γ-Aminobutyric acid (GABA) in primary motor cortex was assessed in young and older adults using magnetic resonance spectroscopy and threshold-tracking paired-pulse transcranial magnetic stimulation. Older adults exhibited reduced extrasynaptic inhibition (short-interval intracortical inhibition at 1 ms) compared with young, whereas GABA concentration and synaptic inhibition were similar between age groups. We demonstrate that magnetic resonance spectroscopy and threshold-tracking provide valid and reliable assessments of primary motor cortex GABA concentration and neurotransmission, respectively.
Keywords: aging, magnetic resonance spectroscopy, transcranial magnetic stimulation, gamma-aminobutyric acid, intracortical inhibition
Abstract
The effects of healthy aging on γ-aminobutyric acid (GABA) within primary motor cortex (M1) remain poorly understood. Studies have reported contrasting results, potentially due to limitations with the common assessment technique. The aim of the present study was to investigate the effect of healthy aging on M1 GABA concentration and neurotransmission using a multimodal approach. Fifteen young and sixteen older adults participated in this study. Magnetic resonance spectroscopy (MRS) was used to measure M1 GABA concentration. Single-pulse and threshold-tracking paired-pulse transcranial magnetic stimulation (TMS) protocols were used to examine cortical silent period duration, short- and long-interval intracortical inhibition (SICI and LICI), and late cortical disinhibition (LCD). The reliability of TMS measures was examined with intraclass correlation coefficient analyses. SICI at 1 ms was reduced in older adults (15.13 ± 2.59%) compared with young (25.66 ± 1.44%; P = 0.002). However, there was no age-related effect for cortical silent period duration, SICI at 3 ms, LICI, or LCD (all P > 0.66). The intersession reliability of threshold-tracking measures was good to excellent for both young (range 0.75–0.96) and older adults (range 0.88–0.93). Our findings indicate that extrasynaptic inhibition may be reduced with advancing age, whereas GABA concentration and synaptic inhibition are maintained. Furthermore, MRS and threshold-tracking TMS provide valid and reliable assessment of M1 GABA concentration and neurotransmission, respectively, in young and older adults.
NEW & NOTEWORTHY γ-Aminobutyric acid (GABA) in primary motor cortex was assessed in young and older adults using magnetic resonance spectroscopy and threshold-tracking paired-pulse transcranial magnetic stimulation. Older adults exhibited reduced extrasynaptic inhibition (short-interval intracortical inhibition at 1 ms) compared with young, whereas GABA concentration and synaptic inhibition were similar between age groups. We demonstrate that magnetic resonance spectroscopy and threshold-tracking provide valid and reliable assessments of primary motor cortex GABA concentration and neurotransmission, respectively.
γ-aminobutyric acid (GABA) is the main inhibitory neurotransmitter within primary motor cortex (M1) and plays an important role in optimizing corticomotor output during functional tasks (Stinear and Byblow 2003; Zoghi et al. 2003). Deficits in motor performance accompany advancing age (Bedard et al. 2002; Calautti et al. 2001), which may, in part, be attributed to altered GABAergic neurotransmission (Levin et al. 2014). GABA-mediated inhibition is also important in the modulation of cortical plasticity (Cash et al. 2016; Ziemann et al. 2001) and may contribute to an age-related diminished capacity of processes important for synaptic plasticity (Zimerman and Hummel 2010). However, the effects of healthy aging on human M1 GABAergic inhibition remain poorly understood, potentially due to limitations with common assessment techniques.
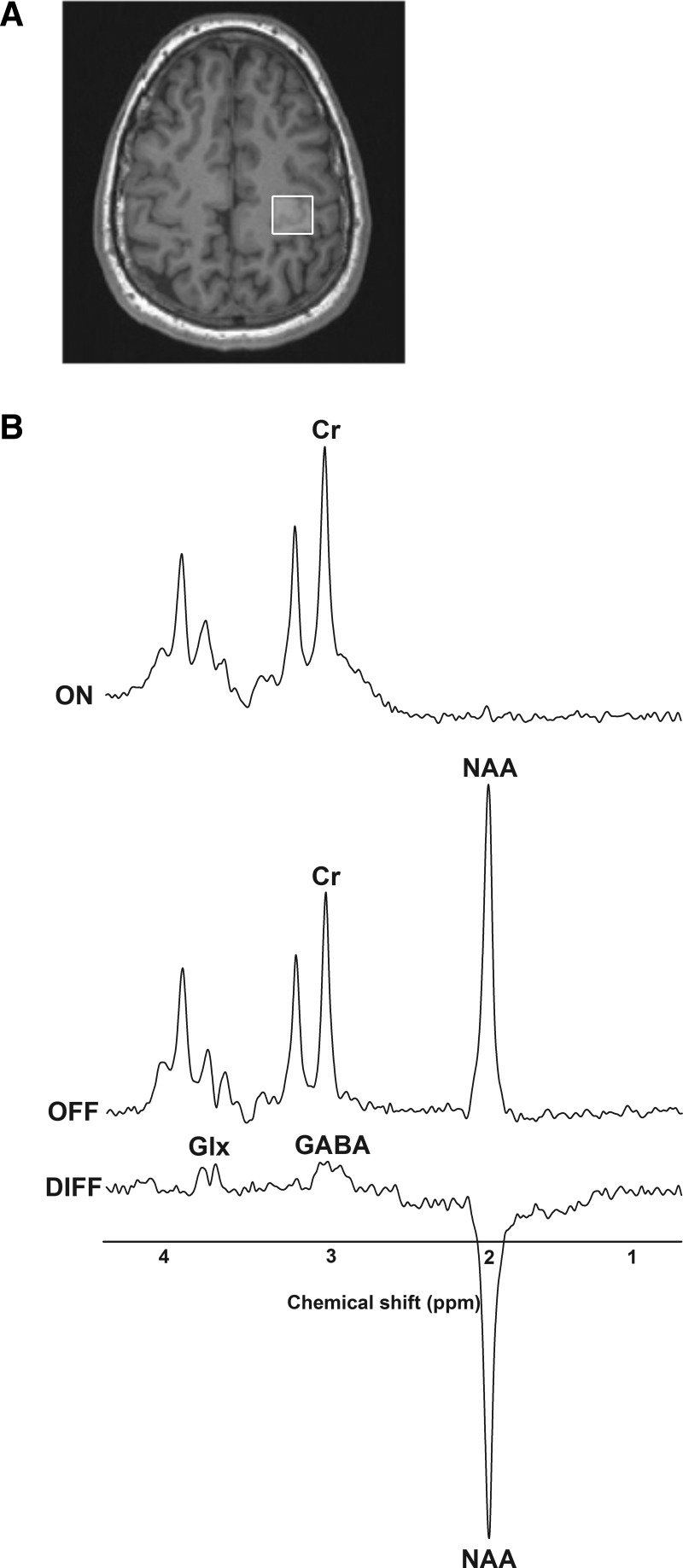
In humans, GABA concentration can be quantified noninvasively using magnetic resonance spectroscopy (Mescher et al. 1998), which uses precisely timed radio frequency pulses to excite hydrogen nuclei within various neurochemicals (Mullins et al. 2014). Frequency spectra are plotted with individual peaks reflecting the quantity of individual chemicals within the cortical region of interest (Fig. 1). There is evidence that GABA concentrations are reduced in older adults compared with young in frontal and parietal cortices (Gao et al. 2013). This finding indicates that there may be a global reduction in cortical GABA concentration with advancing age. However, whether an age-related reduction in GABA is present specifically within M1 remains unknown.
Fig. 1.
T1-weighted anatomic images were acquired to place manually an 18- × 18- × 18-mm voxel over the hand-knob region of left primary motor cortex (A). B: representative ON, OFF, and edited (DIFF) spectra from a young participant showing respective creatine (Cr), N-acetylaspartate (NAA), γ-aminobutyric acid (GABA), and glutamate + glutamine (Glx) peaks.
GABA has a distinct affinity for two receptor subtypes, GABAA and GABAB, which can be assessed using precisely timed paired-pulse transcranial magnetic stimulation (TMS) protocols. Short-interval intracortical inhibition (SICI) is examined by delivering a subthreshold conditioning stimulus before a suprathreshold test stimulus at short (1–5 ms) interstimulus intervals and reflects postsynaptic GABAA receptor-mediated M1 intracortical inhibition (Kujirai et al. 1993). Measures of extrasynaptic (Stagg et al. 2011b) and synaptic (Ziemann et al. 1996) GABAA activity are obtained at intervals of 1 (SICI1ms) and 3 ms (SICI3ms), respectively, and are mechanistically distinct. Long-interval intracortical inhibition (LICI) is examined by delivering two suprathreshold stimuli at longer interstimulus intervals (100–250 ms) and is a marker of postsynaptic GABAB activity (McDonnell et al. 2006). Late cortical disinhibition (LCD) may also be evident at the end of the LICI period, providing a marker of presynaptic GABAB activity (Cash et al. 2010). Therefore, specific conditioning intensities and intervals permit investigation of GABAA- and GABAB-mediated networks in M1.
Conventional paired-pulse TMS uses constant stimulation parameters to probe GABAergic function. In contrast, threshold-tracking TMS (Fisher et al. 2002; Vucic et al. 2006) adjusts the stimulator intensity to maintain a target motor evoked potential (MEP) amplitude in the presence of the conditioning stimulus. Threshold-tracking reduces the confound of MEP variability associated with conventional paired-pulse methods (Kiers et al. 1993). However, both conventional and threshold-tracking techniques are similar in their modes of action (Cirillo and Byblow 2016; Fisher et al. 2002; Murase et al. 2015; Vucic et al. 2006). There are contrasting results about the effect of healthy aging on GABAergic inhibition from studies using conventional paired-pulse TMS (Cirillo et al. 2011; McGinley et al. 2010; Oliviero et al. 2006; Opie et al. 2015; Opie and Semmler 2014; Peinemann et al. 2001; Rogasch et al. 2009; Sale et al. 2015; Smith et al. 2009). Motor evoked potentials are more variable in older vs. younger participants (Pitcher et al. 2003). For this reason, threshold-tracking may offer a preferable alternative to examine M1 inhibition in the elderly.
The aims of the present study were twofold. The first was to investigate the effect of healthy aging on GABA concentration and GABAA- and GABAB-mediated inhibition within M1 using magnetic resonance spectroscopy (MRS) and threshold-tracking TMS, respectively. We hypothesized that overall inhibitory tone would be reduced in older adults compared with young. Second, we evaluated the intersession reliability of threshold-tracking in both young and older adults.
METHODS
Participants
Fifteen neurologically healthy young (four women, mean age 25 ± 1 yr, range 20–31 yr) and sixteen older (seven women, mean age 70 ± 2 yr, range 62–83 yr) adults participated in this study. All participants were right-handed as assessed by the short version of the Edinburgh Handedness Inventory (Veale 2014), with a mean laterality quotient of 89 ± 3 (range 70–100) for young adults and 99 ± 1 (range 92–100) for older adults. Participants completed a transcranial magnetic stimulation safety screening questionnaire that was developed by our institution based on a previous report (Keel et al. 2001), which was screened by a neurologist before participation. Each participant provided written, informed consent, and the study was approved by the University of Auckland Human Participants Research Ethics Committee.
Experimental Design
There were three experimental sessions. In the first session, whole brain structural images and M1 metabolite concentrations were acquired using magnetic resonance imaging and MRS, respectively. In sessions 2 and 3, single- and paired-pulse TMS were used to assess measures of corticomotor excitability and the reliability of threshold-tracking. The grooved pegboard task was used to assess manual dexterity in session 2. Sessions 1 and 2 were separated by a mean of 9 days (range 1–23 days) for young adults and 8 days (range 2–15 days) for the older adults. Sessions 2 and 3 were separated by a mean of 6 days (range 2–7 days) for the young adults and 7 days (range 3–16 days) for the older adults.
Neuroimaging Procedures
Magnetic resonance imaging.
A Siemens MAGNETOM Skyra 3T scanner and 20-channel head coil (Siemens) were used for the neuroimaging session. Relaxation time T1-weighted whole brain structural images were acquired using 1- × 1- × 1-mm voxels and a 256-mm field of view [repetition time (TR) = 1,900 ms, echo time (TE) = 2.07 ms].
Magnetic resonance spectroscopy.
The T1-weighted structural images were used to place manually an 18- × 18- × 18-mm voxel of interest over the left precentral hand knob (Fig. 1A), tangential to the cortical surface. Spectral GABA editing and simultaneous water suppression were then performed using the MEGA-PRESS sequence (TR = 1,500 ms, TE = 68 ms, 96 averages; Mescher et al. 1998). A selective double-banded 180° pulse was created from 20-ms Gaussian pulses. The frequency of the 1st band of this pulse was set to 4.7 parts per million (ppm) for suppression of water. The 2nd band was alternated between 1.9 ppm (ON condition) and 7.5 ppm (OFF condition). The difference spectra (DIFF) between the ON and OFF conditions reveal an edited GABA spectrum without the larger overlapping creatine (Cr) resonance. Representative ON, OFF, and DIFF spectra are shown in Fig. 1B.
Recording and Stimulation Procedures
Surface electromyography.
Surface electromyography (EMG) was recorded from the first dorsal interosseous (FDI) of the dominant right hand using 10-mm-diameter Ag-AgCl recording electrodes (Ambu, Ballerup, Denmark), arranged in a belly-tendon montage. A 20-mm-diameter ground surface electrode (3M Health Care) was positioned on the dorsum of the right hand. EMG signals were amplified (1,000×) and band-pass filtered (10–1,000 Hz) using a CED 1902 amplifier (CED, Cambridge, United Kingdom), sampled at 2 kHz using a CED 1401 interface (CED), and recorded onto a computer for offline analysis using Signal (version 5.03; CED) software.
Transcranial magnetic stimulation.
A MagPro X100+option magnetic stimulator (MagVenture, Farum, Denmark) connected to a figure-of-eight coil (MC-B70; outer wing diameter 97 mm) was used to deliver TMS. The coil was held tangentially to the scalp, handle posterior, ~45° to the midsagittal line, to induce posterior-anterior current flow in the brain (Sakai et al. 1997) using a monophasic waveform (pulse width = 70 μs). The optimal site to elicit consistent MEPs in the resting right FDI muscle was marked on the scalp over the left hemisphere. TMS was delivered at 0.2 Hz, with 20% variation between trials, and optimal coil position was continually monitored throughout the experiment.
Rest motor threshold (RMT) was defined as the minimum stimulus intensity required for eliciting MEPs of ≥50 µV in amplitude in 4 out of 8 trials. Active motor threshold (AMT) was defined as the minimum stimulus intensity required for eliciting MEPs of ≥100 µV in amplitude in 4 out of 8 trials during a low-level voluntary contraction (~10% of maximum voluntary contraction). Measures of cortical silent period duration were obtained while the participant maintained a 10% maximum voluntary contraction. The stimulation intensity was set to 130% RMT, and 16 responses were acquired for each participant.
Protocol
Threshold-tracking a target MEP amplitude of 200 µV (± 20%) was used to quantify the extent of inhibition and disinhibition in M1 in line with previous work (Cirillo and Byblow 2016; Fisher et al. 2002; Vucic et al. 2006). Similar to RMT and AMT, the threshold-tracking target (TTT) was defined as the minimum stimulus intensity required for eliciting MEPs of ≥160 µV in amplitude in four out of eight trials (Cirillo and Byblow 2016). The TTT was determined before and after each paired-pulse protocol.
Short-interval intracortical inhibition.
To investigate SICI1ms and SICI3ms, four conditioning intensities were used ranging from 50 to 95% AMT in 15% AMT steps. In the presence of conditioning, the test stimulus intensity was increased or decreased in 1% maximum stimulator output steps until the TTT was reached. Tracking was deemed successful when the conditioned MEP was above or within 20% of the TTT in two out of three consecutive trials (Cirillo and Byblow 2016). Because of the short interstimulus interval (ISI), a half-sine waveform (pulse width = 70 μs) was used for SICI1ms. Both AMT and TTT were independently determined with a half-sine waveform for SICI1ms.
Long-interval intracortical inhibition and late cortical disinhibition.
Long-interval intracortical inhibition (LICI) and late cortical disinhibition (LCD) were investigated using seven interstimulus intervals (100, 160, 180, 200, 220, 240, and 260 ms). The conditioning stimulus was set to 130% RMT. Identical to the SICI protocol, the test intensity was increased or decreased by 1–2% maximum stimulator output until the TTT was achieved.
Data Analysis
Neuroimaging.
MRS data were processed using the Java Magnetic Resonance User Interface (jMRUI; Naressi et al. 2001). First, the free induction decay signal was corrected for any nonzero direct current offset and smoothed using a 5-Hz Lorentzian filter (Blicher et al. 2015). Next, the residual water peak was filtered using the Hankel-Lanczos singular value decomposition filter. Zero-order phase correction was then manually applied to correct for peak distortion.
Spectral analysis was carried out in the time domain using AMARES, a nonlinear least-squares fitting optimization algorithm (Vanhamme et al. 1997). The OFF spectrum was analyzed first, with peak fitting performed using a fixed Gaussian function to obtain linewidths for N-acetylaspartate (NAA) and Cr. For the DIFF spectrum, a single Gaussian curve was first fitted to the inverted NAA resonance, with the linewidth constrained to that of NAA in the OFF spectrum. Peak fitting the GABA resonance was then performed using two Gaussian curves, with the linewidths separately constrained to that of the Cr resonance from the OFF spectrum (Stagg et al. 2011a). Additionally, peak fitting for the coedited glutamate + glutamine (Glx) resonance was performed in an identical manner. Total amplitude for GABA and Glx was obtained by summing the amplitudes of the two GABA and two Glx peaks, respectively.
T1-weighted structural images were extracted using the Brain Extraction Tool and segmented using Automated Segmentation Tool (Oxford Centre for Functional MRI of the Brain, FMRIB). The relative quantities of gray matter (GM), white matter (WM), and cerebrospinal fluid within the voxel were then calculated for each participant. The NAA and Cr amplitudes were corrected for the proportion of total brain tissue volume (GM + WM) within the voxel, and the GABA and Glx amplitudes were corrected for the proportion of GM volume within the voxel (Stagg et al. 2011a). GABA and Glx concentrations were then calculated as ratios, using the corrected GABA and Glx amplitudes relative to the corrected Cr amplitude and the simultaneously acquired and corrected NAA amplitude (Gao et al. 2013).
Neurophysiology.
The amplitude of the first MEP from the LICI/LCD protocol was used as a measure of corticomotor excitability. Semiautomated methods were used to measure cortical silent period duration. The EMG signal was rectified, and cortical silent period duration was assessed from the point of stimulation until the resumption of EMG activity levels equal to or greater than pretrigger root-mean-squared (rms) EMG (pretrigger rmsEMG window of 50 ms; 5–55 ms before the stimulus). Trials in which participants were not able to maintain the contraction through the perturbation of the stimulus were excluded.
For threshold-tracking, trials that were contaminated by prestimulus EMG activity (rmsEMG >10 μV; 50 ms before stimulation) were rejected online and repeated immediately. SICI1ms, SICI3ms, LICI, and LCD induced by the conditioning stimulus were quantified as the percentage increase or decrease in test stimulus intensity required to evoke the TTT (Fisher et al. 2002):
where positive values indicate inhibition and negative values indicate disinhibition. For both SICI1ms and SICI3ms, the largest threshold change value among conditioned stimulus intensities was determined as maximum inhibition for each participant. Inhibition at an ISI of 100 ms was selected for LICI, whereas the maximum disinhibition observed between the ISIs of 160 and 260 ms was used to index LCD.
Statistical Analysis
Normality was assessed using the Wilk-Shapiro test and homoscedasticity of variance using the Levene test of equality and Mauchly test of sphericity. Nonnormal data were log-transformed. Independent-sample t-tests were used to analyze the effect of AGE (Young, Older) on voxel GM%, WM%, GABA and Glx concentrations, and grooved pegboard task completion times.
A two-way mixed-effects repeated-measures ANOVA was performed to determine the effect of AGE (Young, Older) and TMS SESSION (One, Two) on RMT, AMT, TTT, MEP amplitude, cortical silent period duration, SICI1ms, SICI3ms, LICI, and LCD. Additional one-sample t-tests (hypothesized mean = 0) were performed for SICI1ms, SICI3ms, LICI, and LCD to confirm significant inhibition/disinhibition for both age groups.
Intersession reliability of threshold-tracking TMS was assessed using intraclass correlation coefficients. Reliability estimates were judged as fair (0.40–0.58), good (0.59–0.74), or excellent (>0.75; Cicchetti and Sparrow 1981).
Pearson correlation analyses were used to investigate the relationship among metabolite concentrations, MEP amplitude, inhibition measures, and manual dexterity. The significance level was set at P < 0.05, and group data are presented as means ± SE in the text.
RESULTS
Participants completed all three experimental sessions with no adverse events. MEPs could not be elicited in the right FDI muscle of one older adult. Analysis of the grooved pegboard task data revealed that time to complete a single trial was slower in older adults (78.76 ± 3.27 s) compared with young (60.63 ± 2.27 s; P < 0.001).
Magnetic Resonance Spectroscopy
No differences in voxel GM% and WM%, GABA-to-Cr ratio (GABA:Cr), GABA:NAA, Glx:Cr, or Glx:NAA were observed between young and older adults (all P > 0.10; Table 1).
Table 1.
Participant characteristics and MRS and single-pulse TMS data
| Age Group |
|||
|---|---|---|---|
| Young | Older | P Value | |
| Number of participants | 15 (4 F) | 16 (7 F) | |
| Age, yr | 24.6 (1.1) | 70.3 (1.7) | <0.001 |
| Magnetic resonance spectroscopy | |||
| Voxel GM% | 35.36 (2.00) | 32.66 (1.12) | 0.25 |
| Voxel WM% | 55.65 (2.85) | 49.34 (2.32) | 0.10 |
| GABA:Cr | 0.102 (0.008) | 0.114 (0.008) | 0.29 |
| GABA:NAA | 0.050 (0.005) | 0.061 (0.005) | 0.10 |
| Glx:Cr | 0.094 (0.008) | 0.088 (0.007) | 0.59 |
| Glx:NAA | 0.045 (0.004) | 0.048 (0.005) | 0.68 |
| Transcranial magnetic stimulation | |||
| RMT, %MSO | 49.2 (2.4) | 50.7 (2.9) | 0.70 |
| AMT, %MSO | 38.8 (2.1) | 39.9 (2.3) | 0.73 |
| TTT, %MSO | 53.1 (2.7) | 54.7 (3.5) | 0.72 |
| MEP amplitude, log10 mV | 0.19 (0.07) | 0.15 (0.11) | 0.77 |
| Cortical silent period duration, ms | 180.1 (6.0) | 176.2 (7.9) | 0.70 |
Values are means (SE). F, female; GM, gray matter; WM, white matter; GABA, γ-aminobutyric acid; Cr, creatine; NAA, N-acetylaspartate; RMT, rest motor threshold; AMT, active motor threshold; TTT, threshold-tracking target; MSO, maximum stimulator output; MEP, motor evoked potential.
Transcranial Magnetic Stimulation
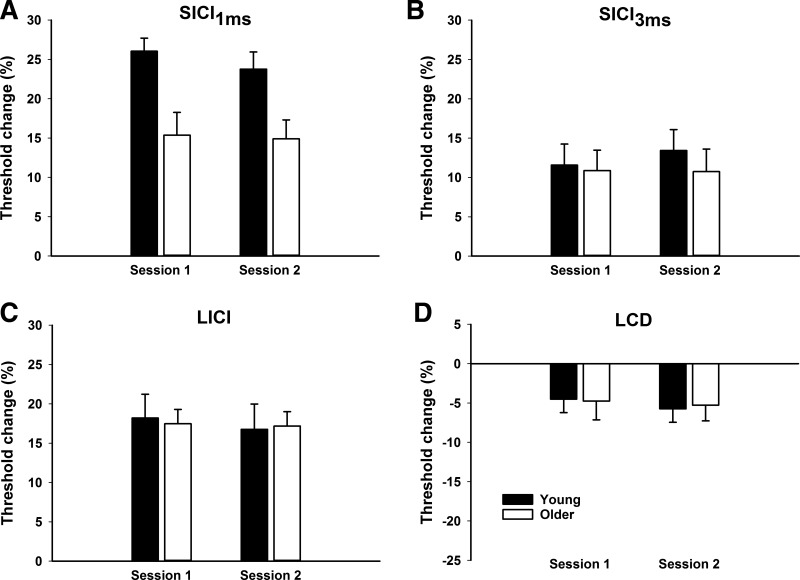
Example EMG traces from a typical participant are shown in Fig. 2. There were no main effects of AGE or TMS SESSION and no interaction for RMT, AMT, TTT, MEP amplitude, and cortical silent period duration (all P > 0.12). For maximum SICI1ms, data from one young participant was deemed an outlier (2 SD outside of the mean) and excluded from analysis. There was a main effect of AGE (F1,27 = 12.14, P = 0.002) for SICI1ms, revealing the extent of inhibition was reduced in older adults compared with young (Fig. 3A). There was no main effect of TMS SESSION and no interaction (both P > 0.57). No main effects of AGE or TMS SESSION and no interactions were observed for SICI3ms, LICI, and LCD (all P > 0.13; Fig. 3, B–D). One-sample t-tests showed that inhibition/disinhibition was present for all paired-pulse TMS protocols in both young (all P < 0.008) and older (all P < 0.037) adults.
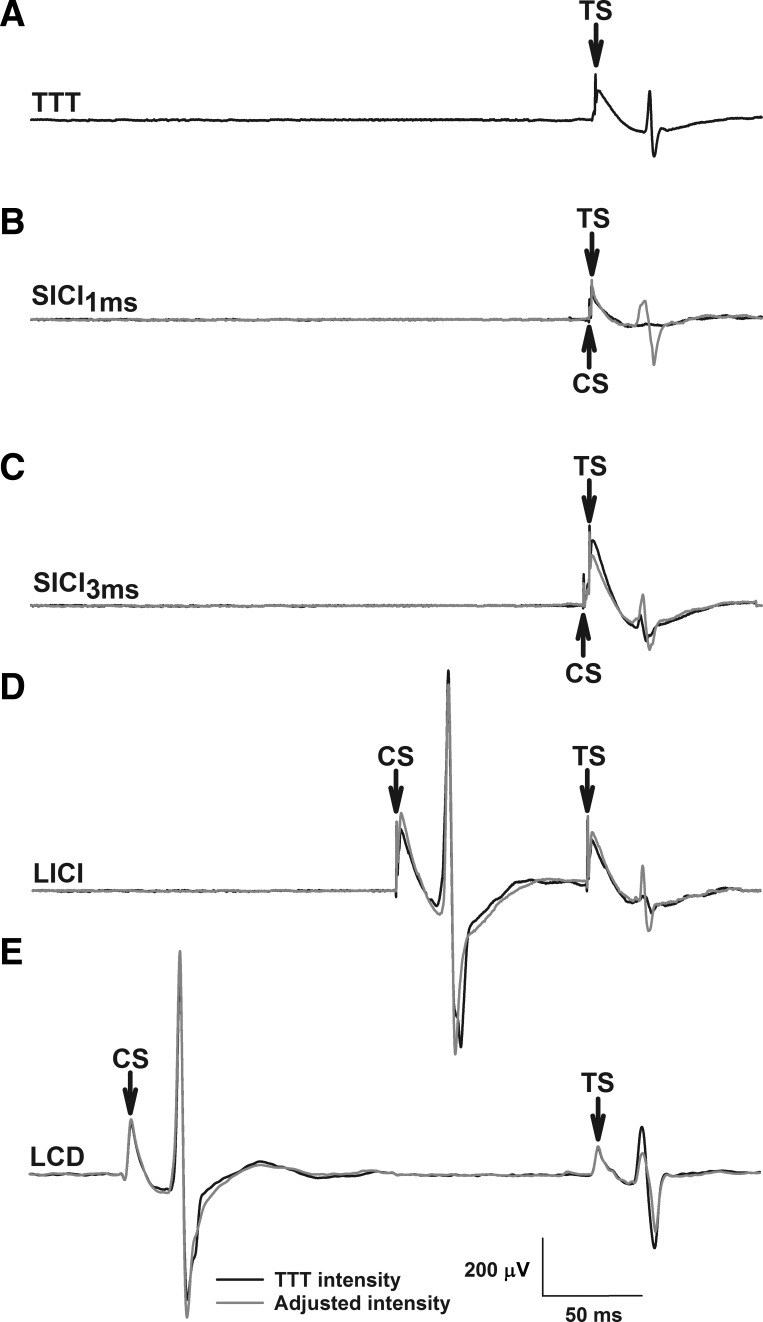
Fig. 2.
Example EMG traces depict motor evoked potentials (MEPs) from an individual young participant. A: TMS intensity required to elicit a fixed MEP amplitude (200 μV) to the single-pulse test stimulus (TS; threshold-tracking target, TTT). B and C: short-interval intracortical inhibition [SICI; conditioning stimulus (CS) = 50–95% AMT, 15% steps] at 1 and 3 ms, respectively. D: long-interval intracortical inhibition (LICI; CS = 130% RMT, ISI = 100 ms). E: late cortical disinhibition (LCD; CS = 130% RMT, ISI = 160–260 ms, 20-ms steps). Threshold-tracking requires an increase or decrease in the TS intensity to evoke the target response in the presence of conditioning (gray traces in B–E).
Fig. 3.
Threshold-tracking values obtained from each paired-pulse protocol. A: short-interval intracortical inhibition (SICI) at 1 ms was reduced in older adults compared with young in both TMS sessions. No differences in SICI at 3 ms (B), long-interval intracortical inhibition (C), or late cortical disinhibition (D) were observed between young and older adults in either session. In A–C, greater inhibition is indicated upward. In D, greater disinhibition is indicated downward. Data are presented as means + SE. N = 15 young and 15 older adults.
Intraclass correlation coefficient values for threshold-tracking SICI1ms, SICI3ms, LICI, and LCD are displayed in Table 2. There was good-to-excellent intersession reliability for all paired-pulse TMS measures in both young (range 0.75–0.96) and older adults (range 0.88–0.93).
Table 2.
Intraclass correlation coefficients
| Age Group |
||
|---|---|---|
| Protocol | Young | Older |
| SICI1ms | 0.75 | 0.89 |
| SICI3ms | 0.93 | 0.92 |
| LICI | 0.96 | 0.88 |
| LCD | 0.89 | 0.93 |
SICI, short-interval intracortical inhibition; LICI, long-interval intracortical inhibition; LCD, late cortical disinhibition.
Linear Regression
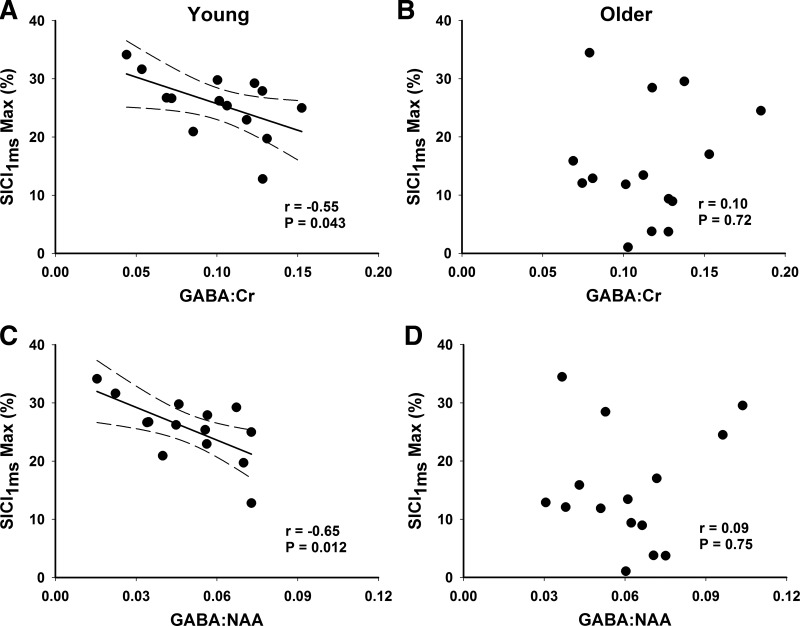
A negative correlation was observed between GABA concentration and maximum SICI1ms for young adults (Fig. 4, A and C), where individuals with higher GABA concentration exhibited lower SICI1ms (GABA:Cr: r = −0.55, P = 0.043; GABA:NAA: r = −0.65, P = 0.012). There was a positive correlation between GM quantity and GABA concentration (GABA:Cr: r = 0.98, P < 0.001; GABA:NAA: r = 0.93, P < 0.001) and a trend for an association between GM quantity and maximum SICI1ms (r = −0.52, P = 0.06). Partial correlation analyses with GM quantity as a controlling variable revealed no association between GABA concentration and maximum SICI1ms (GABA:Cr: r = −0.17, P = 0.59; GABA:NAA: r = −0.51, P = 0.08). No other correlations between GABA or Glx concentrations and single- or paired-pulse TMS measures were observed for young (all P > 0.12) or older adults (all P > 0.11). Similarly, there were no associations between manual dexterity and metabolite concentrations or paired-pulse TMS measures for either age group (all P > 0.26).
Fig. 4.
Correlation analyses between maximal (Max) short-interval intracortical inhibition at 1 ms (SICI1ms) and magnetic resonance spectrometry GABA concentration relative to creatine (Cr) and N-acetylaspartate (NAA) in young (A and C) and older (B and D) adults. There was a negative relationship in young adults, with higher GABA concentration associated with less SICI1ms. Greater inhibition is indicated upward. No relationship was observed in older adults. N = 14 young and 15 older adults.
DISCUSSION
The present study investigated the effect of healthy aging on M1 GABA concentration and GABAergic neurotransmission and the reliability of threshold-tracking. Overall, SICI1ms was reduced in older adults compared with young, but GABA concentration and other measures of GABAergic neurotransmission were not significantly different between age groups. GABA concentration was negatively correlated with SICI1ms in young but not older adults. Threshold-tracking had good-to-excellent intersession reliability in both young and older adults. These findings indicate that M1 GABA concentration and synaptic GABAA and GABAB activity are maintained with advancing age, whereas extrasynaptic GABAA activity is reduced.
M1 GABA Concentration Is Maintained with Advancing Age
GABA concentration within M1 was similar between young and older adults. This finding is in contrast with a previous study that observed lower GABA in the frontal and parietal cortices with advancing age (Gao et al. 2013). This discrepancy between the current study and Gao et al. (2013) may highlight the nonuniform distribution of GABA concentration across the human cortex (Greenhouse et al. 2016). Alternatively, methodological differences in scanning parameters that influence the observed signal, such as the number of averages collected and voxel size (Mullins et al. 2014), may contribute to the disparate findings. A limitation of the present study is a small voxel (18 mm3) was used to optimize the recorded signal for the hand-knob region of M1. Reducing voxel size can be detrimental to the inherently low signal-to-noise ratio associated with quantifying GABA (Mullins et al. 2014). Therefore, scanning parameters and regional variations in metabolite concentrations should be carefully considered in future MRS studies investigating age-related effects.
Healthy Aging Influences GABAergic Neurotransmission Differentially
The extent of age-related changes in M1 intracortical inhibition with TMS is unclear. The current study showed that SICI1ms was reduced in older adults using threshold-tracking, which supports a previous conventional TMS study (Peinemann et al. 2001). However, this finding contrasts recent studies investigating SICI1ms in older adults (Shibuya et al. 2016; Smith et al. 2009). Shibuya et al. (2016) assessed SICI1ms with threshold-tracking across a broad age spectrum (20–83 yr) and demonstrated that extent of inhibition was not altered with advancing age. A key difference between the current study and Shibuya et al. (2016) was the intensity of the conditioning stimulus. Shibuya and colleagues (2016) used a single conditioning intensity (70% of TTT), whereas the current study determined maximum SICI1ms for each participant over a range of conditioning stimulus intensities (50–95% AMT; steps of 15%). It is advantageous to use multiple conditioning intensities because the profile of the SICI1ms curve may differ between individuals (Smith et al. 2009). Interestingly, Smith et al. (2009) observed more SICI1ms in older adults with a conditioning intensity set to 5% maximum stimulator output below AMT. However, AMT was higher in older adults compared with young, and when conditioning stimulus intensities were set relative to AMT for both groups, the age-related increase in inhibition was not evident (Smith et al. 2009). Therefore, utilizing multiple conditioning intensities that are set relative to the threshold of an individual is likely to be advantageous in detecting age-related changes in SICI.
The interpretation of SICI1ms can be somewhat controversial. One proposition is that the inhibition reflects neuronal refractoriness due to activation of low threshold interneurons by the conditioning (Fisher et al. 2002). However, increasing the conditioning intensity reduces SICI1ms, eventually leading to facilitation (Vucic et al. 2009). If neuronal refractoriness was solely responsible for SICI1ms, then greater inhibition would be expected with higher conditioning intensities due to subliminal activation of a larger population of interneurons (Vucic et al. 2009). Alternatively, SICI1ms may reflect extrasynaptic GABAA activity (Stagg et al. 2011b; Vucic et al. 2009). Extrasynaptic GABAA receptors have high sensitivity to ambient extracellular GABA (Belelli et al. 2009) and regulate cortical excitability through tonically active inhibition (Walker and Semyanov 2008). The level of tonic inhibition is likely to be a key factor in neurorehabilitation, with animal models showing reduced inhibition in the subacute phase after stroke promotes motor recovery (Clarkson et al. 2010). Therefore, a better understanding of the mechanism(s) underlying SICI1ms and identifying how healthy aging effects inhibitory tone within M1 may have key implications in older adults, typical of the age requiring neurorehabilitation after stroke.
Although older adults exhibited reduced extrasynaptic GABAA activity, threshold-tracking TMS of synaptic GABAA and GABAB activity were similar between young and older adults. This finding coincides with the majority of previous studies investigating age-related effects using conventional TMS (Cirillo et al. 2010, 2011; Oliviero et al. 2006; Rogasch et al. 2009; Smith et al. 2009). However, an increase (McGinley et al. 2010; Sale et al. 2015) and decrease (Heise et al. 2013; Opie and Semmler 2014; Peinemann et al. 2001) in synaptic GABAergic neurotransmission has also been reported in older adults. Interestingly, Sale et al. (2015) showed that SICI3ms was greater in older adults than young when using anterior-posterior current flow in M1 but not posterior-anterior. Although not used in the present study, threshold-tracking with an anterior-posterior induced current may provide a more robust and sensitive measure of SICI3ms than with a posterior-anterior current (Cirillo and Byblow 2016). It has been shown previously SICI at 3 ms is more robust than 2 ms when using threshold-tracking (Murase et al. 2015). In the present study, the conditioning intensities used to assess SICI3ms were below the level where short-interval intracortical facilitation has been shown to interact with SICI3ms (Peurala et al. 2008). For these reasons, there is no reason to suspect that the absence of an age-related effect for SICI3ms is due to contamination from facilitatory inputs. Whether the target muscle is voluntarily activated may help differentiate age-related changes in inhibition. For example, reduced SICI3ms in older adults was observed during voluntary activation but not resting conditions, whereas LICI was less in older adults at rest but not during muscle contraction (Opie and Semmler 2014). Future studies are required to investigate age-related changes in intracortical inhibition using threshold-tracking with different induced currents and during voluntary activation.
Here, we present evidence that LCD is maintained with healthy aging. To our knowledge, this study is the first to examine age-related effects on LCD, a proposed marker of presynaptic GABAB activity (Cash et al. 2010). We extend findings from previous studies assessing LCD in young adults using conventional TMS (Cash et al. 2010, 2011; Caux-Dedeystère et al. 2014, 2015) by demonstrating LCD in both young and older adults using threshold-tracking. The presence of LCD is not always consistent at rest and appears to be more prominent during voluntary activation of the target muscle (Caux-Dedeystère et al. 2014, 2015). Although LCD was not assessed during voluntary activation in the present study, LCD was observed using threshold-tracking by selecting the interstimulus interval where maximum disinhibition occurred for each participant. We suggest that LCD may be examined in future studies by using threshold-tracking TMS and multiple interstimulus intervals.
Assessment of SICI3ms with threshold-tracking shows good-to-excellent intra- and intersession reliability in young adults (Samusyte et al. 2016). We extend these findings by showing that threshold-tracking SICI1ms, SICI3ms, LICI, and LCD have good-to-excellent intersession reliability in both young and older adults. Conventional TMS also demonstrates good intersession reliability for both SICI3ms and LICI in older adults (Schambra et al. 2015). Overall, Samusyte et al. (2016) found that intra- and intersession reliability was better with threshold-tracking than conventional TMS. The two techniques are presumed to reflect activity within the same cortical networks and differ only in the extent to which they are effected by MEP variability. Our results demonstrate that threshold-tracking is a valid and reliable technique to investigate M1 GABAergic neurotransmission in young and older adults.
GABA Concentration and Paired-Pulse TMS Measures
There was a negative association between GABA concentration and SICI1ms in young but not older adults (i.e., young participants with higher GABA concentration exhibited less inhibition). Interestingly, this association was not observed when controlling for the proportion of GM within the voxel, and therefore this finding must be interpreted with caution. Our finding in young adults and a recent similar finding (Dyke et al. 2017) contrast with a previous study demonstrating a positive relationship between MRS GABA and SICI1ms (Stagg et al. 2011b). Different paradigms to assess SICI1ms between the current study (maximum inhibition using threshold-tracking) and Stagg et al. (2011b; slope of inhibition curve using conventional TMS) may explain the discrepant results. Although SICI1ms was reduced in older adults, no age-related differences in GABA concentration within M1 were found. It is possible that age-related changes in intracellular GABA levels mask a decline in extrasynaptic GABA, which may account for the lack of relationship between SICI1ms and GABA concentration in older adults.
There were no associations between GABA concentration and TMS surrogate measures of synaptic GABAA (SICI3ms) or GABAB (LICI, LCD, and cortical silent period) in young and older adults. These findings are consistent with previous studies focusing on healthy young cohorts (Stagg et al. 2011b; Tremblay et al. 2013). Limited sensitivity of MRS to synaptic GABA may account for the lack of a relationship between GABA concentration and paired-pulse TMS measures of synaptic GABA activity. GABA stores within the presynaptic bouton of inhibitory interneurons comprise ~30% of cortical GABA concentration (Petroff 2002), with the amount of GABA directly related to vesicular release (Golan et al. 1996). Improving the specificity of MRS assessments of GABA concentration will aid interpretation of data from combined MRS and paired-pulse TMS studies.
In summary, threshold-tracking demonstrated that extrasynaptic GABAA activity may be reduced as a consequence of aging. Conversely, GABA concentration and synaptic GABAergic activity may be maintained with aging. Furthermore, threshold-tracking with paired-pulse TMS is a reliable technique for assessing M1 GABAergic function. These findings may have implications for age-related conditions, such as stroke, where tonic inhibition plays an important role in motor recovery.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.A.M., J.C., and W.D.B. conceived and designed research; R.A.M. performed experiments; R.A.M. analyzed data; R.A.M., J.C., and W.D.B. interpreted results of experiments; R.A.M. prepared figures; R.A.M. drafted manuscript; R.A.M., J.C., and W.D.B. edited and revised manuscript; R.A.M., J.C., and W.D.B. approved final version of manuscript.
GRANTS
This study was funded by the Health Research Council of New Zealand (Project 14/136).
ACKNOWLEDGMENTS
We thank the Centre for Advanced MRI for assistance with data acquisition and Terry Corrin for technical assistance.
REFERENCES
- Bedard AC, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. The development of selective inhibitory control across the life span. Dev Neuropsychol 21: 93–111, 2002. doi: 10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci 29: 12757–12763, 2009. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blicher JU, Near J, Næss-Schmidt E, Stagg CJ, Johansen-Berg H, Nielsen JF, Østergaard L, Ho YC. GABA levels are decreased after stroke and GABA changes during rehabilitation correlate with motor improvement. Neurorehabil Neural Repair 29: 278–286, 2015. doi: 10.1177/1545968314543652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti C, Serrati C, Baron JC. Effects of age on brain activation during auditory-cued thumb-to-index opposition: a positron emission tomography study. Stroke 32: 139–146, 2001. doi: 10.1161/01.STR.32.1.139. [DOI] [PubMed] [Google Scholar]
- Cash RF, Murakami T, Chen R, Thickbroom GW, Ziemann U. Augmenting plasticity induction in human motor cortex by disinhibition stimulation. Cereb Cortex 26: 58–69, 2016. doi: 10.1093/cercor/bhu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash RF, Ziemann U, Murray K, Thickbroom GW. Late cortical disinhibition in human motor cortex: a triple-pulse transcranial magnetic stimulation study. J Neurophysiol 103: 511–518, 2010. doi: 10.1152/jn.00782.2009. [DOI] [PubMed] [Google Scholar]
- Cash RF, Ziemann U, Thickbroom GW. Inhibitory and disinhibitory effects on I-wave facilitation in motor cortex. J Neurophysiol 105: 100–106, 2011. doi: 10.1152/jn.00650.2010. [DOI] [PubMed] [Google Scholar]
- Caux-Dedeystère A, Derambure P, Devanne H. Late cortical disinhibition in relaxed versus active hand muscles. Neuroscience 298: 52–62, 2015. doi: 10.1016/j.neuroscience.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Caux-Dedeystère A, Rambour M, Duhamel A, Cassim F, Derambure P, Devanne H. Task-dependent changes in late inhibitory and disinhibitory actions within the primary motor cortex in humans. Eur J Neurosci 39: 1485–1490, 2014. doi: 10.1111/ejn.12505. [DOI] [PubMed] [Google Scholar]
- Cicchetti DV, Sparrow SA. Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic 86: 127–137, 1981. [PubMed] [Google Scholar]
- Cirillo J, Byblow WD. Threshold tracking primary motor cortex inhibition: the influence of current direction. Eur J Neurosci 44: 2614–2621, 2016. doi: 10.1111/ejn.13369. [DOI] [PubMed] [Google Scholar]
- Cirillo J, Rogasch NC, Semmler JG. Hemispheric differences in use-dependent corticomotor plasticity in young and old adults. Exp Brain Res 205: 57–68, 2010. doi: 10.1007/s00221-010-2332-1. [DOI] [PubMed] [Google Scholar]
- Cirillo J, Todd G, Semmler JG. Corticomotor excitability and plasticity following complex visuomotor training in young and old adults. Eur J Neurosci 34: 1847–1856, 2011. doi: 10.1111/j.1460-9568.2011.07870.x. [DOI] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature 468: 305–309, 2010. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke K, Pépés SE, Chen C, Kim S, Sigurdsson HP, Draper A, Husain M, Nachev P, Gowland PA, Morris PG, Jackson SR. Comparing GABA-dependent physiological measures of inhibition with proton magnetic resonance spectroscopy measurement of GABA using ultra-high-field MRI. Neuroimage 152: 360–370, 2017. doi: 10.1016/j.neuroimage.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res 143: 240–248, 2002. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, Zhao B, Wang H, Bai X, Zhao C, Wang X, Barker PB. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 78: 75–82, 2013. doi: 10.1016/j.neuroimage.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan H, Talpalar AE, Scheleifstein-Attias D, Grossman Y. GABA metabolism controls inhibition efficacy in the mammalian CNS. Neurosci Lett 217: 25–28, 1996. doi: 10.1016/0304-3940(96)13061-5. [DOI] [PubMed] [Google Scholar]
- Greenhouse I, Noah S, Maddock RJ, Ivry RB. Individual differences in GABA content are reliable but are not uniform across the human cortex. Neuroimage 139: 1–7, 2016. doi: 10.1016/j.neuroimage.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise KF, Zimerman M, Hoppe J, Gerloff C, Wegscheider K, Hummel FC. The aging motor system as a model for plastic changes of GABA-mediated intracortical inhibition and their behavioral relevance. J Neurosci 33: 9039–9049, 2013. doi: 10.1523/JNEUROSCI.4094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol 112: 720, 2001. doi: 10.1016/S1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- Kiers L, Cros D, Chiappa KH, Fang J. Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 89: 415–423, 1993. doi: 10.1016/0168-5597(93)90115-6. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol 471: 501–519, 1993. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin O, Fujiyama H, Boisgontier MP, Swinnen SP, Summers JJ. Aging and motor inhibition: a converging perspective provided by brain stimulation and imaging approaches. Neurosci Biobehav Rev 43: 100–117, 2014. doi: 10.1016/j.neubiorev.2014.04.001. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res 173: 86–93, 2006. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- McGinley M, Hoffman RL, Russ DW, Thomas JS, Clark BC. Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Exp Gerontol 45: 671–678, 2010. doi: 10.1016/j.exger.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed 11: 266–272, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- Mullins PG, McGonigle DJ, O’Gorman RL, Puts NA, Vidyasagar R, Evans CJ, Edden RA; Cardiff Symposium on MRS of GABA . Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage 86: 43–52, 2014. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase N, Cengiz B, Rothwell JC. Inter-individual variation in the after-effect of paired associative stimulation can be predicted from short-interval intracortical inhibition with the threshold tracking method. Brain Stimul 8: 105–113, 2015. doi: 10.1016/j.brs.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med 31: 269–286, 2001. doi: 10.1016/S0010-4825(01)00006-3. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, Di Lazzaro V. Effects of aging on motor cortex excitability. Neurosci Res 55: 74–77, 2006. doi: 10.1016/j.neures.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Opie GM, Ridding MC, Semmler JG. Age-related differences in pre- and post-synaptic motor cortex inhibition are task dependent. Brain Stimul 8: 926–936, 2015. doi: 10.1016/j.brs.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Opie GM, Semmler JG. Age-related differences in short- and long-interval intracortical inhibition in a human hand muscle. Brain Stimul 7: 665–672, 2014. doi: 10.1016/j.brs.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Conrad B, Siebner HR. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci Lett 313: 33–36, 2001. doi: 10.1016/S0304-3940(01)02239-X. [DOI] [PubMed] [Google Scholar]
- Petroff OA. GABA and glutamate in the human brain. Neuroscientist 8: 562–573, 2002. doi: 10.1177/1073858402238515. [DOI] [PubMed] [Google Scholar]
- Peurala SH, Müller-Dahlhaus JF, Arai N, Ziemann U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF). Clin Neurophysiol 119: 2291–2297, 2008. doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Pitcher JB, Ogston KM, Miles TS. Age and sex differences in human motor cortex input-output characteristics. J Physiol 546: 605–613, 2003. doi: 10.1113/jphysiol.2002.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch NC, Dartnall TJ, Cirillo J, Nordstrom MA, Semmler JG. Corticomotor plasticity and learning of a ballistic thumb training task are diminished in older adults. J Appl Physiol (1985) 107: 1874–1883, 2009. doi: 10.1152/japplphysiol.00443.2009. [DOI] [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res 113: 24–32, 1997. doi: 10.1007/BF02454139. [DOI] [PubMed] [Google Scholar]
- Sale MV, Lavender AP, Opie GM, Nordstrom MA, Semmler JG. Increased intracortical inhibition in elderly adults with anterior-posterior current flow: a TMS study. Clin Neurophysiol 127: 635–640, 2016. doi: 10.1016/j.clinph.2015.04.062. [DOI] [PubMed] [Google Scholar]
- Samusyte G, Bostock H, Rothwell JC, Koltzenburg M. Reproducibility of short interval intracortical inhibition using threshold-tracking. Clin Neurophysiol 127: e67, 2016. doi: 10.1016/j.clinph.2015.11.223. [DOI] [Google Scholar]
- Schambra HM, Ogden RT, Martínez-Hernández IE, Lin X, Chang YB, Rahman A, Edwards DJ, Krakauer JW. The reliability of repeated TMS measures in older adults and in patients with subacute and chronic stroke. Front Cell Neurosci 9: 335, 2015. doi: 10.3389/fncel.2015.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K, Park SB, Geevasinga N, Huynh W, Simon NG, Menon P, Howells J, Vucic S, Kiernan MC. Threshold tracking transcranial magnetic stimulation: effects of age and gender on motor cortical function. Clin Neurophysiol 127: 2355–2361, 2016. doi: 10.1016/j.clinph.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Smith AE, Ridding MC, Higgins RD, Wittert GA, Pitcher JB. Age-related changes in short-latency motor cortex inhibition. Exp Brain Res 198: 489–500, 2009. doi: 10.1007/s00221-009-1945-8. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Curr Biol 21: 480–484, 2011a. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bestmann S, Constantinescu AO, Moreno Moreno L, Allman C, Mekle R, Woolrich M, Near J, Johansen-Berg H, Rothwell JC. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J Physiol 589: 5845–5855, 2011b. doi: 10.1113/jphysiol.2011.216978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Role of intracortical inhibition in selective hand muscle activation. J Neurophysiol 89: 2014–2020, 2003. doi: 10.1152/jn.00925.2002. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Beaulé V, Proulx S, de Beaumont L, Marjanska M, Doyon J, Pascual-Leone A, Lassonde M, Théoret H. Relationship between transcranial magnetic stimulation measures of intracortical inhibition and spectroscopy measures of GABA and glutamate+glutamine. J Neurophysiol 109: 1343–1349, 2013. doi: 10.1152/jn.00704.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129: 35–43, 1997. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- Veale JF. Edinburgh Handedness Inventory - Short Form: a revised version based on confirmatory factor analysis. Laterality 19: 164–177, 2014. doi: 10.1080/1357650X.2013.783045. [DOI] [PubMed] [Google Scholar]
- Vucic S, Cheah BC, Krishnan AV, Burke D, Kiernan MC. The effects of alterations in conditioning stimulus intensity on short interval intracortical inhibition. Brain Res 1273: 39–47, 2009. doi: 10.1016/j.brainres.2009.03.043. [DOI] [PubMed] [Google Scholar]
- Vucic S, Howells J, Trevillion L, Kiernan MC. Assessment of cortical excitability using threshold tracking techniques. Muscle Nerve 33: 477–486, 2006. doi: 10.1002/mus.20481. [DOI] [PubMed] [Google Scholar]
- Walker MC, Semyanov A. Regulation of excitability by extrasynaptic GABA(A) receptors. Results Probl Cell Differ 44: 29–48, 2008. doi: 10.1007/400_2007_030. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol 40: 367–378, 1996. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain 124: 1171–1181, 2001. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]
- Zimerman M, Hummel FC. Non-invasive brain stimulation: enhancing motor and cognitive functions in healthy old subjects. Front Aging Neurosci 2: 149, 2010. doi: 10.3389/fnagi.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghi M, Pearce SL, Nordstrom MA. Differential modulation of intracortical inhibition in human motor cortex during selective activation of an intrinsic hand muscle. J Physiol 550: 933–946, 2003. doi: 10.1113/jphysiol.2003.042606. [DOI] [PMC free article] [PubMed] [Google Scholar]