Abstract
Background
Positive lymph node ratio (LNR), defined as ratio of positive lymph nodes to all lymph nodes removed, is a powerful prognostic factor in invasive breast cancer. Here we focused on the impact of negative lymph node (NLN) count on the prediction of value of LNR in breast cancer survival.
Methods
Of 929 invasive breast cancer patients were enrolled in our retrospective study. We use Kaplan-Meier to calculate the 5-year overall survival (OS) according to different clinicopathologic parameters. The prediction value of NLN count and LNR in OS was examined.
Results
The optimal cutoff of NLN count was designated as 9. Five-year OS was 77.0% and 95.0% in patients with NLN of 0–9 and ≥10, respectively (P<0.001). Among 204 patients who had 0–9 NLN, 25 patients with LNR 0–20.0% had 5-year OS of 95.7%, 104 patients with LNR 20.1–65.0% had 5-year OS of 83.4%, and 75 patients with LNR 65.1–100.0% had 5-year OS of 61.7% (P<0.001); Among 725 patients who had NLN ≥10, 650 patients with LNR 0–20.0% had 5-year OS of 96.1%, 68 patients with LNR 20.1–65.0% had 5-year OS of 86.8%, and 7 patients with LNR 65.1–100% had 5-year OS of 71.4% (P<0.001).
Conclusions
High NLN count is associated with improved survival in invasive breast cancer patients. Combining NLN count with LNR could be considered as an alternative to LNR alone in prediction of postoperative breast cancer survival.
Keywords: Breast cancer, negative lymph node (NLN), positive lymph node, lymph node ratio (LNR), prognosis
Introduction
Breast cancer is the most common cancer in women worldwide. Management of axillary lymph node metastases has been a controversial but evolving area of breast cancer therapy (1-5). Axillary lymph node status is important in predicting local recurrence and long-term outcomes (6,7). Lymph node staging of breast cancer according to the 7th edition of the American Joint Committee on Cancer (AJCC) and International Union Against Cancer (UICC) TNM staging system, is based on positive lymph node count, but not the total number of lymph nodes removed or the negative lymph node (NLN) count. In fact, it is impossible that axillary dissection in every patient is of the same extent, so there must exist heterogeneity if we only use positive lymph node number to classify patients with different prognosis. To improve the efficiency of prognostic system in breast cancer, not only positive lymph node number, but also the number of total lymph nodes should be taken into account (8-10).
In the last decade, many studies have shown that lymph node ratio (LNR) confers prognostic value in breast cancer (8-10). Since total lymph nodes after axillary lymph node dissection (ALND) are composed of both positive and NLNs. It’s necessary to bring NLN count into the system of prognosis prediction in breast cancer. Many researchers have suggested that NLN count is closely related to the prognosis in gastric cancer (11), colon cancer (12), esophageal cancer (13), and cervical cancer (14). However, few data has been published to elucidate the importance of NLN count in breast cancer prognosis.
Here we explored the correlation between NLN count and prognosis of invasive breast cancer, and the value of combining NLN count with LNR in prediction of breast cancer survival.
Methods
Patients
In our study, primary invasive breast cancer patients who underwent surgery (breast-conserving surgery or mastectomy) between January 2005 and December 2007 at the Department of Thyroid and Breast Surgery, West China Hospital, Sichuan University were included. All patients enrolled had ALND, including those who underwent ALND after positive sentinel lymph node biopsies. Patients with previous breast cancer, bilateral breast cancer, distant metastasis, and those who underwent neoadjuvant chemotherapy were excluded. Ultimately 929 female patients aged 22–87 years (49±10.95, median 50 years) were enrolled in our study. Most patients (>90%) received adjuvant chemotherapy, radiotherapy, human epidermal growth factor receptor 2 (HER2) targeted therapy, or hormone therapy after surgery, alone or in combination.
Our study was approved by the Ethics Committee of West China Hospital [No. 2016(27)], and all the participants agreed to participate in this study and signed an informed consent before taking part.
Pathologic analysis
A total of 471 (50.7%) patients had axillary lymph nodes metastasis that was assessed by haematoxylin and eosin staining. The total number of axillary lymph nodes removed from all 929 patients was 15,680 (mean 15, range from 10 to 46), among which, 2,956 lymph nodes were negative. Estrogen receptor (ER), progesterone receptor (PR), ki67, and HER2 were determined by immunohistochemical (IHC) analysis.
Follow-up
After surgery, all the patients were under surveillance every 6 months for 3 years, and then every year after 3 years by hospital visits. The median follow-up was 67 (range 1–94) months. Physical examination and ultrasound for breast, axillary, infraclavicular and supraclavicular lymph nodes were performed at every visit to monitor tumor recurrence. Mammography was performed annually. In addition to passive follow-up, an active one performed each year by the medical staff of West China Hospital Database Office through telephone or letters.
Statistical analysis
In our study, we defined death from breast cancer as an end point event, and patients dying from other causes were censored. The 5-year overall survival (OS) rate was determined by Kaplan-Meier, and statistical differences between groups were calculated with the log-rank test. The following nine variables were included in univariate analysis: (I) age at diagnosis (≤50 or >50 years); (II) T stage (T1, T2, T3, or T4); (III) histologic grade (grades I, II, III); (IV) ER (−, +); (V) PR (−, +);(VI) ki67 (<14%, ≥14%); (VII) HER2 (−, +); (VIII) NLN count (0–9, ≥10); (IX) LNR (0–20.0%, 20.1–65.0% and 65.1–100.0%). Factors of statistically significant difference by univariate analysis then were included in multivariate analysis by Cox regression and by forward LR stepwise procedure for viable selection. Hazard ratios (HR) with 95% confidence intervals (CI) were calculated. For all the statistical analyses, SPSS version 19.0 (SPSS, Chicago, IL, USA) was used.
We adopted the cut-point survival analysis to determine the most appropriate cutoffs for NLN and LNR (10,11,15). According to log rank test χ2 statistics, we compared the ability to differentiate among subgroups.
Results
Cut-point of NLN count and LNR
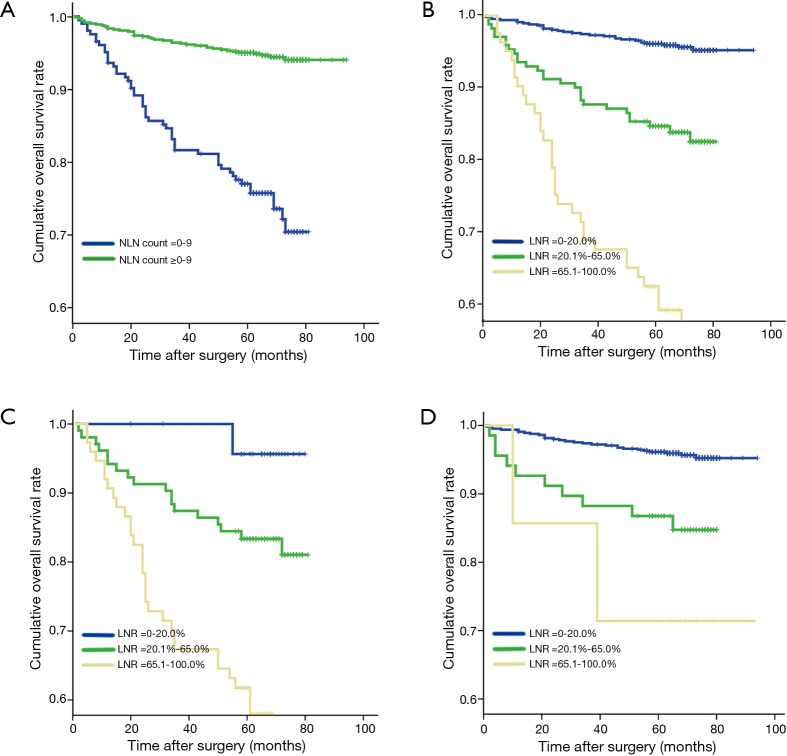
Five-year OS of all the 929 breast cancer patients was 91.1%. Eight hundred and thirty-seven patients were alive when our follow-up completed. Results for all possible NLN count cut points were listed in Table 1.The optimal cutoffs of NLN count for classify patients with different survival were 9. Of 204 patients with 0–9 NLN count had a poorer prognosis compare to 725 patients with ≥10 NLN count (P<0.001) (Figure 1A). Among 929 patients, 675 patients had LNR 0–20.0%, 172 patients had LNR 20.1–65.0% and 82 patients had LNR 65.1–100.0%. The survival of the three subgroups had a statistically significant difference (P<0.001) (Figure 1B).
Table 1. NLN count with 5-year overall survival rate.
| NLN count | N | 5YSR (%) | χ2 | P value |
|---|---|---|---|---|
| 0 | 14 | 50.0 | ||
| 1 | 11 | 35.8 | 0.667 | 0.414 |
| 2 | 7 | 71.4 | 2.143 | 0.143 |
| 3 | 12 | 83.3 | 0.713 | 0.398 |
| 4 | 24 | 78.9 | 0.053 | 0.819 |
| 5 | 22 | 81.8 | 0.027 | 0.869 |
| 6 | 23 | 78.3 | 0.012 | 0.912 |
| 7 | 27 | 96.2 | 2.432 | 0.119 |
| 8 | 30 | 79.9 | 2.993 | 0.084 |
| 9 | 34 | 75.4 | 0.002 | 0.969 |
| 10 | 108 | 94.4 | 9.03 | 0.003 |
| 11 | 70 | 94.3 | 0.016 | 0.898 |
| 12 | 57 | 94.7 | 0.142 | 0.707 |
| 13 | 59 | 100.0 | 1.249 | 0.264 |
| 14 | 56 | 94.6 | 1.215 | 0.270 |
| 15 | 54 | 94.4 | 0.003 | 0.958 |
| 16 | 43 | 93.0 | 0.07 | 0.791 |
| 17 | 50 | 98.0 | 1.301 | 0.254 |
| 18 | 42 | 92.8 | 0.296 | 0.586 |
| 19 | 27 | 88.9 | 1.226 | 0.268 |
| 20 | 29 | 96.6 | 0.213 | 0.644 |
| 21 | 15 | 93.3 | 0.667 | 0.414 |
| 22 | 10 | 100.0 | 0.867 | 0.352 |
| 23 | 15 | 93.3 | – | – |
| 24 | 13 | 100.0 | 0.769 | 0.380 |
| 25 | 10 | 100.0 | 0.692 | 0.405 |
| 26 | 13 | 92.3 | 1 | 0.317 |
| 27 | 9 | 100.0 | 0.061 | 0.806 |
| 28 | 9 | 88.9 | 0.032 | 0.857 |
| 29 | 6 | 83.3 | 0.6 | 0.439 |
| 30 | 6 | 80.0 | – | – |
| 31 | 6 | 100.0 | – | – |
| 32 | 3 | 100.0 | – | – |
| 33 | 3 | 100.0 | – | – |
| 34 | 2 | 100.0 | – | – |
| 35 | 1 | 100.0 | – | – |
| 36 | 2 | 100.0 | – | – |
| 38 | 2 | 100.0 | – | – |
| 40 | 2 | 100.0 | – | – |
| 41 | 1 | 100.0 | – | – |
| 43 | 1 | 0 | 1 | 0.317 |
| 46 | 1 | 100.0 | 1 | 0.317 |
5YSR, 5-year overall survival rate; NLN count, No. of negative lymph nodes.
Figure 1.
Five-year OS of postoperative breast cancer patients. (A) Five-year OS of different subgroup of NLN count (0–9 and ≥10); (B) 5-year OS of different subgroup of LNR (0–20.0%, 20.1–65.0%, 65.1–100%); (C) 5-year OS of 204 breast cancer patients with 0–9 NLN count according to subgroups of LNR (0–20.0%, 20.1–65.0%, 65.1–100%); (D) 5-year OS of 725 breast cancer patients with NLN count ≥10 according to subgroups of LNR (0–20.0%, 20.1–65.0%, 65.1–100%). OS, overall survival; LNR, lymph node ratio; NLN, negative lymph node.
Univariate and multivariate survival analysis of 929 patients
Seven factors were determined to have statistical significance in survival of 929 breast cancer patients after surgery by univariate analysis (Table 2). These are as follows: (I) age: patients with age ≤50 were more likely to have a better 5-year OS compared with age >50; (II) T stage: patients with larger primary tumor had lower 5-year OS; (III) histologic grade: the higher the histologic grade, the lower the 5-year OS; (IV) Ki67: patients with ki67 <14% were more likely to have a better 5-year OS compared with Ki67 ≥14%; (V) HER2: HER2 positive patients were more likely to have a lower 5-year OS compared with HER2 negative patients; (VI) LNR: patients with higher LNR showed lower 5-year OS; (VII) NLN count: patients with higher NLN count had better 5-year OS. All of the seven variables were included in multivariate analysis to adjust for covariate effects (Table 3). In this Cox proportional hazard model, only LNR, T stage, and histologic grade remained statistically significant in correlation with 5-year OS of postoperative invasive breast cancer.
Table 2. Univariate analysis of factors affecting survival of 929breast cancer patients by Kaplan-Meier method.
| Factor | N | 5YSR (%) | χ2 | P value |
|---|---|---|---|---|
| Age | 6.108 | 0.013 | ||
| ≤50 | 507 | 93.3 | ||
| >50 | 422 | 88.5 | ||
| T stage | 74.755 | <0.001 | ||
| T1 | 373 | 96.0 | ||
| T2 | 450 | 91.7 | ||
| T3 | 69 | 73.6 | ||
| T4 | 37 | 67.5 | ||
| Histological stage | 65.675 | <0.001 | ||
| Grade 1 | 318 | 99.0 | ||
| Grade 2 | 223 | 94.5 | ||
| Grade 3 | 388 | 82.6 | ||
| ER | 0.724 | 0.395 | ||
| (−) | 365 | 89.8 | ||
| (+) | 564 | 92.0 | ||
| PR | 3.836 | 0.050 | ||
| (−) | 285 | 87.9 | ||
| (+) | 644 | 92.5 | ||
| Ki 67 | 5.053 | 0.025 | ||
| <14% | 388 | 93.8 | ||
| ≥14% | 541 | 89.2 | ||
| HER2 | 10.224 | 0.001 | ||
| (−) | 695 | 92.8 | ||
| (+) | 234 | 86.3 | ||
| NLN count | 78.815 | <0.001 | ||
| 0–9 | 204 | 77.0 | ||
| ≥10 | 725 | 95.0 | ||
| LNR | 155.758 | <0.001 | ||
| 0–20.0% | 675 | 96.1 | ||
| 20.1–65.0% | 172 | 84.7 | ||
| 65.1–100.0% | 82 | 62.6 |
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; LNR, ratio between positive and examined lymph nodes.
Table 3. Multivariate analysis of factors affecting survival of 929breast cancer patients by Cox proportional hazard model.
| Factor | P value | HR | 95% CI |
|---|---|---|---|
| T stage | <0.001 | 1.811 | 1.441–2.276 |
| Histologic stage | <0.001 | 3.235 | 2.173–4.817 |
| LNR | <0.001 | 2.951 | 2.310–3.771 |
HR, hazard ratio, CI, confidence interval.
Combining NLN count with LNR in survival prediction
Among 204 patients who had NLN count 0–9, 25 patients had LNR 0–20.0% with 5-year OS of 95.7%, 104 patients had LNR 20.1–65.0% with 5-year OS of 83.4%, 75 patients had LNR 65.1–100% with 5-year OS of 61.7% (Table 4). We demonstrated that in this case 5-year OS would decrease significantly as LNR increased (P<0.001) (Figure 1C). In addition, of 725 patients who had more than 9 NLN, 650 patents had LNR 0–20.0% with 5-year OS of 96.1%, 68 had LNR 20.1–65.0% with 5-year OS of 86.8%, and 7 had LNR 65.1–100.0% with 5-year OS of 71.4% (Table 4). Similarly, we found that 5-year OS decreased as LNR increased (P<0.001) (Figure 1D). Moreover, in patients who had comparatively high LNR (>65%), their survival increased along with the elevated NLN count (Table 4).
Table 4. Combining the NLN count with LNR to predict prognosis of 929 breast cancer patients after surgery.
| LNR (%) | NLN count and 5YSR | ||||
|---|---|---|---|---|---|
| 0–9 | ≥10 | ||||
| N | % | N | % | ||
| 0–20.0 | 25 | 95.7 | 650 | 96.1 | |
| 20.1–65.0 | 104 | 83.4 | 68 | 86.8 | |
| 65.1–100.0 | 75 | 61.7 | 7 | 71.4 | |
| 5YSR | 77.0 | 95.0 | |||
To elucidate whether there is a direct relationship between LNR and NLN count, we performed a linear regression analysis using LNR as a dependent variable and NLN count as an independent predictor. We indicated that LNR decreased as NLN count increased (P<0.001) (Figure 2).
Figure 2.
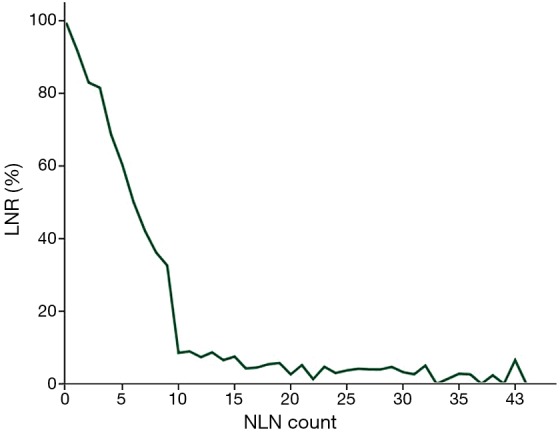
Correlation between LNR and NLN count.
Discussion
The AJCC/UICC pN classification is based on the number of positive lymph nodes£¨which is easily affected by the extend of ALND and the number of lymph nodes removed, especially if the retrieved number is small. Recent research emphasized the advantage of LNR over the number of positive lymph nodes in cases with few nodes removed (10). Others reported that LNR also improved the comparability between different centers (16). Furthermore, LNR could eliminate the stage migration phenomenon to some extent (17,18). In our study, LNR also showed statistically significant correlation with prognosis of postoperative invasive breast cancer. A review of literature showed that cutoff points of LNR differed widely: 0.10/0.50 (19), 0.50/0.75 (20), 0.25/0.55 (21), 0.1/0.65 (22), 0.3 (23). Vinh-Hung et al. (10) assessed the prognostic value of LNR in 1,829 breast cancer patients. They classified patients into three groups: low (≤0.20), intermediate (>0.20 and ≤0.65), and high-risk (>0.65) group, according to the cutoff points of LNR 0.20 and 0.65, The cutoff points by Vinh-Hung were widely accepted because of the large number of population studied and accurate follow-up data. Therefore, we divided LNR into three groups according to Vinh-Hung.
Total lymph nodes after ALND are composed of both positive and NLNs. Recently, several studies about NLN count and its predictive value of survival for cancer patients have been down. Schwarz et al. (24) showed that higher NLN count in patients underwent gastric cancer curative resection was correlated with improved postoperative survival. Johnson et al. (12) also demonstrated that increased NLN count in stage IIIB and IIIC colon cancer patients associated with decreased mortality.
However, the predictive value of NLN count in breast cancer patients is still an open question. Mersin et al. (25) analyzed 270 patients with axillary lymph node-negative breast cancer, and they concluded that the increase in number of NLN count was associated with poorer prognosis. The 5-year event-free and OS was 92.5% and 98.3% for patients who had 18 lymph nodes or less, and 70% and 86.7% for those who had more than 18 negative nodes, respectively (P<0.00001). Later, Moorman et al. (26) studied the relationship between the number of lymph nodes and breast carcinoma survival among 911 women with lymph node-negative breast carcinoma. They concluded that NLNs number examined was associated with neither 5-year nor long-term survival. Therefore, the prognostic value of NLN count still remains controversial in node-negative breast cancer patients.
Different from their studies, we included both lymph node negative and positive patients in our study. We explored prognostic value of NLN count and LNR in 929 invasive breast cancer patients after surgery. We found that patients with more NLN count (≥10) and lower LNR showed improved survival. After combining LNR with NLN count, we found among patients in the same LNR subgroup, those with more NLN count had a better prognosis (Table 4). Therefore, combining NLN count with LNR could be considered as an alternative to LNR alone in survival prediction.
The reason why NLN count is associated with survival has not been fully explained. There are several possible mechanisms. Firstly, the evaluation of high numbers of lymph nodes may decrease stage-migration phenomenon£¨which means survival on base of disease stage improves due to a better classification, though maybe no improvement in overall outcome or for a given individual. For instance£¨low number of lymph nodes harvested related to poor survival due to misclassification of node-positive patients as node-negative. Secondly, NLN count may reflect adequacy of total lymph nodes evaluation and excellent quality of surgical care (12). Thirdly, the NLN count would be a potential marker of tumor-host interactions, and might influence the presence and functions of circulating cancer cells (CTC), which has an independent effect on survival (27). In addition, breast cancer patients with more positive axillary lymph nodes are more susceptible to local recurrence and distant metastasis than those with more NLNs (28).
However, it has been reported by ACSOG Z0011 randomized trial that ALND can’t increase the survival of breast cancer patients with negative or less than three positive sentinel lymph nodes after breast-conserving therapy. In those patients, it has been proposed that ALND could be avoided (29). Moreover, radical axillary dissection may lead to more morbidity. So ALND was controversial in breast cancer patients at present and the balance between benefits and risks of extensive ALND should be individualized.
In summary, we have shown in this study that NLN count is associated with breast cancer survival. Combining NLN count with LNR has a better ability to discriminate populations with different survival than LNR alone. There are limitations in our study. This is a retrospective study conducted at a single center. Further prospective multicenter studies are required to confirm the exact value of NLN count and the most appropriate cut-off point of NLN and LNR.
Acknowledgements
Authors would like to express our sincere appreciation to Dr. Jingyu Deng for his valuable comments on our paper.
Ethical Statement: Our study was approved by the Ethics Committee of West China Hospital [No. 2016(27)], and all the participants agreed to participate in this study and signed an informed consent before taking part.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Jaffer S, Bleiweiss IJ. Evolution of sentinel lymph node biopsy in breast cancer, in and out of vogue? Adv Anat Pathol 2014;21:433-42. 10.1097/PAP.0000000000000041 [DOI] [PubMed] [Google Scholar]
- 2.Pilewskie ML, Morrow M. Management of the clinically node-negative axilla: what have we learned from the clinical trials? Oncology (Williston Park) 2014;28:371-8. [PubMed] [Google Scholar]
- 3.Caudle AS, Cupp JA, Kuerer HM. Management of axillary disease. Surg Oncol Clin N Am 2014;23:473-86. 10.1016/j.soc.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 4.Joslyn SA, Konety BR. Effect of axillary lymphadenectomy on breast carcinoma survival. Breast Cancer Res Treat 2005;91:11-8. 10.1007/s10549-004-6276-7 [DOI] [PubMed] [Google Scholar]
- 5.Bachleitner-Hofmann T, Gnant M. Surgical axilla dissection. Technical standard or obsolete method? Zentralbl Chir 2000;125:822-9. 10.1055/s-2000-10053 [DOI] [PubMed] [Google Scholar]
- 6.Smith JA, 3rd, Gamez-Araujo JJ, Gallager HS, et al. Carcinoma of the breast: analysis of total lymph node involvement versus level of metastasis. Cancer 1977;39:527-32. [DOI] [PubMed] [Google Scholar]
- 7.Brooks AD. When it comes to breast cancer staging, lymph node status is king. J Surg Res 2010;164:67-8. 10.1016/j.jss.2009.11.711 [DOI] [PubMed] [Google Scholar]
- 8.Dings PJ, Elferink MA, Strobbe LJ, et al. The prognostic value of lymph node ratio in node-positive breast cancer: a Dutch nationwide population-based study. Ann Surg Oncol 2013;20:2607-14. 10.1245/s10434-013-2932-7 [DOI] [PubMed] [Google Scholar]
- 9.Elkhodary TR, Ebrahim MA, Hatata EE, et al. Prognostic value of lymph node ratio in node-positive breast cancer in Egyptian patients. J Egypt Natl Canc Inst 2014;26:31-5. 10.1016/j.jnci.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 10.Vinh-Hung V, Verkooijen HM, Fioretta G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol 2009;27:1062-8. 10.1200/JCO.2008.18.6965 [DOI] [PubMed] [Google Scholar]
- 11.Deng J, Liang H, Wang D, et al. Enhancement the prediction of postoperative survival in gastric cancer by combining the negative lymph node count with ratio between positive and examined lymph nodes. Ann Surg Oncol 2010;17:1043-51. 10.1245/s10434-009-0863-0 [DOI] [PubMed] [Google Scholar]
- 12.Johnson PM, Porter GA, Ricciardi R, et al. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J Clin Oncol 2006;24:3570-5. 10.1200/JCO.2006.06.8866 [DOI] [PubMed] [Google Scholar]
- 13.Zhu Z, Chen H, Yu W, et al. Number of negative lymph nodes is associated with survival in thoracic esophageal squamous cell carcinoma patients undergoing three-field lymphadenectomy. Ann Surg Oncol 2014;21:2857-63. 10.1245/s10434-014-3665-y [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Zhang L, Tian J, et al. Combining the negative lymph nodes count with the ratio of positive and removed lymph nodes can better predict the postoperative survival in cervical cancer patients. Cancer Cell Int 2013;13:6. 10.1186/1475-2867-13-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng JY, Liang H, Sun D, et al. The most appropriate category of metastatic lymph nodes to evaluate overall survival of gastric cancer following curative resection. J Surg Oncol 2008;98:343-8. 10.1002/jso.21119 [DOI] [PubMed] [Google Scholar]
- 16.Truong PT, Woodward WA, Thames HD, et al. The ratio of positive to excised nodes identifies high-risk subsets and reduces inter-institutional differences in locoregional recurrence risk estimates in breast cancer patients with 1-3 positive nodes: an analysis of prospective data from British Columbia and the M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys 2007;68:59-65. 10.1016/j.ijrobp.2006.12.017 [DOI] [PubMed] [Google Scholar]
- 17.Hua Y, Hu Q, Tang Q, et al. Prognostic significance of the number of positive lymph nodes, number of involved regions and metastatic lymph node ratio in hypopharyngeal cancer. Zhonghua Zhong Liu Za Zhi 2014;36:783-7. [PubMed] [Google Scholar]
- 18.Tol JA, Brosens LA, van Dieren S, et al. Impact of lymph node ratio on survival in patients with pancreatic and periampullary cancer. Br J Surg 2015;102:237-45. 10.1002/bjs.9709 [DOI] [PubMed] [Google Scholar]
- 19.Tsuchiya A, Kanno M, Abe R. The impact of lymph node metastases on the survival of breast cancer patients with ten or more positive lymph nodes. Surg Today 1997;27:902-6. 10.1007/BF02388136 [DOI] [PubMed] [Google Scholar]
- 20.Walker MJ, Osborne MD, Young DC, et al. The natural history of breast cancer with more than 10 positive nodes. Am J Surg 1995;169:575-9. 10.1016/S0002-9610(99)80224-4 [DOI] [PubMed] [Google Scholar]
- 21.Kim JY, Ryu MR, Choi BO, et al. The prognostic significance of the lymph node ratio in axillary lymph node positive breast cancer. J Breast Cancer 2011;14:204-12. 10.4048/jbc.2011.14.3.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Holmes E, Shah K, et al. The prognostic value of lymph node cross-sectional cancer area in node-positive breast cancer: a comparison with N stage and lymph node ratio. Patholog Res Int 2012;2012:161964. 10.1155/2012/161964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang C, Liu F, Li S, et al. Lymph node ratio: a new feature for defining risk category of node-positive breast cancer patients. Int J Surg Pathol 2012;20:546-54. 10.1177/1066896912451323 [DOI] [PubMed] [Google Scholar]
- 24.Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol 2007;14:317-28. 10.1245/s10434-006-9218-2 [DOI] [PubMed] [Google Scholar]
- 25.Mersin H, Yildirim E, Bulut H, et al. The prognostic significance of total lymph node number in patients with axillary lymph node-negative breast cancer. Eur J Surg Oncol 2003;29:132-8. 10.1053/ejso.2002.1285 [DOI] [PubMed] [Google Scholar]
- 26.Moorman PG, Hamza A, Marks JR, et al. Prognostic significance of the number of lymph nodes examined in patients with lymph node-negative breast carcinoma. Cancer 2001;91:2258-62. [DOI] [PubMed] [Google Scholar]
- 27.Vinh-Hung V, Burzykowski T, Cserni G, et al. Functional form of the effect of the numbers of axillary nodes on survival in early breast cancer. Int J Oncol 2003;22:697-704. [PubMed] [Google Scholar]
- 28.Wu SG, Wang Y, Zhou J, et al. Number of negative lymph nodes should be considered for incorporation into staging for breast cancer. Am J Cancer Res 2015;5:844-53. [PMC free article] [PubMed] [Google Scholar]
- 29.Giuliano AE, McCall L, Beitsch P, et al. Locoregional Recurrence After Sentinel Lymph Node Dissection With or Without Axillary Dissection in Patients With Sentinel Lymph Node Metastases: Long-term Follow-up From the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized Trial. Ann Surg 2016;264:413-20. 10.1097/SLA.0000000000001863 [DOI] [PMC free article] [PubMed] [Google Scholar]