Abstract
Background
Functional condition is crucial for operability of patients with lung cancer and/or chronic respiratory diseases. The aim of the study was to measure changes of functional and quality of life parameters in terms of the effectiveness of perioperative pulmonary rehabilitation (PR).
Methods
A total of 208 COPD patients (age: 63±9 years, man/woman: 114/94, FEV1: 62±14%pred) participated in a perioperative PR program. The indication was primary lung cancer in 72% of the patients. The 68 patients participated in preoperative (PRE) rehabilitation, 72 in a pre- and postoperative rehabilitation (PPO) and 68 patients only in postoperative rehabilitation (POS). PR program included respiratory training techniques, individualized training and smoking cessation. Lung function tests, 6 minutes walking distance (6MWD) were measured before and after the rehabilitation. Quality of life tests [COPD Assessment Test (CAT) and Modified Medical Research Council Dyspnoea Scale (mMRC)] were evaluated as well.
Results
There was a significant improvement in FEV1 (PRE: 64±16 vs. 67±16%pred; PPO: 60±13 vs. 66±13%pred before the operation, 48±13 vs. 52±13%pred after the operation; POS: 56±16 vs. 61±14%pred, P<0.05) and 6MWD (PRE: 403±87 vs. 452±86 m; PPO: 388±86 vs. 439±83 m before, 337±111 vs. 397±105 m after the operation; POS: 362±89 vs. 434±94 m, P<0 0001). Significant improvement was detected in FVC, grip strength, mMRC and CAT questionnaires as an effectiveness of PR, also. Average intensive care duration was 3.8±5.2 days with vs. 3.1±3.6 without preoperative PR.
Conclusions
Improvements in exercise capacity and quality of life were seen following PR both before and after thoracic surgery.
Keywords: Perioperative pulmonary rehabilitation (perioperative PR), thoracic surgery, lung cancer, chest physiotherapy, endurance training, chronic obstructive pulmonary disease (COPD)
Introduction
Severe chronic obstructive pulmonary disease (COPD), severely impaired functional capacity, low physical activity, obesity, smoking and comorbidities are significant factors for risk stratification before thoracic operation (1-3). Preoperative rehabilitation can improve functional condition. Improvement of cardiovascular function, metabolism, muscle-function and lung mechanics can be achieved by pulmonary rehabilitation (4).
More interest is focusing the non-pharmacological interventions, such as exercise and improving functional capacity during, before and after thoracic surgery and cancer treatment. Exercise is proved to be successful intervention that makes physical and psychological health better in different cancer states, including lung cancer (5,6). Cardiopulmonary rehabilitation is part of the management of COPD. It can reduce symptoms and minimize the exacerbation rate of the disease (5,6). Smoking cessation is important in terms of reduction of postoperative complications as well (7).
It has been shown, that pulmonary rehabilitation (PR) can improve exercise capacity and Health Related Quality of Life (HRQOL), and can reduce the main symptoms such as dyspnoea, fatigue and depression (8). There is limited availability of specific exercise intervention for patients with lung cancer (8). It is important to define the optimal design of exercise intervention that will be feasible, acceptable and positively affect under perioperative conditions.
Our aim was to investigate the effectiveness of perioperative rehabilitation by monitoring the parameters of lung function, lung mechanics, chest kinematics, exercise capacity and quality of life.
Methods
Study subjects
A total of 208 COPD patients participated in the perioperative pulmonary rehabilitation program in connection with thoracic operations between 2012 and 2015 at Department of Thoracic Surgery in National Koranyi Institute for Pulmonology, Hungary. Indication of the operation was primary lung cancer in 150, pulmonary metastasis in 11, benign disease in 10, infection in 16, other cause in 21 cases. The patient’s characteristic is presented in Table 1. All of the patients gave consent for the study in the Department of Pulmonary Rehabilitation. It was an observational study using the general management of the patient, it was a non-interventional study. There was no significant difference between the groups in terms of patient’s characteristics (Table 1).
Table 1. Patient’s characteristics.
| Characteristics, N=208 | Group PRE, n=68 | Group POS, n=68 | Group PPO, n=72 | Significance |
|---|---|---|---|---|
| Age (years) | 65±6 | 60±11 | 65±7 | NS |
| Male:female | 45:23 (66%:34%) | 35:33 (51%:49%) | 34:38 (47%:53%) | NS |
| BMI (kg/m2) | 27±5 | 25±5 | 26±6 | NS |
| FEV1 (%pred) | 64±16 | 55±16 | 60±13 | NS |
| Hypertension | 46 (68%) | 43 (68%) | 46 (72%) | NS |
| Diabetes | 25 (37%) | 25 (37%) | 23 (32%) | NS |
| Atherosclerosis | 22 (32%) | 23 (34%) | 24 (33%) | NS |
| Pulmonary hypertension | 10 (15%) | 9 (13%) | 8 (11%) | NS |
| Quitting rate of smoking cessation | 52 (76%) | 51 (75%) | 54 (75%) | NS |
Our patients participated in three groups. The 68 patients performed only preoperative pulmonary rehabilitation (PRE). In PPO group, 72 patients performed pre- and postoperative rehabilitation also, and 68 patients had postoperative rehabilitation only (POS) (Figure 1).
Figure 1.
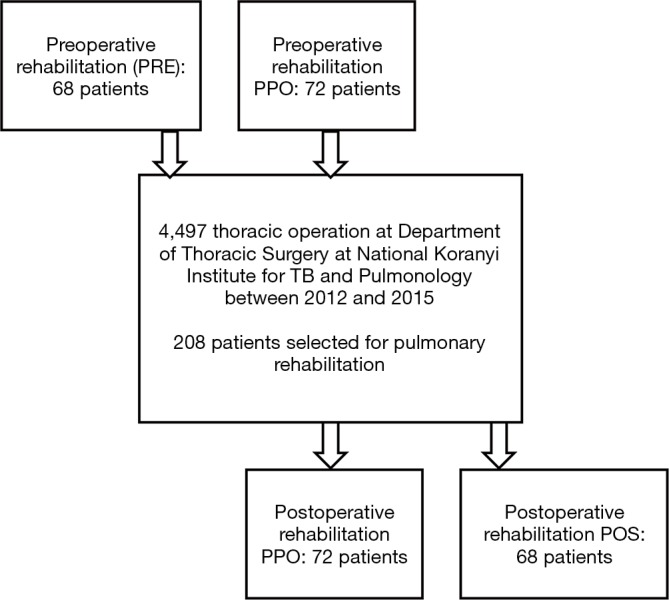
Flow of participants in the perioperative rehabilitation program.
Pulmonary function
According to ATS/ERS guidelines all patients underwent post-bronchodilator pulmonary function testing (Vmax 229 and Autobox 6200, Sensormedics) including spirometry measurements (9). COPD patients inhaled 400 µg salbutamol 20 minutes before testing.
Functional follow-up and quality of life questionnaire
Functional follow-up included measurement of lung functions, 6 minutes walking distance (6MWD) test (10) and quality of life tests such as COPD Assessment Test (CAT) (11) and Modified Medical Research Council Dyspnoea Scale (mMRC) (12).
Personalized training programs
Our pulmonary rehabilitation program includes 30 minutes of respiratory training in the morning, chest wall mobilization, learning the controlled breathing techniques, inhalation, expectoration, improving the psychological condition, smoking cessation and a personalized training. Patients participate an individualized continuous or interval type of cycle- and/or treadmill training for 10–30 minutes, 2–3 times a day at a level of 60–80% of maximal intensity. The duration of the rehabilitation program is 3 weeks (13). The intensity of the training was progressive from 60–80% of peak work rate, the intensity was increased based on maintaining Borg dyspnea scale breathlessness and leg fatigue both on grade No. 7.
Smoking cessation
Smoking cessation is an important part of the perioperative rehabilitation program. Our institute has a special smoking cessation program for the patients once per week for 45 minutes, with help of psychologists (7).
Statistical analysis
Subject’s characteristics, lung function and exercise physiologic variables were compared by paired t-test and non-parametric sign test and Wilcoxon test. Significance was accepted at the P<0.05 level. The distribution around the mean was expressed as ± SD, in tables and the figures also. Distributions were tested for normality by the Kolmogorov-Smirnov test.
Results
Clinically significant improvement was detected in FEV1, FVC, 6MWD, grip strength, in PRE, in PPO before and after the operation and in POS group (Tables 2-4, P<0.05). The level of dyspnoea (measured by the mMRC Dyspnoea Scale) and quality of life (measured by CAT) improved significantly in PRE group, in the PPO group before and after the operation, and in POS group, also (Tables 2-4; P<0.001). There was no significant difference between the result of cancer and non-cancer patients in functional parameters. Average intensive care duration was 3.8±5.2 days with vs. 3.1±3.6 without preoperative pulmonary rehabilitation. The quitting rate was high, and not significantly different between groups (Table 1) (Figures 2,3).
Table 2. Changes in functional parameters as the effectiveness of preoperative pulmonary rehabilitation (PRE group).
| Parameter | PRE (preoperative pulmonary rehabilitation), n=68 | ||
|---|---|---|---|
| Before rehab. | After rehab. | Change, significance | |
| FEV1 | 1.75±0.58 L (63.7±16.0%pred) | 1.90±0.58 L (67.4±16.3%pred) | +5.89% (P=0.0025) |
| FVC | 2.92±0.90 L (85.8±17.6%pred) | 3.12±0.89 L (90.6±16.8%pred) | +5.66% (P=0.0109) |
| 6MWD (m) | 403±87 | 452±86 | +12.07% (P<0.0001) |
| mMRC | 1.0±0.7 | 0.7±0.6 | −31.71% (P=0.0004) |
| Grip strength (kg) | 19.9±14.4 | 21.8±15.5 | +10.02% (P=0.0002) |
| CAT | 8.4±5.3 | 5.4±4.7 | −35.47% (P<0.0001) |
6MWD, 6 minutes walking distance; mMRC, Modified Medical Research Council Dyspnoea Scale; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease.
Table 3. Changes of functional parameters as the effectiveness of postoperative pulmonary rehabilitation (POS group).
| Parameter | POS (postoperative pulmonary rehabilitation), n=68 | ||
|---|---|---|---|
| Before rehab. | After rehab. | Change, significance | |
| FEV1 | 1.50±0.49 L (55.6±16.2%pred) | 1.75±0.61 L (60.8±14.2%pred) | +9.34% (P=0.0044) |
| FVC | 2.19±0.74 L (66.8±21.3%pred) | 2.54±0.83 L (74.9±19.6%pred) | +12.09% (P=0.0001) |
| 6MWD (m) | 362±89 | 434±94 | +19.88% (P<0.0001) |
| mMRC | 1.5±1.0 | 1.0±0.8 | −32.31% (P<0.0001) |
| Grip strength | 19.2±12.3 | 21.2±13.2 | +10.14% (P=0.0008) |
| CAT | 17.6±9.0 | 12.8±8.8 | −27.00% (P<0.0001) |
6MWD, 6 minutes walking distance; mMRC, Modified Medical Research Council Dyspnoea Scale; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease.
Table 4. Changes of functional parameters as the effectiveness of pre- and postoperative pulmonary rehabilitation (PPO group).
| Parameter | PPO (pre- and postoperative pulmonary rehabilitation), n=72 | ||||||
|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative | ||||||
| Before rehab. | After rehab. | Change, significance | Before rehab. | After rehab. | Change, significance | ||
| FEV1 | 1.49±0.53 L (60.1±12.8%pred) | 1.68±0.53 L (66.3±12.9%pred) | +10.39% (P<0.0001) | 1.21±0.43 L (48.4±12.7%pred) | 1.30±0.40 L (51.8±13,0%pred) | +7.14% (P=0.0247) | |
| FVC | 2.57±0.81 L (88.7±14.7%pred) | 2.86±0.82 L (97.9±14.7%pred) | +10.41% (P=0.0001) | 2,.00±0.72 L (63.6±16.1%pred) | 2.13±0.68 L (67.7±17.7%pred) | +6.57% (P=0.1126) | |
| 6MWD (m) | 388±86 | 439±83 | +13.06% (P<0.0001) | 337±111 | 397±105 | +17.74% (P<0.0001) | |
| mMRC | 1.2±1.0 | 0.8±0.8 | −35.30% (P=0.0002) | 1.8±0.9 | 1.4±0.8 | −18.47% (P=0.0017) | |
| Grip strength (kg) | 22.0±12.0 | 23.3±12.8 | +5.69% (P=0.0057) | 20.2±14.1 | 21.1±14.5 | +4.23% (P=0.3658) | |
| CAT | 12.0±6.9 | 8.4±5.5 | −30.08% (P<0.0001) | 16.0±6.2 | 11.4±5.3 | −28.75% (P<0.0001) | |
6MWD, 6 minutes walking distance; mMRC, Modified Medical Research Council Dyspnoea Scale; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease.
Figure 2.
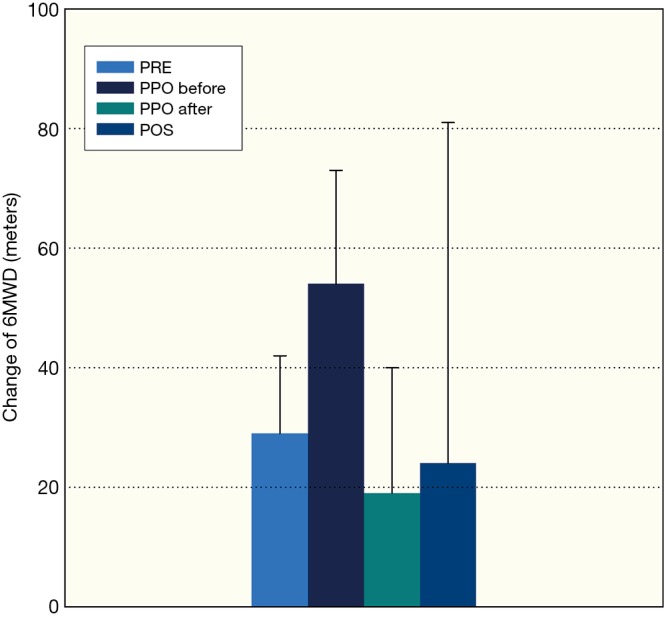
Improvement of maximal exercise capacity measured by 6-minute walking test, PRE-preoperative rehabilitation group, PPO-perioperative rehabilitation group, PPO-before: pulmonary rehabilitation before thoracic surgery, PPO-after: pulmonary rehabilitation after thoracic surgery, POS-postoperative rehabilitation group, mean ± standard deviation were presented in the columns.
Figure 3.
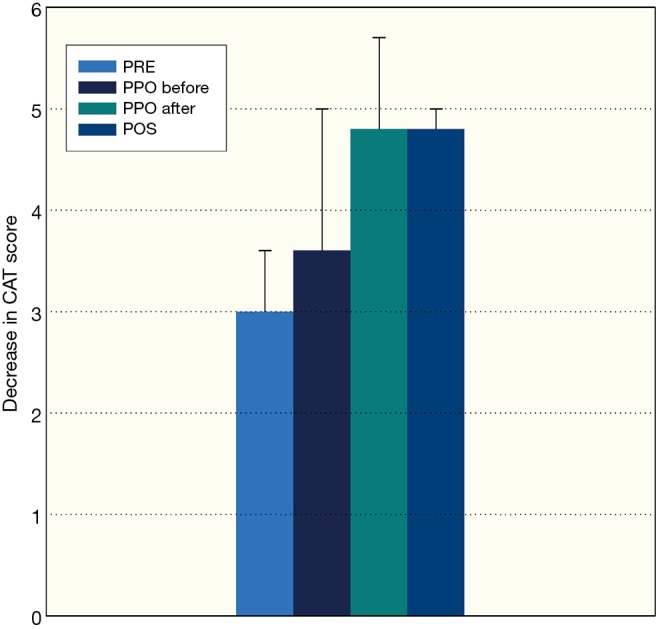
Improvement of quality of life measured by COPD Assessment Test (CAT), PRE-preoperative rehabilitation group, PPO-perioperative rehabilitation group, PPO-before: pulmonary rehabilitation before thoracic surgery, PPO-after: pulmonary rehabilitation after thoracic surgery, POS-postoperative rehabilitation group, mean ± standard deviation were presented in the columns. COPD, chronic obstructive pulmonary disease.
Discussion
Perioperative pulmonary rehabilitation was performed before and/or after thoracic surgery in patients with COPD. Pre- and postoperative pulmonary rehabilitation resulted significant improvement in FEV1, FVC, 6MWD and quality of life questionnaires (CAT, mMRC). There was no significant difference between intensive care duration with or without preoperative pulmonary rehabilitation.
Exercise tolerance is a crucial part of risk stratification before surgical resection. Exercise interventions can lead to improve exercise capacity parallel with reduction of risk in cardiopulmonary function (14). Reduction in post-operative complications and length of hospital stay can be detected as a result of pre-surgical interventions, which may be in connection with increased exercise capacity (14). We detected significant improvement in exercise capacity as a potential predictor of reduction of postoperative complication, but we did not collect the complication rate in this study.
Pulmonary rehabilitation can improve lung mechanics, exercise capacity and quality of life using controlled breathing techniques, chest wall mobilization and specific training modalities. Specialized training modalities are favourable for respiratory and peripheral muscles as well (15). The effectiveness of our rehabilitation program was supported by improvement of lung mechanics, exercise capacity and quality of life.
Clinical data underline that exercise intervention compared with usual care both pre and post-surgery is a safe, feasible and acceptable method, which can increase exercise capacity, reduce postoperative complications and hospital stay (16). Exercise can increase muscle strength and reduce fatigue, as well (16). In the other hand, the improvement in pulmonary function, quality of life, and blood gas analysis is not consistent. Our exercise interventions were safe, feasible and effective for the patients.
As a result of rehabilitation, increased muscle strength and reduced fatigue are important findings. Resistance training can improve muscle strength, highlighting the importance of interventions (17). Exercise intervention suggests that physical exercise can help to reduce fatigue both during and after treatment for cancer including lung cancer (18,19). However, there is a question about the component of rehabilitation against fatigue.
Different types of exercise, aerobic exercise in comparison with resistance exercise alone, may have superior effect for improving exercise capacity and oxygen uptake (VO2 max), but there are not so many data about (17) resistance exercise. Resistance training can result (17) significant improvement in muscle strength. The combination of resistance and aerobic exercise training may result the optimal training program for this population. Our rehabilitation program included strength and endurance part, also.
Endurance training is an important component of pulmonary rehabilitation. Supervised high intensity interval training can be more effective compared to continuous training in healthy subjects. In patients with COPD, these two methods have no significant difference in results (13,20). Both methods have advantages according to self-paced training (13,20). It is important to consider the patient`s functional capacities, significant co-morbidities (like impaired pulmonary hemodynamics) and desaturation index during exercise to find the optimal, personalized training program (20). In our program, patients performed continuous and interval training based on functional capacity and comorbidities.
Improvements in exercise capacity, together with no change in pulmonary function may be an unexpected findings in this type of patients like other pulmonary diseases, for example, COPD and restrictive disorders (21,22). Overall there is a question about the evidence that exercise may or may not improve pulmonary function in this population. We found significant improvement in lung function using these rehabilitation techniques.
Quality of life has a non-significant change in one part of the studies. Preoperative rehabilitation period (23) may result no change in QOL due to a possible ceiling effect. Randomized controlled trials (24-26) for post-operative interventions resulted no significant differences in QOL between groups, however there were certain limitations of the studies. In the other hand, in the study by Arbane et al. (27), there was no data about the adherence to home-based exercise and how much exercise was undertaken, in another clinical study (18) the intervention group was significantly fitter at baseline, and finally, Gao et al. (28) did not use a disease specific tool to measure QOL. Differences in tools, design of intervention and extent of surgery made comparisons and conclusions concerning QOL difficult. There is a need for future studies to use equal measurement instruments for which reliability and validity (27,28). We detected significant improvement in quality of life and dyspnoea measured by CAT and mMRC.
Our perioperative pulmonary rehabilitation program was shorter compared to general duration of pulmonary rehabilitation program based on ATS, ERS, BTS guidelines. These types of patients performed the pulmonary rehabilitation before thoracic surgery because of lung cancer or other severe thoracic diseases. We needed to reduce the time before thoracic surgery and there are some studies about a shorter duration of pulmonary rehabilitation effective for improvement of cardiovascular response, metabolism, muscle function and lung mechanics.
Our study had limitations as well. This was a cohort study and there was no control group. We hadn’t collected postoperative complications in this study. We can choose a wide range of other quality of life scores to evaluate symptoms, like Saint George Respiratory Questionnaire, Chronic Clinical Questionnaire (CCQ). We may focus the change of depression and anxiety in the future as a result of perioperative pulmonary rehabilitation, as well.
Pulmonary rehabilitation had positive effects before lung surgery by improving exercise capacity and functional reserves. Thoracic surgical functional operability and postoperative management was promoted by the conditioning effect of pulmonary rehabilitation. Positive effects of pulmonary rehabilitation appeared objectively in all three groups. There was significant improvement in lung function, chest kinematics, exercise capacity and quality of life, also. We consider this rehabilitation program as an effective management of pulmonary rehabilitation in perioperative condition.
Acknowledgements
The authors would like to thank you Zsuzsanna Balogh to work with the patients and measure functional parameters as a physiotherapist, Csaba Feher and Miklos Molnar thoracic surgeons to help about the thoracic surgical database management, Istvan Gaudi for the statistical analysis, Zsofia Hodovan to work with the patients as a psychologist and Erika Pataki for coordinating the smoking cessation program.
Ethical Statement: The study was approved by Institutional Review Board and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Levett DZ, Grocott MP. Cardiopulmonary exercise testing, prehabilitation, and Enhanced Recovery After Surgery (ERAS). Can J Anaesth 2015;62:131-42. 10.1007/s12630-014-0307-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hillas G, Perlikos F, Tsiligianni I, et al. Managing comorbidities in COPD. Int J Chron Obstruct Pulmon Dis 2015;10:95-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunelli A, Charloux A, Bolliger CT, et al. European Respiratory Society; European Society of Thoracic Surgeons Joint Task Force on Fitness For Radical Therapy. The European Respiratory Society and European Society of Thoracic Surgeons clinical guidelines for evaluating fitness for radical treatment (surgery and chemoradiotherapy) in patients withlung cancer. Eur J Cardiothorac Surg 2009;36:181-4. 10.1016/j.ejcts.2009.04.022 [DOI] [PubMed] [Google Scholar]
- 4.Licker M, Schweizer A, Ellenberger C, et al. Perioperative medical management of patients with COPD. Int J Chron Obstruct Pulmon Dis 2007;2:493-515. [PMC free article] [PubMed] [Google Scholar]
- 5.Koelwyn GJ, Jones LW, Hornsby W, et al. Exercise therapy in the management of dyspnea in patients with cancer. Curr Opin Support Palliat Care 2012;6:129-37. 10.1097/SPC.0b013e32835391dc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asher A, Myers JS. The effect of cancer treatment on cognitive function. Clin Adv Hematol Oncol 2015;13:441-50. [PubMed] [Google Scholar]
- 7.Theadom A, Cropley M. Effects of preoperative smoking cessation on the incidence and risk of intraoperative and postoperative complications in adult smokers: a systematic review. Tob Control 2006;15:352-8. 10.1136/tc.2005.015263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corhay JL, Dang DN, Van Cauwenberge H, et al. Pulmonary rehabilitation and COPD: providing patients a good environment for optimizing therapy. Int J Chron Obstruct Pulmon Dis 2014;9:27-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatoryflows. Report Working Party Standardization of LungFunction Tests, European Communityfor Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J 2003;Suppl 16:5-40. [PubMed] [Google Scholar]
- 10.Balke B. A simple filed test for the assessment of physical fitness. Rep 63-6. Rep CivAeromed Res Inst US 1963:1-8. [PubMed] [Google Scholar]
- 11.Jones PW, Agusti AG. Outcomes and markers in the assessment of chronic obstructive pulmonary disease. Eur Respir J 2006;27:822-32. 10.1183/09031936.06.00145104 [DOI] [PubMed] [Google Scholar]
- 12.Fletcher CM, Elmes PC, Fairbairn MB, et al. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J 1959;2:257-66. 10.1136/bmj.2.5147.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.VargaJ, PorszaszJ, BodaK, et al. Supervised high intensity continuous and interval training vs. self-paced training in COPD. Respir Med 2007;101-11:2297-304. [DOI] [PubMed]
- 14.Hoogeboom TJ, Dronkers JJ, Hulzebos EH, et al. Merits of exercise therapy before and after major surgery. Curr Opin Anaesthesiol 2014;27:161-6. 10.1097/ACO.0000000000000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. ATS/ERS Task Force on Pulmonary Rehabilitation. Am J Respir Crit Care Med 2013;188:e13-64. 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 16.Perry R, Scott LJ, Richards A, et al. Pre-admission interventions to improve outcome after elective surgery-protocol for a systematic review. Syst Rev 2016;5:88. 10.1186/s13643-016-0266-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogdanis GC. Effects of physical activity and inactivity on muscle fatigue. Front Physiol 2012;3:142. 10.3389/fphys.2012.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crandall K, Maguire R, Campbell A, et al. Exercise intervention for patients surgically treated for Non-Small Cell LungCancer (NSCLC): A systematic review. Surg Oncol 2014;23:17-30. 10.1016/j.suronc.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 19.Riesenberg H, Lübbe AS. In-patientrehabilitationoflung cancerpatients--a prospective study. Support Care Cancer 2010;18:877-82. 10.1007/s00520-009-0727-y [DOI] [PubMed] [Google Scholar]
- 20.Kortianou EA, Nasis IG, Spetsioti ST, et al. Effectiveness of Interval Exercise Training in Patients with COPD. Cardiopulm Phys Ther J 2010;21:12-9. [PMC free article] [PubMed] [Google Scholar]
- 21.Haave E, Hyland ME, Engvik H. Improvements in exercise capacity during a 4-weeks pulmonary rehabilitation program for COPD patients do not correspond with improvements in self-reported health status or quality of life. Int J Chron Obstruct Pulmon Dis 2007;2:355-9. [PMC free article] [PubMed] [Google Scholar]
- 22.Casaburi R, Porszasz J, Burns MR, et al. Physiologic benefits of exercise training in rehabilitation of patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997;155:1541-51. 10.1164/ajrccm.155.5.9154855 [DOI] [PubMed] [Google Scholar]
- 23.Ambrosino N, Strambi S. New strategies to improve exercise tolerance in chronic obstructive pulmonary disease. Eur Respir J 2004;24:313-22. 10.1183/09031936.04.00002904 [DOI] [PubMed] [Google Scholar]
- 24.Salhi B, Huysse W, Van Maele G, et al. The effect of radical treatment andrehabilitationon muscle mass and strength: a randomized trial in stages I-IIIlung cancerpatients. Lung Cancer 2014;84:56-61. 10.1016/j.lungcan.2014.01.011 [DOI] [PubMed] [Google Scholar]
- 25.Lübbe AS, Riesenberg H, Baysal B, et al. Rehabilitationoflung cancerpatients. Pneumologie 2008;62:502-6. [DOI] [PubMed] [Google Scholar]
- 26.Arbane G, Douiri A, Hart N, et al. Effect of postoperative physical training on activity after curativesurgeryfor non-small celllungcancer: a multicentrerandomised controlled trial. Physiotherapy 2014. [Epub ahead of print]. 10.1016/j.physio.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 27.Cavalheri V, Tahirah F, Nonoyama M, et al. Exercise training undertaken by people within 12 months oflungresection for non-small celllungcancer. Cochrane Database Syst Rev 2013;(7):CD009955. [DOI] [PubMed] [Google Scholar]
- 28.Gao W, Bennett MI, Stark D, et al. Psychological distress incancerfrom survivorship to end of life care: prevalence, associated factors and clinical implications. Eur J Cancer 2010;46:2036-44. 10.1016/j.ejca.2010.03.033 [DOI] [PubMed] [Google Scholar]


