Abstract
Programmed death 1 (PD-1) inhibitors have been shown to increase overall survival in non-small cell lung cancer (NSCLC) patients. HIV patients have increased expression of PD-1 on their T-cell surfaces. We describe a patient with well-controlled HIV and NSCLC who underwent treatment with nivolumab and had a durable complete response (CR) with his viral load remaining undetectable. To date there is only one case report of a cancer patient with melanoma and with HIV treated with a programmed death ligand 1 (PD-L1) inhibitor. The majority of clinical trials involving PD-1 and PD-L1 inhibitors exclude HIV patients.
Keywords: Nivolumab, metastatic non-small cell lung cancer (NSCLC), HIV
Introduction
Immune checkpoint blockade has been recently studied in various cancers. Programmed death 1 (PD-1) receptor on T-cell surfaces and programmed death ligand 1 (PD-L1) are part of an inhibitory checkpoint that helps protect against autoimmunity by acting as negative regulators of activated T cells (1,2). Use of PD-1 inhibitors has shown increased survival in non-small cell lung cancer (NSCLC) (3). PD-1 expression is also increased on the surface of CD4 T cells in HIV-infected patients (4). Most of the clinical trials using PD-1 and PDL-1 inhibitors have excluded HIV-infected patients (5).
Case presentation
A 61-year-old male with a history of tobacco abuse and 8 years of well-controlled HIV (CD4 count of 684 and an undetectable viral load) on anti-retroviral therapy with emtricitabine-tenofovir and lopinavir-ritonavir initially presented with a right upper lobe mass (see Figure 1). The patient underwent a CT-guided biopsy which showed poorly differentiated, squamous cell carcinoma of the lung. A PET scan demonstrated increased FDG avidity in the right upper lobe nodule, which was approximately 10 mm, as well in the ipsilateral mediastinal lymph nodes and subcarinal lymph nodes. Staging was completed with an endobronchial ultrasound (EBUS) with lymph node biopsy, and MRI of the brain. Patient was determined to be stage IIIa-T1a, N2, M0. He was initially treated with one cycle of carboplatin/paclitaxel while waiting radiation planning and then received definitive chemoradiation with cisplatin and etoposide and achieved an 80–90% response on CT in March of 2015. On 6-month follow-up scans he had developed new mediastinal lymphadenopathy which was biopsy confirmed recurrent NSCLC. Genomic profiling failed to demonstrate targetable lesions and radiation oncology did not feel that he was a candidate for further therapy. The patient was started on nivolumab in January 2016 and achieved a complete response (CR) on CT scan in March 2016, which was confirmed on repeat scans in June 2016. His experienced no side effects and his CD4 count remained stable and his viral load undetectable during and after treatment.
Figure 1.
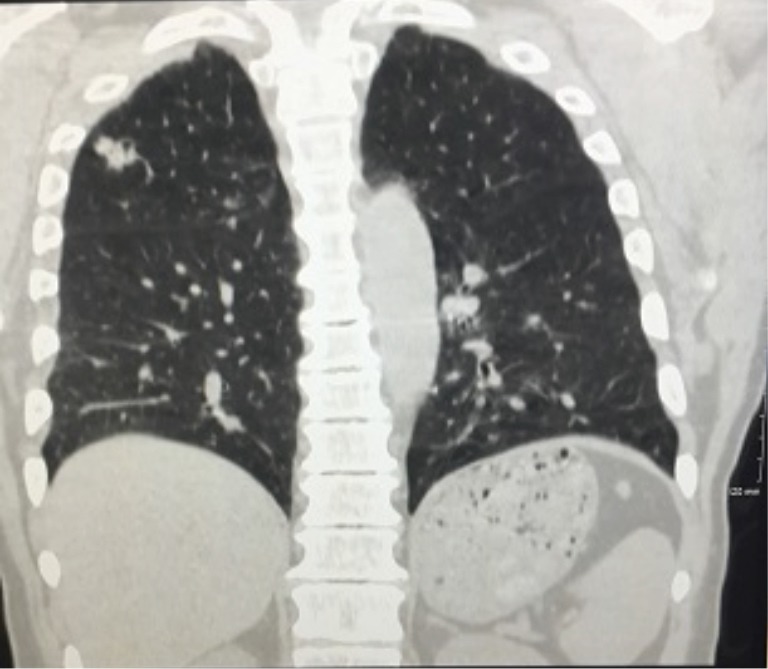
Chest X-ray showing right upper lobe mass.
Discussion
Nivolumab is a monoclonal antibody that binds to and inhibits PD-1 receptors. This type of immune checkpoint blockade has shown promise in some types of cancer. Two randomized phase III trials compared nivolumab to docetaxel after platinum based chemotherapy in squamous and non-squamous NSCLC. Both trials demonstrated a significant increase in overall survival compared to docetaxel (3,6). This increase in overall survival leads to the FDA approval of nivolumab for the treatment of metastatic squamous NSCLC after prior platinum-based chemotherapy (7). However, patients with HIV were excluded from both of these trials. In a further trial that tested the safety of PD-1 inhibitors in advanced cancers, only 5% of patients had serious adverse events (8). However, HIV-infected patients were also excluded from this trial.
HIV-infected patients have increased expression of PD-1 on their T-cell surfaces inhibiting T-cell activation, implying T-cell exhaustion (9). PD-1 up-regulation on HIV-specific CD4 T cells correlates with viremia (10). PD-1 impairs HIV-specific T helper responses by limiting cell proliferation and cytokine secretion (9). A study at Massachusetts General Hospital demonstrated that PD-L1 blockade can lead to enhanced HIV-specific CD4 T-cell proliferation as well as effector functions (11). However, clinical trials using PD-1 and PD-L1 inhibitors often exclude HIV-infected patients as there are hypothesized risks that these checkpoint inhibitors may exacerbate autoimmune diseases and chronic viral infections (12).
There has been a case report of a patient with HIV and melanoma who underwent treatment with the PD-1 inhibitor pembrolizumab (12). In this case, there was no exacerbation of his underlying well-controlled HIV with viral load remaining undetectable (12). The majority of clinical trials testing PD-1 inhibitors in melanoma patients excluded those with HIV (13,14). On clinicaltrials.gov there were 97 studies testing PD-1 or PDL-1 monoclonal antibodies and 12 of these studies do not specifically list HIV in the exclusion criteria (5). Nivolumab as a potential immunotherapy option in HIV-infected patients still needs to be further studied.
Conclusions
Here, we report a case of a patient with HIV and metastatic, squamous NSCLC who had a durable CR to nivolumab without adverse effect on the control of his HIV. This case illustrates the potential of nivolumab in HIV-infected patients. Exploring the use of nivolumab in cancer patients with HIV could be an area of future research.
Acknowledgements
None.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sundar R, Cho BC, Brahmer JR, et al. Nivolumab in NSCLC: latest evidence and clinical potential. Ther Adv Med Oncol 2015;7:85-96. 10.1177/1758834014567470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macatangay BJ, Rinaldo CR. PD-1 blockade: A promising immunotherapy for HIV? Cellscience 2009;5:61-5. [PMC free article] [PubMed] [Google Scholar]
- 5.Available online: https://clinicaltrials.gov/
- 6.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kazandjian D, Suzman DL, Blumenthal G, et al. FDA Approval Summary: Nivolumab for the Treatment of Metastatic Non-Small Cell Lung Cancer With Progression On or After Platinum-Based Chemotherapy. Oncologist 2016;21:634-42. 10.1634/theoncologist.2015-0507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabmeier-Pfistershammer K, Steinberger P, Rieger A, et al. Identification of PD-1 as a unique marker for failing immune reconstitution in HIV-1-infected patients on treatment. J Acquir Immune Defic Syndr 2011;56:118-24. 10.1097/QAI.0b013e3181fbab9f [DOI] [PubMed] [Google Scholar]
- 10.Velu V, Shetty RD, Larsson M, et al. Role of PD-1 co-inhibitory pathway in HIV infection and potential therapeutic options. Retrovirology 2015;12:14. 10.1186/s12977-015-0144-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porichis F, Kwon DS, Zupkosky J, et al. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood 2011;118:965-74. 10.1182/blood-2010-12-328070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davar D, Wilson M, Pruckner C, et al. PD-1 Blockade in Advanced Melanoma in Patients with Hepatitis C and/or HIV. Case Rep Oncol Med 2015;2015:737389. 10.1155/2015/737389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. 10.1056/NEJMoa1305133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908-18. 10.1016/S1470-2045(15)00083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]


