Abstract
Background
Prolonged air leak and high-volume pleural drainage are the most common causes for delays in chest tube removal following lung resection. While digital pleural drainage systems have been successfully used in the management of post-operative air leak, their effect on pleural drainage and inflammation has not been studied before. We hypothesized that digital drainage systems (as compared to traditional analog continuous suction), using intermittent balanced suction, are associated with decreased pleural inflammation and postoperative drainage volumes, thus leading to earlier chest tube removal.
Methods
One hundred and three [103] patients were enrolled and randomized to either analog (n=50) or digital (n=53) drainage systems following oncologic lung resection. Chest tubes were removed according to standardized, pre-defined protocol. Inflammatory mediators [interleukin-1B (IL-1B), 6, 8, tumour necrosis factor-alpha (TNF-α)] in pleural fluid and serum were measured and analysed. The primary outcome of interest was the difference in total volume of postoperative fluid drainage. Secondary outcome measures included duration of chest tube in-situ, prolonged air-leak incidence, length of hospital stay and the correlation between pleural effusion formation, degree of inflammation and type of drainage system used.
Results
There was no significant difference in total amount of fluid drained or length of hospital stay between the two groups. A trend for shorter chest tube duration was found with the digital system when compared to the analog (P=0.055). Comparison of inflammatory mediator levels revealed no significant differences between digital and analog drainage systems. The incidence of prolonged post-operative air leak was significantly higher when using the analog system (9 versus 2 patients; P=0.025). Lobectomy was associated with longer chest tube duration (P=0.001) and increased fluid drainage when compared to sub-lobar resection (P<0.001), regardless of drainage system.
Conclusions
Use of post-lung resection digital drainage does not appear to decrease pleural fluid formation, but is associated with decreased prolonged air leaks. Total pleural effusion volumes did not differ with the type of drainage system used. These findings support previously established benefits of the digital system in decreasing prolonged air leaks, but the advantages do not appear to extend to decreased pleural fluid formation.
Keywords: Lung cancer, pleural drainage, inflammation, digital, analog, resection, chest tube, cytokine
Introduction
Pleural fluid is continuously formed in the pleural cavity, where a constant balance between formation and absorption is physiologically maintained (1). Following lung resection, normal fluid equilibrium is disrupted resulting in a significant increase in the formation and accumulation of pleural fluid (2). Excessive pleural fluid production is influenced by multiple factors, such as increased pleural permeability and intra-thoracic hydrostatic pressure, decreased fluid resorption, and the degree of post-operative inflammation (1,3).
The accumulation of pleural effusion after lung resection may induce dyspnea and lead to atelectasis and pneumonia. Based on anecdotal experience, it is generally accepted that negative pressure suction is required for effective drainage of post-operative effusions, adequate control of parenchymal air leaks, and expansion of the remaining lung (4,5). Two different pleural suction modalities are approved for use in Canada: a traditional analog constant suction system and a digital intermittent suction system. The traditional analog system applies a fixed and constant amount of negative suction, without any capacity for feedback between the chest cavity and the drainage system. Continuous suction of the pleural space without feedback increases the pressure gradient for fluid filtration across the pleural membrane and can potentiate a local inflammatory response. Recent research has demonstrated an association between increased pleural inflammation and pleural effusion (6,7). Conversely, the digital suction system maintains a pre-specified intra-pleural negative pressure by applying intermittent negative suction, depending on real-time measurements of intra-pleural pressures (8). While previous studies have evaluated the effect of digital system on post-operative air leak outcomes, there has been no prior research evaluating the effect on pleural fluid volumes or inflammatory mediators. We hypothesize that, compared to constant negative suction applied by the analog system; intermittent digital negative suction might lead to decreased local inflammation and earlier removal of chest tubes due to decreased post-resection pleural fluid formation.
Methods
Between April and November 2013, patients undergoing lung resection for primary or secondary lung malignancies at a tertiary thoracic surgery center were screened for study enrollment. Patients were included in the trial if they were 18 years of age or older undergoing lung resection via thoracotomy or video-assisted thoracoscopic surgery (VATS). Anatomic (segmentectomy, lobectomy and bilobectomy) and non-anatomic wedge resection were included. Exclusion criteria consisted of: resections requiring pneumonectomy and/or chest wall resection; history of chronic cardiac, renal or hepatic dysfunction; excessive intraoperative adhesions requiring adhesiolysis; prior ipsilateral lung surgery; neoadjuvant chemotherapy or radiation; and failure for provide consent. Each patient had one or two 28-French chest tubes placed at the completion of the index operation. When two chest tubes were placed (for large anatomic resections—lobectomy and bilobectomy), one was curved and positioned along the diaphragm and the other straight tube was placed along the posterior mediastinum towards the apex of the chest. The quantity of pleural fluid drained within the first hour postoperatively was documented but not included in outcome calculations, given that it likely represents intra-operative irrigation fluid.
Study participants were randomized to one of two intervention arms at the conclusion of the index operation using a sealed envelope method with random blocks of 4 and 6 following a 1:1 allocation ratio, stratified by participating surgeon. Patients in the control arm had chest tubes connected to the traditional analog Express (Atrium® Medical Corporation, New Hampshire, USA) underwater pleural drainage system, connected to −20 cm H2O of negative pressure wall suction. Patients in the Intervention arm had chest tubes connected to the digital Thopaz pleural drainage system (Medela International, Baar, Switzerland), set to intermittent negative suction to maintain a pleural negative pressure of −20 cm H2O. The latter system only utilized intermittent suction (and not constant suction) to maintain a negative intra-pleural pressure. Due to the nature of the intervention, only outcomes assessors were blinded to patient allocation.
Criteria for chest tube removal were defined a priori, and included the absence of a measurable air leak (defined as drainage of <40 mL/min over 8 hours in the digital drainage arm, and less than grade 2 based on the previously reported Cerfolio scale for analog drainage systems) (9), pleural fluid drainage of <350 mL per 24 hours or <150 mL within the last registered 8 hours, and complete lung expansion on chest X-ray. Patients were discharged from hospital when they met standard practice discharge criteria and were seen in clinic at 28 days post-operatively for study measurements, and subsequently at 90 days. Fluid output was recorded in 8 hour intervals starting one hour postoperatively and until chest tube removal.
The primary outcome of interest was total quantity of pleural drainage until chest tube removal. Secondary outcomes included: duration of chest tube in situ, hospital length of stay (LOS), 90-day mortality and postoperative morbidity [as defined by the Ottawa Thoracic Morbidity and Mortality classification system (10)], rate of re-intervention (thoracentesis or chest tube insertion), 30-day hospital readmission and pleural inflammatory maker levels interleukin-1B (IL-1B), IL-6, IL-8 and tumour necrosis factor-alpha (TNF-α). Results were also analysed based on surgical approach (thoracotomy vs. thoracoscopy), type of resection (anatomic vs. wedge resection)
Pleural fluid analysis was conducted using ELISA (R&D Systems Complete) and cytology evaluations. Levels were compared to baseline serum values. Samples were coded and technicians were blinded to subject allocation. Cell counts were performed in non-dispersed fluid in standard Neubauer counting chambers. Collected fluid samples were spun at 4,000 rpm for 15 min in aliquot tubes and the supernatant was stored in clearly labeled eppendorf tubes at −70 °C for measurement of intrapleural cytokine levels. Multiplex assays using validated beads for IL-6, Il-8, TNF-α, and IL-1B were used to quantify cytokine levels respectively (Meso Scale Diagnostics, Human Proinflammatory 4-Plex II multiplex panel; Rockville, MD, USA).
Based on institutional estimates of postoperative pleural drainage of 1,000±350 mL per patient, a desired 20% reduction, with 80% power and an α-value of 0.05; 49 patients were requited in each arm. Categorical data was reported as proportions and continuous data as means (standard deviation) or medians (range). Unadjusted comparisons of continuous outcome measures were made using student’s t-test or Wilcoxon rank-sum test (after testing for normal distribution). Categorical outcome measures were compared using chi-square or Fisher’s exact test. A two-sided P value of 0.05 was considered statistically significant. Data was analyzed using SPSS for Windows, Version 20.0 (SPSS, Chicago, IL, USA) statistical software. The trial was registered with clinicatrials.gov under trial number NCT01776372, and was approved by the Hamilton Integrated Research Ethics Board (No. 12-3800). Written informed consent was obtained from study patients.
Results
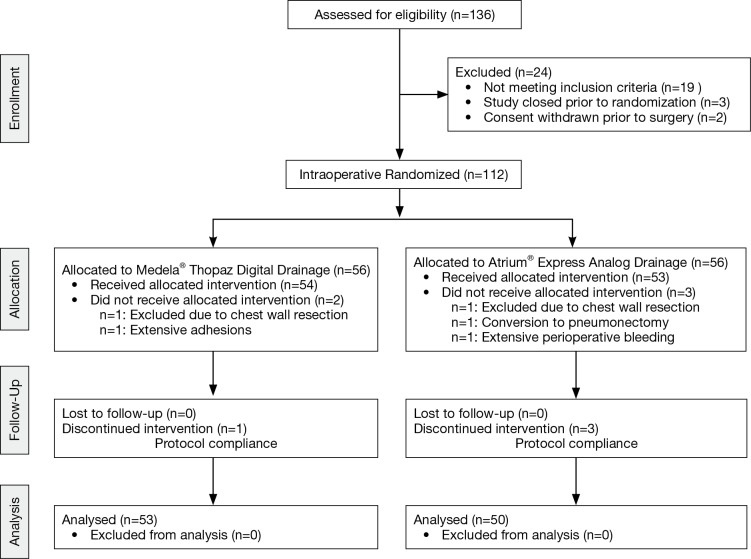
Of 136 patients assessed for eligibility, 24 were excluded due to specific exclusion criteria (n=19), inability to randomize (n=3) and withdrawal of consent prior to surgery (n=2). A total 112 patients were randomized, 56 allocated to the analog drainage arm and 56 randomized to the digital drainage group. Nine patients were excluded peri-operatively due to post-operative bleeding (n=1), unanticipated chest wall resection (n=2), unplanned pneumonectomy (n=1), extensive adhesions (n=1), and noncompliance with study protocol (n=4). (Figure 1) Ultimately, 103 patients were included in the final analysis (50 patients in the analog arm and 53 patients in the digital arm).
Figure 1.
CONSORT diagram of study population.
There was no statistically significant difference in baseline characteristics between both groups, including age, smoking history, extent of resection, surgical approach and use of non-steroidal anti-inflammatory drugs (NSAIDs) (Table 1). Indications for lung resection were similar for both group, and included: primary lung cancer (analog n=36, digital n=45), lung metastases (analog n=9, digital n=7) and benign conditions (analog n=6, digital n=1). Follow-up was completed for all randomized patients.
Table 1. Patient characteristics.
| Study variable | Drainage system, n (%) | P value | |
|---|---|---|---|
| Atrium (Analog) | Thopaz (Digital) | ||
| Sample size | N=50 | N=53 | |
| Gender | 0.488 | ||
| Male | 27 (51.9) | 25 (48.1) | |
| Female | 23 (45.1) | 28 (54.9) | |
| Age, mean, SD | 64.8, 10.6 | 68.5, 10.3 | 0.077 |
| Previous cancer | 0.653 | ||
| Yes | 22 (51.2) | 21 (48.8) | |
| No | 28 (46.7) | 32 (53.3) | |
| Cardiac diseases (MI or arrhythmia) | 0.850 | ||
| Yes | 15 (50.0) | 15 (50.0) | |
| No | 35 (47.9) | 38 (52.1) | |
| COPD | 0.480 | ||
| Yes | 12 (42.9) | 16 (57.1) | |
| No | 38 (50.7) | 37 (49.3) | |
| Obstructive sleep apnea | 0.111 | ||
| Yes | 3 (100.0) | 0 | |
| No | 47 (47.0) | 53 (53.0) | |
| Hypercholesterol | 0.283 | ||
| Yes | 8 (38.1) | 13 (61.9) | |
| No | 42 (51.2) | 40 (48.8) | |
| Diabetes | 0.474 | ||
| Yes | 6 (40.0) | 9 (60.0) | |
| No | 44 (50.0) | 44 (50.0) | |
| VTE | 0.243 | ||
| Yes | 0 | 3 (100.0) | |
| No | 50 (50.0) | 50 (50.0) | |
| Current NSAID use | 1.00 | ||
| Yes | 2 (40.0) | 3 (60.0) | |
| No | 48 (49.0) | 50 (51.0) | |
| Smoking | 0.195 | ||
| Current | 14 (53.8) | 12 (46.2) | |
| Quit less than 1 year ago | 8 (66.7) | 4 (33.3) | |
| Quit more than 1 year ago | 14 (35.9) | 25 (64.1) | |
| Never | 14 (53.8) | 12 (46.2) | |
| Smoking pack years | 0.388 | ||
| 0 | 14 (53.8) | 12 (46.2) | |
| <10 | 1 (20.0) | 4 (80.0) | |
| 10–20 | 5 (33.3) | 10 (66.7) | |
| 20–40 | 15 (48.4) | 16 (51.6) | |
| >40 | 15 (57.7) | 11 (42.3) | |
| FEV1 (%) | 0.793 | ||
| >80 | 37 (49.3) | 38 (50.7) | |
| <80 | 13 (46.4) | 15 (53.6) | |
| DLCO (%) | 0.399 | ||
| >80 | 16 (55.2) | 13 (44.8) | |
| <80 | 34 (45.9) | 40 (54.1) | |
| BMI | 0.407 | ||
| <18.5 | 0 | 2 (100) | |
| 18.5–25 | 15 (50.0) | 15 (50.0) | |
| 25–30 | 21 (55.3) | 17 (44.7) | |
| >30 | 14 (42.4) | 19 (57.6) | |
| Surgeon identification | 0.853 | ||
| 1 | 20 (48.8) | 21 (51.2) | |
| 2 | 12 (44.4) | 15 (55.6) | |
| 3 | 18 (51.4) | 17 (48.6) | |
| Procedure | 0.664 | ||
| Lobectomy | 32 (49.2) | 33 (50.8) | |
| Bilobectomy | 4 (80.0) | 1 (20.0) | |
| Segmentectomy | 6 (42.9) | 8 (57.1) | |
| Single wedge | 4 (40.0) | 6 (60.0) | |
| Multiple wedge | 4 (44.4) | 5 (55.6) | |
| Procedure type | 0.363 | ||
| Open | 29 (52.7) | 26 (47.3) | |
| VATS | 21 (43.8) | 27 (56.2) | |
| Resection type | 0.394 | ||
| Lobe | 36 (51.4) | 34 (48.6) | |
| Sublobar | 14 (42.4) | 19 (57.6) | |
| Side of resection | 0.286 | ||
| Left | 26 (54.2) | 22 (45.8) | |
| Right | 24 (43.6) | 31 (56.4) | |
NSAID, non-steroidal anti-inflammatory drug; VATS, video-assisted thoracoscopic surgery.
Within the entire patient cohort, there were two postoperative deaths (1.9% mortality). One patient developed a post-operative broncho-pleural fistula and empyema and passed away due to respiratory compromise, and the other suffered a massive pulmonary embolus post-hospital discharge. Complications occurred in 19.4% of all cases. Five patients were discharged on home oxygen for a duration of 1 to 4 weeks and seven patients had prolonged air leak (>5 days). One patient in the analog group was readmitted with a pneumothorax requiring chest tube insertion, while one patient in the digital arm was readmitted with radiographic evidence of a pleural effusion and was treated with insertion of a small bore chest tube.
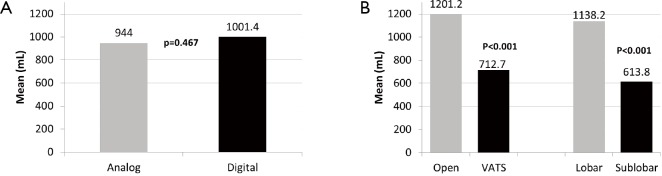
There was no significant difference with regards to mean volume of total pleural drainage using the analog vs. digital drainage systems (analog =944.0 mL vs. digital =1,001.4 mL, P=0.467). Secondary analysis demonstrated significant differences in total drainage volumes between open thoracotomy and VATS procedures, as well as lobar versus sub-lobar resection. Thoracotomy resulted in significantly larger amounts of chest tube drainage when compared to VATS (1,201.2 vs. 712.7 mL, P<0.001). Similarly, lobar resections were associated with greater quantity of post-operative pleural fluid when compared to sub-lobar resections (1,138.2 vs. 613.8 mL, P<0.001) (Figure 2). These differences existed regardless of the type of system used.
Figure 2.
Total drainage volume. (A) Total volume of pleural fluid drained in analog and digital study arms; (B) total volume of pleural fluid drainage between open versus VATS and lobar versus sub-lobar resection stratifications. VATS, video-assisted thoracoscopic surgery.
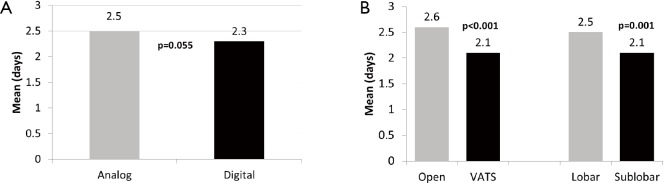
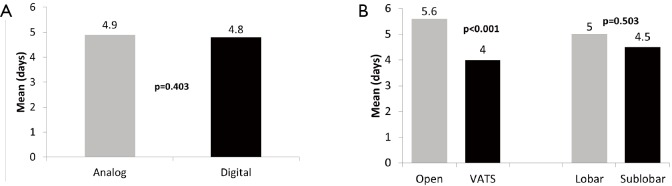
A trend towards significantly shorter chest tube duration was found with the digital arm at 2.3 days, when compared to the analog arm at 2.5 days (P=0.055). A significant difference in chest tube duration was also noted when comparing open vs. VATS resections (P<0.001) and lobar versus sub-lobar procedures, with a shorter duration in favour of VATS procedures (2.6 vs. 2.1 days, P=0.001) and sub-lobar resections (2.5 vs. 2.1 days, P=0.001), respectively (Figure 3). Prolonged air leak greater than 5 days post-operatively was seen in 11 patients overall (9 in the analog arm; and 2 in the digital arm). The incidence of prolonged post-operative air leak was significantly higher when using the analog system compared to the digital system (P=0.025). There was no significant difference detected with regard to length of hospital stay when comparing digital versus non-digital drainage systems, with a mean of 4.9 days reported in the analog group and 4.8 days in the digital group (P=0.403). Patients undergoing an open procedure, regardless of drainage system used, had a significant longer hospital stay than with VATS approach (5.6 vs. 4.0 days, P<0.001) (Figure 4).
Figure 3.
Time to chest tube removal. (A) Duration of time (days) chest tubes remained in situ for analog and digital study arms; (B) duration of time (days) chest tubes remained in situ between open versus VATS and lobar versus sub-lobar resections. VATS, video-assisted thoracoscopic surgery.
Figure 4.
Length of stay. (A) Length of hospital stay (days) in analog and digital study arms; (B) length of hospital stay (days) between open versus VATS and lobar versus sublobar resections. VATS, video-assisted thoracoscopic surgery.
Analysis of pleural inflammatory mediators demonstrated elevated IL-8 and TNF-α levels with the usage of analog compared to digital drainage systems on the first postoperative day (908.12 vs. 575.67 pg/mL, P=0.009; and 3.10 versus 1.21 pg/mL digital, P=0.001). A significant increase in pleural fluid IL-8 concentration (790.20 pg/mL) was seen between POD 2 and POD3 in the digital group however. Conversely, pleural IL-8 levels decreased in the analog arm to 588.58 pg/mL in the same time period (P=0.034). Comparative baseline serum analysis demonstrated no significant difference in inflammatory markers between the two groups. Altogether, no differences were found between the two groups when comparing inflammatory mediators, regardless of the type of drainage system used, extent of resection and open resections vs. VATS procedures.
Discussion
This study is the first randomized prospective trial comparing digital and analog drainage systems with regards to pleural effusion and inflammation. To date, drainage systems have only been compared with relation to postoperative air leak (11-15). Whilst this is an important aspect of the decision to remove chest tubes, the volume of fluid extracted is another major determinant, that continues to garner increased attention amongst research circles (2,16-18). Pleural fluid formation is greatly influenced by pleural permeability and shift in oncotic and hydrostatic pressures. Perhaps this could be mediated via an inflammatory cascade potentiated by a release of cytokines and influenced by intrapleural pressure controls. Our trial attempts to evaluate whether intermittent (as opposed to traditional continuous) controlled suctioning provided via the mechanism of digital drainage decreases pleural irritation and inflammation and therefore decreases the amount of pleural drainage.
Previously published reports have demonstrated that digital drainage systems are superior in reducing the incidence of post-operative prolonged air leak (12-14). Several articles describe the advantage of a digital system in eliminating the inter-observer differences related to air leak grading, as the system quantifies the precise amount of air leak (13-15). Moreover, the effect of continuous vs. intermittent suction on postoperative air leak may also account for differences in air leak duration. Our findings do not support an association between the type of drainage system used and the total volume of pleural drainage or cytokine release. Recently, Pompili et al. reported an international multicenter prospective randomised trial, evaluating the impact of a digital versus traditional drainage systems on chest tube removal and patient satisfaction (19). The use of a digital system resulted in significantly less prolonged air leaks (P=0.005), shorter duration of chest tube in situ (P<0.001), shorter length of hospital stay (P=0.001) and better mobility (P=0.008). Pleural fluid formation was not compared between the two systems.
Our study showed similar results regarding air leaks with the added value of assessing pleural drainage as part of the outcome analysis. The rate of prolonged air leak was statistically lower in the digital system, while chest tube duration as well as overall LOS did not reach statistical significance. Since the trends for chest tube duration and LOS were similar to the findings reported by Pompili et al., it is possible that a small sample size may contribute to a lack of statistical significance in our report. Our sample size calculations were based on quantity of postoperative pleural fluid drainage and not these other metrics. While this study was not powered to measure differences relating to extent of resection and type of surgical approach, the results pertaining to those specific outcomes are consistent with those reported in the literature (20,21).
The inflammatory process plays a significant role in the creation of pleural effusion. Inflammation is a balance between pro- and anti-inflammatory mediators and appears to play a large role in tumour behavior and physiological responses to surgery. Lung cancer tumour cells, as with other malignant tumours, have higher baseline levels of TNF-α, IL-1 and IL-6 prior to surgery compared to non-malignant controls, indicating that inflammatory changes are innate to the malignant process (22,23). Inflammation has also been significantly linked to physiological surgical stress in lung surgery, and an increased risk of post-surgical complications (24,25). An increase in TNF-α levels was found to increase pleural vascular permeability in a murine model, a key factor in the accumulation of fluid in the pleura (22). Takenaka et al. found that patients undergoing thoracic surgery who developed symptoms of systemic inflammatory response syndrome (with associated elevation of IL-6 concentrations) were admitted 4 days longer with increased pleural drainage volumes (7). Minimally invasive surgical thoracic approaches were found to be associated with lower cytokine concentrations in comparison with open procedures, indicating that less trauma possibly triggers less inflammation, with improved patients outcomes (24-26). The results of this trial did not demonstrate an association between the type of drainage system and the corresponding early levels of intrapleural cytokines, disproving the association between dynamic intermittent intrapleural suction control with digital drainage and the potentiation of a decreased inflammatory cascade.
Prospective trials evaluating interventions in decreasing postoperative pleural drainage are sparse. This report provides the advantage of being the first of its kind, with effective randomization, study design, and complete follow-up. Moreover, the methodology and techniques used to quantify pleural inflammation and cytokine levels can serve as a tool for future research. Despite the novel approach attempting to evaluate the effect of intermittent vs. constant suction on post-operative pleural inflammation and effusion, this study has several limitations. Firstly, the sample size calculations (which were appropriately met) were based on total volume of pleural drainage, and not on other metrics such as rate of re-intervention or incidence of elevated postoperative fluid drainage. While the latter metrics have increased clinical utility, the relative lower incidence would require tremendously high sample sizes, not feasible for the purpose of this study. Secondly, the total drainage volume is correlated to the duration of chest tube in situ, and the two variables could possibly demonstrated confounding effects. We attempted to control for this by using standardized indications for chest tube removal, and ensuring an aggressive approach towards early chest tube discontinuation. Thirdly, the reported LOS for both open and thoracoscopic procedures is slightly higher than would be expected—particularly given the short duration of chest tube in situ. This is likely the result of logistical issues with coordination of patient discharge and disposition planning. Lastly, the biologic plausibility of the effect of intermittent vs. constant suction on pleural effusion via a decreased inflammatory cascade is largely deductive, based on limited literature. This study served the dual purpose of evaluating the biologic plausibility and the effect to dynamic digital drainage on post-lung resection pleural fluid drainage.
Conclusions
Postoperative digital pleural drainage has been proven to offer a wide array of benefits to patients and healthcare institutions. The advantage of decreased prolonged air leak and chest tube duration does not appear extend to decreased postoperative pleural inflammation and effusion. More research is require to accurately describe the inflammatory mechanisms in the pleural space following lung resection.
Acknowledgements
This investigator-initiated trial was funded by the Division of Thoracic Surgery, McMaster University, with an additional grant designated for pleural cytokine analysis being provided by Medela AG.
Ethical Statement: The study was approved by the Hamilton Integrated Research Ethics Board (No. 12-3800) and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: This study was presented on 22nd European Conference on General Thoracic Surgery, Copenhagen, Denmark, 15th−18th June, 2014. Presented June 17th, 2014.
References
- 1.Mehran RJ, Deslauriers J. Anatomy and Physiology of the Pleural Space. In: Deslauriers J, Meyerson SL, Patterson A, et al. editors. Pearson’s Thoracic and Esophageal Surgery, 3rd Edition. Philadelphia: Elsevier, 2008:1001-7. [Google Scholar]
- 2.Brunelli A, Beretta E, Cassivi SD, et al. Consensus definitions to promote an evidence-based approach to management of the pleural space. A collaborative proposal by ESTS, AATS, STS, and GTSC. Eur J Cardiothorac Surg 2011;40:291-7. 10.1016/j.ejcts.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 3.Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG ZOO30 trial. Ann Thorac Surg 2006;81:1013-9. 10.1016/j.athoracsur.2005.06.066 [DOI] [PubMed] [Google Scholar]
- 4.Deng B, Tan QY, Zhao YP, et al. Suction or non-suction to the underwater seal drains following pulmonary operation: meta-analysis of randomised controlled trials. Eur J Cardiothorac Surg 2010;38:210-5. 10.1016/j.ejcts.2010.01.050 [DOI] [PubMed] [Google Scholar]
- 5.Coughlin SM, Emmerton-Coughlin HM, Malthaner R. Management of chest tubes after pulmonary resection: a systematic review and meta-analysis. Can J Surg 2012;55:264-70. 10.1503/cjs.001411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szczesny TJ, Slotwinski R, Stankiewicz A, et al. Interleukin 6 and interleukin 1 receptor antagonist as early markers of complications after lung cancer surgery. Eur J Cardiothorac Surg 2007;31:719-24. 10.1016/j.ejcts.2007.01.027 [DOI] [PubMed] [Google Scholar]
- 7.Takenaka K, Ogaa E, Wada H, et al. Systemic inflammatory response syndrome and surgical stress in thoracic surgery. J Crit Care 2006;21:48-53; discussion 53-5. 10.1016/j.jcrc.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 8.Medela USA. Thopaz digital chest drainage system: Instructions for use. Accessed 12 Mar 2015. Available online: http://www.medela-healthcare.us/healthcare/products/cardiothoracic-drainage
- 9.Cerfolio RJ. Chest tube management after pulmonary resection. Chest Surg Clin N Am 2002;12:507-27. 10.1016/S1052-3359(02)00015-7 [DOI] [PubMed] [Google Scholar]
- 10.Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42. 10.1016/j.athoracsur.2010.05.014 [DOI] [PubMed] [Google Scholar]
- 11.Brunelli A, Varela G, Refai M, et al. A scoring system to predict the risk of prolonged air leak after lobectomy. Ann Thorac Surg 2010;90:204-9. 10.1016/j.athoracsur.2010.02.054 [DOI] [PubMed] [Google Scholar]
- 12.Filosso PL, Ruffini E, Solidoro P, et al. Digital air leak monitoring after lobectomy for primary lung cancer in patients with moderate COPD: can a fast-tracking algorithm reduce postoperative cost and complications? J Cardiovasc Surg (Torino) 2010;51:429-33. [PubMed] [Google Scholar]
- 13.Brunelli A, Salati M, Al Refai M, et al. Evaluation of a new chest tube removal protocol using digital air leak monitoring after lobectomy: a prospective randomised trial. Eur J Cardiothorac Surg 2010;37:56-60. 10.1016/j.ejcts.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 14.Cerfolio RJ, Bryant AS. The benefits of continuous and digital air leak assessment after pulmonary resection: a prospective study. Ann Thorac Surg 2008;86:396-401. 10.1016/j.athoracsur.2008.04.016 [DOI] [PubMed] [Google Scholar]
- 15.Varela G, Jimenez MJ, Novoa NM, et al. Postoperative chest tube management: measuring air leak using an electronic device decreases variability in the clinical practice. Eur J Cardiothorac Surg 2009;35:28-31. 10.1016/j.ejcts.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 16.Bjerregaard LS, Jensen K, Petersen RH, et al. Early chest tube removal after video-assisted thoracic surgery lobectomy with serous fluid production up to 500 ml/day. Eur J Cardiothorac Surg 2014;45:241-6. 10.1093/ejcts/ezt376 [DOI] [PubMed] [Google Scholar]
- 17.Cerfolio RJ, Bryant AS. The management of chest tubes after pulmonary resection. Thorac Surg Clin 2010;20:399-405. 10.1016/j.thorsurg.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 18.Hristova R, Pompili C, Begum S, et al. An aggregate score to predict the risk of large pleural effusion after pulmonary lobectomy. Eur J Cardiothorac Surg 2015;48:72-6 10.1093/ejcts/ezu413 [DOI] [PubMed] [Google Scholar]
- 19.Pompili C, Detterbeck F, Papagiannopoulos K, et al. Multicenter International Randomized Comparison of Objective and Subjective Outcomes Between Electronic and Traditional Chest Drainage Systems. Ann Thorac Surg 2014;98:490-6; discussion 496-7. 10.1016/j.athoracsur.2014.03.043 [DOI] [PubMed] [Google Scholar]
- 20.Grogan EL, Jones DR. VATS lobectomy is better than open thoracotomy: what is the evidence for short-term outcomes? Thorac Surg Clin 2008;18:249-58. 10.1016/j.thorsurg.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 21.Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16. 10.1016/j.athoracsur.2008.07.009 [DOI] [PubMed] [Google Scholar]
- 22.Stathopoulos GT, Kollintza A, Moschos C, et al. Tumor necrosis factor-α promotes malignant pleural effusion. Cancer Res 2007;67:9825-34. 10.1158/0008-5472.CAN-07-1064 [DOI] [PubMed] [Google Scholar]
- 23.Arias-Díaz J, Vara E, Torres-Melero J, et al. Nitrite/nitrate and cytokine levels in bronchoalveolar lavage fluid of lung cancer patients. Cancer 1994;74:1546-51. [DOI] [PubMed] [Google Scholar]
- 24.Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: A comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. 10.1016/S0003-4975(01)02804-1 [DOI] [PubMed] [Google Scholar]
- 25.Friscia ME, Zhu J, Kolff JW, et al. Cytokine response is lower after lung volume reduction through bilateral thoracoscopy versus sternotomy. Ann Thorac Surg 2007;83:252-6. 10.1016/j.athoracsur.2006.08.012 [DOI] [PubMed] [Google Scholar]
- 26.Yim AP, Wan S, Lee TW, et al. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg 2000;70:243-7. 10.1016/S0003-4975(00)01258-3 [DOI] [PubMed] [Google Scholar]