Abstract
Background
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) have high mortality and health care costs. The platelet to lymphocyte ratio (PLR) is a marker of inflammation and has been reported to be elevated in patients with AECOPD compared to stable state. In this study, we sought to evaluate the association of the PLR in patients with AECOPD with 90-day mortality.
Methods
We conducted a retrospective cohort study of patients admitted to our institution with a primary diagnosis of AECOPD between January 2014 and July 2014. Blood test results on admission were recorded. The primary outcomes were 90-day mortality.
Results
One hundred and eighty-one AECOPD patients were considered for the study. Death had occurred in 16 (9%) patients within 90 days of hospital discharge. Univariable analysis identified age, haemoglobin, neutrophil count, and urea level, neutrophil lymphocyte ratio (NLR) and PLR as being associated with increased 90-day mortality. Multivariable logistic regression analysis variables demonstrated that only PLR (P=0.03) was significantly associated with death at 90 days. Receiver operator characteristic analysis identified PLR ≥235 had a sensitivity of 63% and specificity of 74% in predicting 90-day mortality. PLR was ≥235 was also associated with worse survival (days) [mean ± standard deviation (SD): PLR ≥235 vs. PLR <235: 512±358 vs. 626±346, P=0.004].
Conclusions
A PLR ≥235 was significantly associated with 90-day mortality, which may provide prognostic guidance to clinicians.
Keywords: Chronic obstructive pulmonary disease (COPD), blood tests, platelet lymphocyte ratio, prognosis
Introduction
Chronic obstructive pulmonary disease (COPD) poses a significant public health challenge, as it is associated with increased mortality and health care costs. COPD is the third leading cause of death worldwide and the mortality rates are projected to increase (1,2). The disease also has a large economic burden from hospitalization, time off work and disability (2). Acute exacerbations of COPD (AECOPD) are part of the natural history of this disease where a large proportion of mortality and health expenditure is encountered. It is defined as a change from the patient’s baseline dyspnoea, cough and/or sputum (3). We have previously identified that following a hospital admission for AECOPD, the 12-month re-admission and mortality rates are 38% and 8% respectively (4). Longitudinal studies have demonstrated increasing severity of COPD, prior exacerbations, history of heartburn, poor quality of life and elevated white cell count are predictors of future exacerbations and possibly increased mortality as well (5). Considerable research has been conducted into identifying clinically applicable biomarkers in patients with stable and during exacerbations of COPD (6). The goal of the biomarkers would be to identify patients who would benefit from additional risk factor management with the goal of improving prognosis. Prognostic stratification is also beneficial for the patient and family so they are better informed of their condition.
Several recent studies have evaluated the utility of blood based molecules as diagnostic and prognostic biomarkers for patients with AECOPD (7-11). A recent systematic review of 24 observation studies found that CRP, leukocytes, IL-6, IL-8 and fibrinogen are associated with COPD (12). However, the authors concluded that while these biomarkers may be helpful, they have not been validated in large scale prospective studies and also some of the biomarker tests are not readily clinically available. Hence there is a need for the identification on a simple point of care prognostic biomarker for AECOPD patients. One such biomarker may be the platelet lymphocyte ratio (PLR). The PLR has been extensively evaluated as a surrogate maker of inflammation in many diseases (13,14). An elevated PLR is associated with worse prognosis in patients with many solid organ malignancies (15,16), cardiovascular disease (14) and pulmonary embolism (17). More recently two studies have evaluated the PLR in patients with COPD, with only 1 study evaluating PLR in patients with AECOPD (18,19). Karadeniz et al. evaluated 60 patients with stable COPD and 50 patients with AECOPD and found that the PLR was negatively correlated with the forced expiratory volume in 1 second (FEV1) (18). To the best of our knowledge no previous studies have investigated the prognostic utility of PLR in hospitalized AECOPD patients. The aims of this study were to determine if the PLR has any relationship to mortality within 90 days and survival in hospitalized AECOPD patients.
Methods
Our retrospective, observational cohort study included consecutive AECOPD-related admissions to the Gold Coast University Hospital (GCUH) which is the major hospital of the Gold Coast Hospitals and Health Service (GCHHS). The GCHHS provides care for 500,000 residents and the GCUH is a 570-bed regional teaching hospital with access to all major specialities. In the year 2013–2014, there were 40,868 medical admissions to the GCUH. For the purposes of this study we only considered patients who presented to the emergency department and were subsequently required an admission to the GCUH for ongoing management.
Participants that presented during the calendar months of January 2014 to July 2014 were enrolled. Adults with AECOPD-related diagnosis at the time of discharge were identified by International Classification of Diseases-10 (ICD-10) Principal Diagnosis codes. GCHHS Health Analytics team used admission and discharge codes to provide the study investigators with patient data. All medical entries were made in electronic medical records and the radiology and pathology data was available on a centralised database. Eligibility criteria included adults >40 years, primary diagnosis of AECOPD and patients with a spirometry confirmed diagnosis of COPD, i.e., FEV1/FVC ratio <70% and FEV1 <80%. Lung function measured at disease stability (defined as either within 6 months prior to the exacerbation or 6 weeks after recovery) was used for to establish diagnosis of COPD. Exclusion criteria included age <40 years and airway disease primarily due to other cause (e.g., community acquired pneumonia, interstitial lung disease). Written informed consent was obtained from all participants and the study received ethics committee approval (HREC/15/QGC/20).
The complete blood count parameters, including the white blood cell (WBC), neutrophil, lymphocyte and platelet counts were retrospectively reviewed from the medical records. The PLR was calculated as the ratio of platelets to lymphocytes. Information on age, gender, smoking status, BMI, documented co-morbidities, AECOPD specific medications (bronchodilators, steroids and antibiotics) provided during hospital admission, number of hospital admissions in the 12-month preceding the index admission, hospital length of stay, COPD related readmission to hospital within 12 months of discharge and survival within 90-days of discharge were retrieved. Survival at 90 days after the date of initial admission was ascertained from review of electronic medical records. If death had occurred, electronic death certification was linked to the patients’ electronic medical record and this information was recorded by the study investigators.
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 20.0 (SPSS, Chicago, IL, USA). Kolmogorov-Smirnov and Shapiro-Wilks tests were used to assess for normality of the data. Normally distributed data were analysed for differences between individual groups using the Student’s t-test and presented as mean and standard error. Nonparametric data was analysed for differences between groups using the Mann-Whitney U-test and results expressed as median and interquartile range. Logistic regression analysis was used to identify factors associated with 90-day survival. Variables from the univariable analysis that had P values of <0.05 were evaluated for the multivariate logistic regression analysis. Statistical significance was indicated by a P value of less than 0.05.
Results
During the four-month study period, one hundred and ninety-nine presentations were identified using the ICD-10 codes. After review of the medical case records, 18 presentations were excluded from analysis as they were found to be either incorrectly coded or AECOPD was not the primary reason for hospitalization. Only the 181 patients with spirometry confirmed diagnosis of COPD were included in the analysis.
Patient characteristics are presented in Table 1. The mean age [± standard deviation (SD)] of the patients was 71±10 years and females accounted for 49% of the study cohort. Twenty-four percent of patients stated that they were current smokers and 73% stated that they were reformed smokers. Among the common co-morbid illnesses documented, coronary artery disease was the most common (25%), followed by depression (20%) and osteoporosis (17%). Thirty-four (19%) patients were using home oxygen at time of hospital admission. The mean (± SD) number of hospital admissions in the previous 12 months prior to presentation for the study cohort was 0.92±1.8. The majority of patients had GOLD stage III severity of COPD (42%). Following admission to hospital, an overwhelming majority of patients had received systemic corticosteroids, antibiotics and bronchodilators.
Table 1. Patient characteristics.
| Characteristics | All (N=181) |
|---|---|
| Age in year, mean (± SD) | 71±10 |
| Female gender, n (%) | 88 (49%) |
| Smoking status, n (%) | |
| Current smoker | 43 (24%) |
| Former smoker | 132 (73%) |
| Never smoker | 6 (3%) |
| Body mass index (kg/m2), mean (± SD) | 26±7 |
| Comorbidity, n (%) | |
| Coronary artery disease | 45 (25%) |
| Congestive heart failure | 29 (16%) |
| Depression | 36 (20%) |
| Arrhythmia | 30 (17%) |
| Chronic kidney disease | 16 (9%) |
| Osteoporosis | 30 (17%) |
| Total number of comorbidities, mean (± SD) | 5±3 |
| Pulmonary function test results*, n (%) | |
| % predicted, FEV1, mean (± SD) | 44±18 |
| GOLD stage I | 8 (4%) |
| GOLD stage II | 53 (29%) |
| GOLD stage III | 76 (42%) |
| GOLD stage IV | 44 (24%) |
| Treatment provided during hospital admission, n (%) | |
| Antimicrobial therapy | 148 (82%) |
| Systemic corticosteroid therapy | 152 (84%) |
| Bronchodilator therapy | 181 (100%) |
*, pulmonary function tests performed during period of stability defined as either within 6 months prior to the exacerbation or 6 weeks after recovery. SD, standard deviation; FEV1, forced expiratory volume in 1 second.
Among the 181 patients, 58 patients required a COPD related readmission to hospital within 30 days of discharge. At the end of 12 months, 72 patients had presented admitted to hospital at least once for management of an acute exacerbation of COPD. Death had occurred in 16 (9%) patients within 90 days of hospital discharge and by the end of 12 months since discharge, a total of 32 patients had died. We then evaluated patient characteristics and biomarkers in relation to death at 90 days following hospital discharge (Table 2). Univariable analysis of clinical and laboratory parameters between patients who had died (N=16) or were alive (n=165) at 90 days since hospital discharge, there was a difference in age (P=0.004), haemoglobin (P<0.001), neutrophil count (P=0.023), urea level (P=0.015), neutrophil lymphocyte ratio (NLR) (P=0.004) and PLR (P=0.010). There was also no difference in the treatments provided to patients in hospital. Multivariable logistic regression analysis of the significant variables demonstrated that only PLR (P=0.03) was significantly associated with death at 90 days since discharge (Table 3).
Table 2. Demographic and laboratory characteristics of patients according to survival status within 90 days of discharge following hospitalization for AECOPD.
| Characteristics | Died within 90 days months post discharge (N=16) | Alive at 90 months post discharge (N=165) | P value |
|---|---|---|---|
| Demographics | |||
| Age | 78±9 | 70±10 | 0.004 |
| Sex (male/female) | 12/4 | 81/84 | 0.066 |
| BMI (kg/m2) | 23±5 | 26±7 | 0.090 |
| FEV1 (% predicted) | 41±19 | 44±18 | 0.349 |
| BMI (kg/m2), mean (±SD) | 23.3±5.8 | 26.1±6.8 | 0.090 |
| Comorbidity, n (%) | |||
| Coronary artery disease | 5 (31%) | 40 (24%) | 0.550 |
| Congestive heart failure | 4 (25%) | 25 (15%) | 0.294 |
| Depression | 3 (19%) | 33 (20%) | 1.00 |
| Arrhythmia | 4 (25%) | 26 (16%) | 0.309 |
| Chronic kidney disease | 5 (31%) | 11 (7%) | 0.069 |
| Osteoporosis | 4 (25%) | 26 (16%) | 0.309 |
| Total number of comorbidities, mean (± SD) | 5.1±1.8 | 4.6±2.7 | 0.15 |
| Index hospital admission | |||
| Antimicrobial therapy | 13 | 135 | 1.00 |
| Systemic corticosteroid therapy | 13 | 138 | 1.00 |
| Bronchodilator therapy | 16 | 165 | 1.00 |
| Length of stay (days)* | 5.5 [2.3–11] | 3 [1–6] | 0.313 |
| Biomarkers | |||
| Haemoglobin (g/dL) | 118±15 | 137±20 | 0.000 |
| Neutrophil | 13±11 | 8±4 | 0.023 |
| NLR | 13±10 | 7±8 | 0.004 |
| PLR | 348±228 | 208±164 | 0.010 |
| Urea | 11±7 | 7±7 | 0.015 |
Data is presented as mean ± standard deviation for normally distributed data. *, median (range) for non-Gaussian distributed data. AECOPD, acute exacerbation of COPD; CI, confidence interval; OR, odds ratio; BMI, body mass index; FEV1, forced expiratory volume in 1 second; SD, standard deviation; NLR, neutrophil lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
Table 3. Logistic regression analysis of independent factors associated with survival at 90 days post hospital admission for AECOPD.
| Variables | OR | 95% CI for OR | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 1.11 | 1.03 | 1.20 | 0.011 |
| Haemoglobin | 0.96 | 0.93 | 1.00 | 0.052 |
| Neutrophil | 1.00 | 0.99 | 1.01 | 0.13 |
| NLR | 0.95 | 0.84 | 1.08 | 0.46 |
| PLR | 1.15 | 1.02 | 1.30 | 0.03 |
| Urea | 1.03 | 0.97 | 1.09 | 0.36 |
AECOPD, acute exacerbation of COPD; CI, confidence interval; OR, odds ratio; NLR, neutrophil lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
We conducted post-hoc analysis to determine the prognostic utility of PLR by calculating its sensitivity and specificity using the ROC curve. The area under the ROC curve for death at 90 days and PLR was 0.695 (95% CI, 0.551–0.840), P=0.010 (Figure 1). ROC curve analysis was further analysed to identify the greatest sum of sensitivity and specificity for distinguishing death at 90 days from the group that was still alive at 90 days post hospital discharge. PLR cut-off level of 235 had a sensitivity of 63% and specificity of 74% in predicting death at 90 days. PLR was ≥235 in 54 patients and patients with a PLR ≥235 had a trend for lower survival (survival, days (mean ± SD): PLR ≥235 vs. PLR <235: 512±358 vs. 626±346, P=0.004) (Figure 2).
Figure 1.
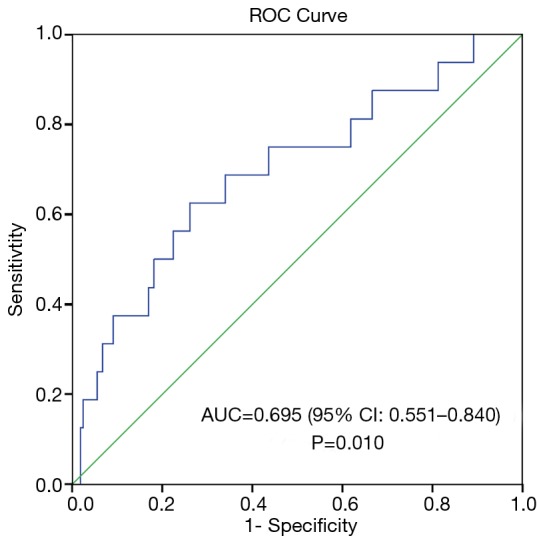
Receiver operating characteristic curve of PLR and 90-day mortality in patients with AECOPD. PLR, platelet to lymphocyte ratio; AECOPD, acute exacerbations of COPD.
Figure 2.
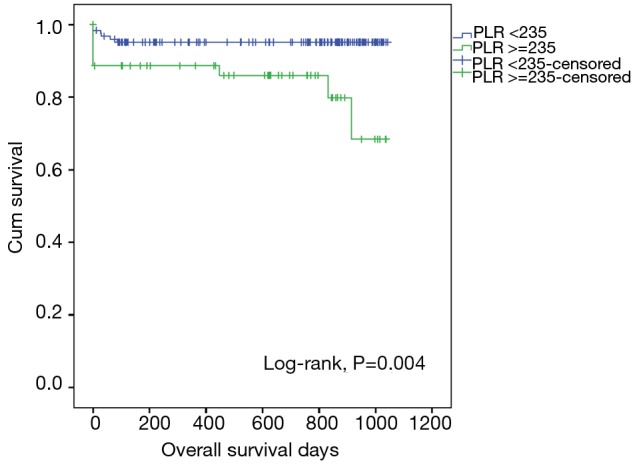
Kaplan-Meier survival curves for overall survival of patients with AECOPD with platelet-to-lymphocyte ratio (PLR) <235 and PLR ≥235. AECOPD, acute exacerbations of COPD; PLR, platelet to lymphocyte ratio.
Discussion
The purpose of the present study was to evaluate the utility of a simple and widely available biomarker, the PLR and its association with both short term prognosis and survival of patients hospitalized for management of AECOPD. We found on multivariable analysis that in hospitalized AECOPD patients PLR is associated with increased 90-day mortality. Furthermore, a PLR cut-off of >235 is associated with reduced survival. Our results are a novel contribution to the growing scientific body of knowledge about the prognostic utility of the PLR in not only solid organ malignancies but also chronic inflammatory disorders, such as COPD.
COPD is best described as a “…disease characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases” (3). COPD is already a leading cause of morbidity and mortality in both developed and developing nations worldwide, afflicting approximately 20% of the world’s population (20). Immunopathology studies have demonstrated that there is activation of both the innate and adaptive immune systems in patients with COPD, most likely due to persistent exposure to cigarette smoke (21). Previous studies have demonstrated that the inflammation in patients with COPD is not limited to the lungs alone but that the level of circulating systemic inflammatory markers is higher in patients with COPD than in healthy control subjects (22-25). Indeed, the studies have also shown that that patients with COPD, even during stable periods, have higher values of CRP, fibrinogen, interleukin (IL)-6, and tumour necrosis factor (TNF)-α as compared with those in healthy controls, and elevated levels of these markers of inflammation are associated with an increased risk of disease exacerbations and death (22-25).
The prognostic utility of routine blood based biomarkers in patients with AECOPD has been noted by Asiimwe et al. (26). Here the authors performed a retrospective analysis and reported that serum urea, albumin and arterial PaCO2 could predict in-hospital mortality in COPD patients. Similarly, other investigators have noted that WBC count and its subtypes also have prognostic utility in not only increasing risk of future exacerbations (27,28) but also mortality (29). More recently Harrison et al. reported that thrombocytosis could predict 1-year mortality and in-hospital mortality in large retrospective study of AECOPD patients (30). It is also being increasingly recognised that lymphopenia is associated with an adverse outcome in patients with COPD, such that it may be related to increase all-cause mortality (31,32). It is therefore reasonable to postulate that the PLR, which integrates thrombocytosis and lymphopenia, reflects the severity and activity of COPD. Two recent studies with relatively small sample sizes have specifically evaluated PLR values in patients with COPD (18,19). While both studies reported that the PLR was higher in AECOPD patients compared to stable COPD patients, neither specifically addressed the association between an elevated PLR and adverse clinical outcomes. To our knowledge, our study is the first to demonstrate the association between the PLR during the AECOPD state and relate it to short term mortality and survival. We believe that our results combined with those of the previous studies demonstrate that PLR may be used as convenient peripheral blood marker to reflect the severity and activity of the inflammation in patients with COPD, particularly during periods of an acute exacerbation.
Elevated PLR has been shown in recent studies to indicate inflammation in AECOPD (19). Its role in predicting mortality may be explained by similar mechanisms that have been used for thrombocytosis. Hypoxia in COPD may lead to increased production of immature platelets that are more prone to aggregate, elevating atherothrombotic risk (30,33). Combined with an increased baseline inflammatory state, COPD patients are at higher risk of ischemic heart disease. Statins (which have an anti-inflammatory effect) as well as anti-platelets (such as aspirin) have been found to reduce mortality in COPD. To this extent, the PLR finding in this study does support the view in the literature that platelets and WBCs can be simple and cost-effective markers for prognosis.
The strengths of our study were its spirometry confirmed COPD cohort with admission for AECOPD. Furthermore, it included a tertiary centre and despite the retrospective nature, the records had comprehensive data. Weaknesses of the study include its relatively small sample size and only using one measure in time rather than serial measures. However, the latter was purposeful, as we wanted to investigate the utility of the measures on admission so it could guide clinical decision-making from presentation. Confirmatory studies will need to be performed, particularly in a large cohort of COPD patients. Consequently, we are planning a large-scale prospective study to evaluate PLR in COPD patients, to validate the findings of our current study.
Future research in this field could be directed towards a meta-analysis of biomarkers. This type of study could provide a more meaningful multivariate analysis, helping to determine the clinical significance of effect sizes. Furthermore, continuing research into other candidates may be readily accessible, cheap and reliable to provide new tools for clinicians to use.
Conclusions
In conclusion, the results of our retrospective study have demonstrated a possible association between an elevated PLR and 90-day mortality. The results of our study can only be considered as pilot data and the results of our study will need to be validated in other independent and prospective studies. If confirmed, the PLR may potentially be simple, inexpensive and potentially useful biomarker for identifying patients at increased risk of adverse outcomes and may benefit for regular ongoing clinical surveillance.
Acknowledgements
None.
Ethical Statement: The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Burney PG, Patel J, Newson R, et al. Global and regional trends in COPD mortality, 1990-2010. Eur Respir J 2015;45:1239-47. 10.1183/09031936.00142414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rycroft CE, Heyes A, Lanza L, et al. Epidemiology of chronic obstructive pulmonary disease: a literature review. Int J Chron Obstruct Pulmon Dis 2012;7:457-94. 10.2147/COPD.S32330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vestbo J, Hurd SS, Agusti AG, et al. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease, GOLD Executive Summary. Am J Respir Crit Care Med 2013;187:347-65. 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 4.Wijayaratne K, Wilson J, Sivakumaran P, et al. Differences in care between general medicine and respiratory specialists in the management of patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Thorac Med 2013;8:197-203. 10.4103/1817-1737.118499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müllerova H, Maselli DJ, Locantore N, et al. Hospitalized Exacerbations of COPD: Risk Factors and Outcomes in the ECLIPSE Cohort. Chest 2015;147:999-1007. 10.1378/chest.14-0655 [DOI] [PubMed] [Google Scholar]
- 6.Agusti A, Gea J, Faner R. Biomarkers, the control panel and personalized COPD medicine. Respirology 2016;21:24-33. 10.1111/resp.12585 [DOI] [PubMed] [Google Scholar]
- 7.Chen Y-WR, Leung JM, Sin DD. A Systematic Review of Diagnostic Biomarkers of COPD Exacerbation. PLoS One 2016;11:e0158843. 10.1371/journal.pone.0158843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavord ID, Jones PW, Burgel P-R, et al. Exacerbations of COPD. Int J Chron Obstruct Pulmon Dis 2016;11:21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grolimund E, Kutz A, Marlowe RJ, et al. Long-term Prognosis in COPD Exacerbation: Role of Biomarkers, Clinical Variables and Exacerbation Type. COPD 2015;12:295-305. 10.3109/15412555.2014.949002 [DOI] [PubMed] [Google Scholar]
- 10.Chang CL, Robinson SC, Mills GD, et al. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax 2011;66:764-8. 10.1136/thx.2010.155333 [DOI] [PubMed] [Google Scholar]
- 11.Stolz D, Meyer A, Rakic J, et al. Mortality risk prediction in COPD by a prognostic biomarker panel. Eur Respir J 2014;44:1557-70. 10.1183/09031936.00043814 [DOI] [PubMed] [Google Scholar]
- 12.Su B, Liu T, Fan H, et al. Inflammatory Markers and the Risk of Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0150586. 10.1371/journal.pone.0150586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akboga MK, Canpolat U, Yuksel M, et al. Platelet to lymphocyte ratio as a novel indicator of inflammation is correlated with the severity of metabolic syndrome: A single center large-scale study. Platelets 2016;27:178-83. 10.3109/09537104.2015.1064518 [DOI] [PubMed] [Google Scholar]
- 14.Balta S, Ozturk C. The platelet-lymphocyte ratio: A simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets 2015;26:680-1. 10.3109/09537104.2014.979340 [DOI] [PubMed] [Google Scholar]
- 15.Zhou X, Du Y, Huang Z, et al. Prognostic Value of PLR in Various Cancers: A Meta-Analysis. PLoS One 2014;9:e101119. 10.1371/journal.pone.0101119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Templeton AJ, Ace O, McNamara MG, et al. Prognostic Role of Platelet to Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. Cancer Epidemiol Biomarkers Prev 2014;23:1204-12. 10.1158/1055-9965.EPI-14-0146 [DOI] [PubMed] [Google Scholar]
- 17.Kundi H, Balun A, Cicekcioglu H, et al. The relation between platelet-to-lymphocyte ratio and Pulmonary Embolism Severity Index in acute pulmonary embolism. Heart Lung 2015;44:340-3. 10.1016/j.hrtlng.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 18.Karadeniz G, Aktoğu S, Erer OF, et al. Predictive value of platelet-to-lymphocyte ratio in exacerbation of chronic obstructive pulmonary disease. Biomark Med 2016;10:701-10. 10.2217/bmm-2016-0046 [DOI] [PubMed] [Google Scholar]
- 19.Kurtipek E, Bekci TT, Kesli R, et al. The role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in exacerbation of chronic obstructive pulmonary disease. J Pak Med Assoc 2015;65:1283-87. [PubMed] [Google Scholar]
- 20.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765-73. 10.1016/S0140-6736(07)61380-4 [DOI] [PubMed] [Google Scholar]
- 21.Caramori G, Casolari P, Barczyk A, et al. COPD immunopathology. Semin Immunopathol 2016;38:497-515. 10.1007/s00281-016-0561-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agustí A, Edwards LD, Rennard SI, et al. Persistent Systemic Inflammation is Associated with Poor Clinical Outcomes in COPD: A Novel Phenotype. PLoS One 2012;7:e37483. 10.1371/journal.pone.0037483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomsen M, Dahl M, Lange P, et al. Inflammatory Biomarkers and Comorbidities in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2012;186:982-8. 10.1164/rccm.201206-1113OC [DOI] [PubMed] [Google Scholar]
- 24.Dahl M, Vestbo J, Lange P, et al. C-reactive Protein As a Predictor of Prognosis in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2007;175:250-5. 10.1164/rccm.200605-713OC [DOI] [PubMed] [Google Scholar]
- 25.Walter RE, Wilk JB, Larson MG, et al. Systemic Inflammation and COPD: The Framingham Heart Study. Chest 2008;133:19-25. 10.1378/chest.07-0058 [DOI] [PubMed] [Google Scholar]
- 26.Asiimwe AC, Brims FJ, Andrews NP, et al. Routine laboratory tests can predict in-hospital mortality in acute exacerbations of COPD. Lung 2011;189:225-32. 10.1007/s00408-011-9298-z [DOI] [PubMed] [Google Scholar]
- 27.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to Exacerbation in Chronic Obstructive Pulmonary Disease. N Engl J Med 2010;363:1128-38. 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 28.Thomsen M, Ingebrigtsen T, Marott J, et al. INflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA 2013;309:2353-61. 10.1001/jama.2013.5732 [DOI] [PubMed] [Google Scholar]
- 29.Celli BR, Locantore N, Yates J, et al. Inflammatory Biomarkers Improve Clinical Prediction of Mortality in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2012;185:1065-72. 10.1164/rccm.201110-1792OC [DOI] [PubMed] [Google Scholar]
- 30.Harrison MT, Short P, Williamson PA, et al. Thrombocytosis is associated with increased short and long term mortality after exacerbation of chronic obstructive pulmonary disease: a role for antiplatelet therapy? Thorax 2014;69:609-15. 10.1136/thoraxjnl-2013-203996 [DOI] [PubMed] [Google Scholar]
- 31.Günay E, Sarinc Ulasli S, Akar O, et al. Neutrophil-to-lymphocyte ratio in chronic obstructive pulmonary disease: a retrospective study. Inflammation 2014;37:374-80. 10.1007/s10753-013-9749-1 [DOI] [PubMed] [Google Scholar]
- 32.Sørensen AK, Holmgaard DB, Mygind LH, et al. Neutrophil-to-lymphocyte ratio, calprotectin and YKL-40 in patients with chronic obstructive pulmonary disease: correlations and 5-year mortality – a cohort study. J Inflamm (Lond) 2015;12:20. 10.1186/s12950-015-0064-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fimognari FL, Scarlata S, Conte ME, et al. Mechanisms of atherothrombosis in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2008;3:89-96. 10.2147/COPD.S1401 [DOI] [PMC free article] [PubMed] [Google Scholar]


