioxidant actions represent two of the most important mechanisms by which genistein protect genioglossus myoblasts against a hypoxic injury. The antioxidant property of genistein has been demonstrated in several studies and is reported to be more effective than that of antioxidant vitamins and estrodiol in scavenging ROS and lipid peroxidation41–43. However, other research indicates that genistein stimulates the expression of apoptotic markers such as caspase-3. Taken together, these observations implicate that variation in the dosage of genistein and cell type used in experiments can result in a totally distinct outcome. The antioxidant property probably accounts for the protective effect of genistein on genioglossus fatigue which was found in a previous study44. Furthermore, the result is consistent with the finding that superoxide scavengers attenuate rat pharyngeal dilator muscle fatigue in hypoxia45.
It has been determined that the MAPK and PI3K/Akt signaling pathways regulate a variety of cellular activities including proliferation, differentiation, survival, and death46, 47. The pathways play a central role in orchestrating growth, proliferation, and anti-apoptotic mechanisms to promote cell cycle and survival. These cellular processes are activated by diverse extracellular and intracellular stimuli including peptide growth factors, cytokines, hormones, and various cellular stressors such as oxidative stress and endoplasmic reticulum stress. The mammalian family of MAPK includes extracellular signal-regulated kinase (ERK1/2), p38, and c-Jun NH2-terminal kinase (JNK). In this study, we found that p38 and JNK MAPK were not involved in hypoxia-induced genioglossus myoblast injury. This finding is different from previous research showing that p38 and JNK are involved in hypoxia-induced hepatocyte injury, which suggests that the signaling pathways related to hypoxia are cell-specific36. Hypoxia decreased the expression of the phospho-Akt and phospho-ERK1/2, and Akt and ERK inhibitor (wortmannin and U0126) partially reversed the protection of Gen on the hypoxia-induced injury. This result suggests that the PI3K/Akt and ERK MAPK pathways are involved in the hypoxia injury and protective effect of genistein on genioglossus myoblasts. The conclusion can be verified by the result that genistein increased the expression of phospho-Akt and phospho-ERK1/2, and that wortmannin and U0126 reversed the effect of genistein on Bcl-2, caspase-3, and MDA. Therefore, the effects of genistein on hypoxia-induced genioglossus myoblast injury are presumably through non-genomic pathways rather than ER-mediated genomic pathways. Signaling pathways including the ERK1/2 and PI3K/Akt cascades permit non-genomic actions of genistein.
In conclusion, genistein protects primary cultured genioglossus myoblasts against hypoxia-induced oxidative injury and cell apoptosis independent of ER. The PI3K-Akt and ERK1/2-MAPK signaling pathways are involved in the antioxidant and anti-apoptotic effects of genistein on genioglossus myoblasts under hypoxic conditions.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China, (81301669 and 81400538), Natural Science Foundation of Zhejiang Province (LY16C070001) and Medical Science and Technology Fund of Zhejiang Province (2013 KYA118).
Author Contributions
Wanghui Ding and Jiejun Shi planned the experiments. Xiaoyan Chen and Wanghui Ding performed cell studies. Zhen Fu and Wen Li performed Westernblot and PCT. All authors contributed to result analysis and interpretation, and Wanghui Ding wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Wanghui Ding and Xiaoyan Chen contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ayas NT, Taylor CM, Laher I. Cardiovascular consequences of obstructive sleep apnea. Curr Opin Cardiol. 2016;31:599–605. doi: 10.1097/HCO.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 2.Ding W, et al. Adiponectin protects rat myocardium against chronic intermittent hypoxia-induced injury via inhibition of endoplasmic reticulum stress. PLoS One. 2014;9:e94545. doi: 10.1371/journal.pone.0094545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bu XL, et al. Serum amyloid-beta levels are increased in patients with obstructive sleep apnea syndrome. Sci Rep. 2015;9:13917. doi: 10.1038/srep13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding W, Liu Y. Genistein attenuates genioglossus muscle fatigue under chronic intermittent hypoxia by down-regulation of oxidative stress level and up-regulation of antioxidant enzyme activity through ERK1/2 signaling pathway. Oral Dis. 2011;17:677–684. doi: 10.1111/j.1601-0825.2011.01822.x. [DOI] [PubMed] [Google Scholar]
- 5.McGuire M, MacDermott M, Bradford A. The effects of chronic episodic hypercapnic hypoxia on rat UA muscle contractile properties and fiber-type distribution. Chest. 2002;122:1400–1406. doi: 10.1378/chest.122.4.1400. [DOI] [PubMed] [Google Scholar]
- 6.Williams R, et al. Chronic intermittent hypoxia increases rat sternohyoid muscle NADPH oxidase expression with attendant modest oxidative stress. Front Physiol. 2015;30(6):15. doi: 10.3389/fphys.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrof BJ, Pack AI, Kelly AM, Eby J, Hendricks JC. Pharyngeal myopathy of loaded UA in dogs with sleep apnea. J. Appl Physiol. 1994;76:1746–1752. doi: 10.1152/jappl.1994.76.4.1746. [DOI] [PubMed] [Google Scholar]
- 8.Lewis P, Sheehan D, Soares R, Varela Coelho A, O’Halloran KD. Chronic sustained hypoxia-induced redox remodeling causes contractile dysfunction in mouse sternohyoid muscle. Front Physiol. 2015;20:122. doi: 10.3389/fphys.2015.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malhotra A, et al. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med. 2000;62:1058–1062. doi: 10.1164/ajrccm.162.3.9912067. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Kang J, Kong D. The central motor conductivity of genioglossus in obstructive sleep apnoea. Respirology. 2010;15:1209–1214. doi: 10.1111/j.1440-1843.2010.01858.x. [DOI] [PubMed] [Google Scholar]
- 11.Jia SS, Liu YH. Down-regulation of hypoxia inducible factor-1alpha: a possible explanation for the protective effects of estrogen on genioglossus fatigue resistance. Eur J. Oral Sci. 2010;118:139–144. doi: 10.1111/j.1600-0722.2010.00712.x. [DOI] [PubMed] [Google Scholar]
- 12.Ding WH, Li W, Chen XY, Shi JJ. The study of genistein attenuating genioglossus muscle fatigue under chronic intermittent hypoxia. Zhonghua Kou Qiang Yi Xue Za Zhi. 2016;51:46–50. doi: 10.3760/cma.j.issn.1002-0098.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Hopps E, et al. Analysis of the correlations between oxidative stress, gelatinases and their tissue inhibitors in the human subjects with obstructive sleep apnea syndrome. J. Physiol Pharmacol. 2015;66:803–810. [PubMed] [Google Scholar]
- 14.Sunnetcioglu A, Alp HH, Sertogullarından B, Balaharoglu R, Gunbatar H. Evaluation of Oxidative Damage and Antioxidant Mechanisms in COPD, Lung Cancer, and Obstructive Sleep Apnea Syndrome. Respir Care. 2016;61:205–211. doi: 10.4187/respcare.04209. [DOI] [PubMed] [Google Scholar]
- 15.Asker S, et al. Oxidative stress parameters and their correlation with clinical, metabolic and polysomnographic parameters in severe obstructive sleep apnea syndrome. Int J Clin Exp Med. 2015;8:11449–11455. [PMC free article] [PubMed] [Google Scholar]
- 16.Spagnuolo C, et al. Genistein and cancer: current status, challenges, and future directions. Adv Nutr. 2015;6:408–419. doi: 10.3945/an.114.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmoud AM, Yang W, Bosland MC. Soy isoflavones and prostate cancer: a review of molecular mechanisms. J Steroid Biochem Mol Biol. 2014;140:116–132. doi: 10.1016/j.jsbmb.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: an update. Arch Toxicol. 2015;89:867–882. doi: 10.1007/s00204-015-1472-2. [DOI] [PubMed] [Google Scholar]
- 19.Polivka J, Jr., Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2014;142:164–175. doi: 10.1016/j.pharmthera.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Ciruelos GEM. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev. 2014;40:862–871. doi: 10.1016/j.ctrv.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Zhai X, et al. Dietary flavonoid genistein induces Nrf2 and phase II detoxification gene expression via ERKs and PKC pathways and protects against oxidative stress in Caco-2 cells. Mol Nutr Food Res. Mol Nutr Food Res. 2013;57:249–259. doi: 10.1002/mnfr.201200536. [DOI] [PubMed] [Google Scholar]
- 22.Xi YD, et al. Flavonoids protect cerebrovascular endothelial cells through Nrf2 and PI3K from β-amyloid peptide-induced oxidative damage. Curr Neurovasc Res. 2012;9:32–41. doi: 10.2174/156720212799297092. [DOI] [PubMed] [Google Scholar]
- 23.Ganai AA, Khan AA, Malik ZA, Farooqi H. Genistein modulates the expression of NF-κB and MAPK (p-38 and ERK1/2), thereby attenuating d-Galactosamine induced fulminant hepatic failure in Wistar rats. Toxicol Appl Pharmacol. 2015;283:139–146. doi: 10.1016/j.taap.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Qian Y, et al. Protection by genistein on cortical neurons against oxidative stress injury via inhibition of NF-kappaB, JNK and ERK signaling pathway. Pharm Biol. 2015;53:1124–1132. doi: 10.3109/13880209.2014.962057. [DOI] [PubMed] [Google Scholar]
- 25.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 26.Wagner U, Burkhardt E, Failing K. Evaluation of canine lymphocyte proliferation: comparison of three different colorimetric methods with the 3H-thymidine incorporation assay. Vet Immunol Immunopathol. 1999;70:151–159. doi: 10.1016/S0165-2427(99)00041-0. [DOI] [PubMed] [Google Scholar]
- 27.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Method Ezymol. 1990;86:421–431. doi: 10.1016/0076-6879(90)86135-I. [DOI] [PubMed] [Google Scholar]
- 28.Enari M, et al. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 29.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu S, et al. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature. 1995;374:811–813. doi: 10.1038/374811a0. [DOI] [PubMed] [Google Scholar]
- 31.Szegezdi E, Macdonald DC, Ní Chonghaile T, Gupta S, Samali A. Bcl-2 family on guard at the ER. Am J Physiol Cell Physiol. 2009;296:941–953. doi: 10.1152/ajpcell.00612.2008. [DOI] [PubMed] [Google Scholar]
- 32.Dunleavy M, Bradford A, O’Halloran KD. Oxidative stress impairs upper airway muscle endurance in an animal model of sleep-disordered breathing. Adv Exp Med Biol. 2008;605:458–462. doi: 10.1007/978-0-387-73693-8_80. [DOI] [PubMed] [Google Scholar]
- 33.Hira HS, Samal P, Kaur A, Kapoor S. Plasma level of hypoxanthine/xanthine as markers of oxidative stress with different stages of obstructive sleep apnea syndrome. Ann Saudi Med. 2014;34:308–313. doi: 10.5144/0256-4947.2014.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, et al. CIH-induced neurocognitive impairments are associated with hippocampal Ca(2+) overload, apoptosis, and dephosphorylation of ERK1/2 and CREB that are mediated by overactivation of NMDARs. Brain Res. 2015;1625:64–72. doi: 10.1016/j.brainres.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Gutowski M, Kowalczyk S. A study of free radical chemistry: their role and pathophysiological significance. Acta Biochim Pol. 2013;60:1–16. [PubMed] [Google Scholar]
- 36.Lee MY, Jung SC, Lee JH, Han HJ. Estradiol-17β protects against hypoxia-induced hepatocyte injury through ER-mediated upregulation of Bcl-2 as well as ER-independent antioxidant effects. Cell Res. 2008;18:491–499. doi: 10.1038/cr.2008.42. [DOI] [PubMed] [Google Scholar]
- 37.Xu SZ, et al. Multiple mechanisms of soy isoflavones against oxidative stress-induced endothelium injury. Free Radic Biol Med. 2009;47:167–175. doi: 10.1016/j.freeradbiomed.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 38.Milanesi L, Russo, de Boland. A, Boland R. Expression and localization of estrogen receptor alpha in the C2C12 murine skeletal muscle cell line. J Cell Biochem. 2008;104:1254–1273. doi: 10.1002/jcb.21706. [DOI] [PubMed] [Google Scholar]
- 39.Milanesi L, Vasconsuelo A, de Boland AR, Boland R. Expression and subcellular distribution of native estrogen receptor beta in murine C2C12 cells and skeletal muscle tissue. Steroids. 2009;74:489–497. doi: 10.1016/j.steroids.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Wiik A, et al. Oestrogen receptor beta is expressed in adult human skeletal muscle both at the mRNA and protein level. Acta Physiol Scand. 2003;179:381–387. doi: 10.1046/j.0001-6772.2003.01186.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee YS, Chen XW, Anderson JJB. Physiological concentrations of genistein stimulate the proliferation and protect against free radical-induced oxidative damage of MC3T3-E1 osteoblast-like cells. Nutr Res. 2001;21:1287–1298. doi: 10.1016/S0271-5317(01)00340-2. [DOI] [Google Scholar]
- 42.Exner M, et al. Genistein prevents the glucose autoxidation mediated atherogenic modification of low density lipoprotein. Free Radic Res. 2001;34:101–112. doi: 10.1080/10715760100300101. [DOI] [PubMed] [Google Scholar]
- 43.Sierens J, Hartley JA, Campbell MJ, Leathem AJ, Woodside JV. Effect of phytoestrogen and antioxidant supplementation on oxidative DNA damage assessed using the comet assay. Mutat Res. 2001;485:169–176. doi: 10.1016/S0921-8777(00)00069-0. [DOI] [PubMed] [Google Scholar]
- 44.Velders M, et al. Estradiol and genistein antagonize the ovariectomy effects on skeletal muscle myosin heavy chain expression via ER-beta mediated pathways. J Steroid Biochem Mol Biol. 2010;120:53–59. doi: 10.1016/j.jsbmb.2010.03.059. [DOI] [PubMed] [Google Scholar]
- 45.Skelly JR, Bradford A, O’Halloran KD. Intermittent hypoxia impairs pharyngeal dilator muscle function in male but not female rats. Adv Exp Med Biol. 2010;669:285–287. doi: 10.1007/978-1-4419-5692-7_58. [DOI] [PubMed] [Google Scholar]
- 46.Hassan B, Akcakanat A, Holder AM, Meric-Bernstam F. Targeting the PI3-kinase/Akt/mTOR signaling pathway. Surg Oncol Clin N Am. 2013;22:641–664. doi: 10.1016/j.soc.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim HJ, Crowe P, Yang JL. Current clinical regulation of PI3K/PTEN/Akt/mTOR signalling in treatment of human cancer. J Cancer Res Clin Oncol. 2015;141:671–689. doi: 10.1007/s00432-014-1803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


