Abstract
Background
This study’s objective was to evaluate the change in sarcopenia score following neoadjuvant chemotherapy (NAC) and to correlate both sarcopenia and change in score with perioperative outcomes in patients with advanced resected gastric cancer.
Methods
Multi-institutional analysis of patients with gastric cancer who underwent NAC and resection from 2000–2015 was performed. Demographic and perioperative data were included. Sarcopenia score was defined as CT measurement of total psoas muscle at L3, stratified by height (m). Sarcopenia was defined as a score <385 mm2/m2 in women and <545 mm2/m2 in men.
Results
Of 36 patients, 19% were sarcopenic prior to NAC. Following NAC, 31% were sarcopenic, with 14% developing sarcopenia during NAC. One patient (3%) became non-sarcopenic. There were no significant differences in patient, disease, or surgery characteristics between patients who were sarcopenic vs. not. Patients with sarcopenia were more likely to have post-operative complications (P=0.05). There was no significant difference in hospital stay (P=0.7402) or survival (P=0.2317).
Conclusions
A significant number of patients with gastric cancer become sarcopenic during NAC. Although patients with sarcopenia were nearly twice as likely to develop post-operative complications, this did not appear to impact length of stay (LOS) or survival.
Keywords: Gastric cancer, sarcopenia, neoadjuvant chemotherapy (NAC)
Introduction
Although gastric cancer is one of the most common cancers worldwide, it is not seen commonly in the U.S. Only approximately 22,000 patients are diagnosed with gastric cancer in the U.S. annually (1). Neoadjuvant chemotherapy (NAC) to improve survival has emerged as the standard of care in gastric cancer treatment in the U.S (2,3). However, some patients with gastric cancer present with frailty so severe, they may not tolerate NAC.
Biological age, as opposed to chronological age, has garnered increasing attention as a descriptor of functional status. Sarcopenia, defined as decreased skeletal muscle mass and strength two standard deviations below that of healthy adults, has been used as a clinically significant marker of biological age, and has been found to be associated with functional impairment, perioperative complications, and decreased survival in patients with nonmalignant conditions (4-6). Changes in body composition on computed topography (CT) imaging occur after neoadjuvant treatment in many cancers, including esophageal and breast cancer (7-10).
This study sought to understand the impact of NAC on sarcopenia and to evaluate whether sarcopenia is correlated with perioperative outcomes in patients with advanced resected gastric cancer.
Methods
Patient selection
This was a multi-institutional, retrospective cohort study. Records of patients who underwent NAC and surgery for gastric adenocarcinoma at Penn State Hershey Medical Center and Moffitt Cancer Center from March 2000 to April 2015 were reviewed. Only patients who had both pre-NAC and post-NAC CT or positron emission tomography (PET-CT) scans, were included in final analysis.
Outcomes and covariates
Patient characteristics [including age, sex, body mass index (BMI), comorbidities, pre-operative weight], disease characteristics [American Joint Committee on Cancer (AJCC) T and N stage, tumor size, lymph nodes], and treatment characteristics (surgical approach, type of resection, NAC regimen, surgical margins) were retrospectively collected from the electronic medical record. Post-operative complications, hospital length of stay (LOS), and overall survival were the primary outcomes. Surgical complications were classified by the Clavien-Dindo Classification of Surgical Complications Scale, which grades complications based on deviation from the normal post-operative course and their requirement of therapeutic measures.
Imaging studies
Patients underwent contrast-enhanced abdominal/pelvic CT as part of routine preoperative work-up. All perioperative studies were collected. Only patients with CT imaging before and after neoadjuvant treatment were included. Imaging studies were analyzed using Advantage Workstation server 2.0 (GE Healthcare Waukesha, WI, USA) and Aquarius Intuition software, version 4.4.12, by Tera Recon Inc., Forest City, California, USA. Measurements of the psoas muscle surface area were performed on transverse axial slices at the caudal level of the third lumbar vertebra. Two consecutive slices were measured and averaged. Total cross-sectional area was calculated automatically and presented in square centimeters. Medically trained investigators independently carried out the image analysis at each institution. Cross sectional muscle measurements were corrected for patient height resulting in a sarcopenia score (mm2/m2). Sarcopenia was defined as less than 385 mm2/m2 in women and less than 545 mm2/m2 in men, as defined by an international consensus of experts on cancer cachexia (11).
Statistical analysis
All statistical analyses were performed with STATA software (version 12.1, StataCorp, College Station, TX, USA). Univariate statistical tests were used to compare baseline patient, disease, and surgery characteristics between patients with and without sarcopenia, using chi-square and t-tests where appropriate. Post-operative complications, LOS, and survival were compared using chi-square and t-tests where appropriate. Survival was modeled using Kaplan-Meier analyses.
Results
Patient characteristics
Of 41 patients who underwent NAC for advanced gastric adenocarcinoma, 36 had evaluable sarcopenia scores from both before and after NAC. Median follow-up from diagnosis was 17.8 months. This cohort was predominantly female (n=23, 64%), with median age of 64.5 years. There were no significant differences in sex, age, BMI classifications, or co-morbidities, between patients who were never sarcopenic (n=24, 66%) and those who were ever sarcopenic (n=12, 33%) (Table 1). Of the patients who were sarcopenic, 50% (n=6) were overweight, and 17% (n=2) met definition for class I obesity.
Table 1. Patient demographics.
| Variable | Never sarcopenic (N=24) (%) | Ever sarcopenic (N=12) (%) | P value |
|---|---|---|---|
| Sex | 0.4860 | ||
| Male | 33.3 | 38.5 | |
| Female | 50.0 | 61.5 | |
| Age, mean | 61.7 | 67.58 | 0.1027 |
| ≤50 | 10.0 | 7.7 | |
| 51–60 | 26.7 | 7.7 | |
| 61–70 | 30.0 | 46.2 | |
| 71+ | 33.3 | 38.5 | |
| BMI, mean | 29.61 | 25.43 | 0.0845 |
| Underweight | 6.7 | 7.7 | |
| Normal | 0.0 | 0.0 | |
| Overweight | 16.7 | 53.8 | |
| Class I obesity | 16.7 | 15.4 | |
| Class II obesity | 13.3 | 0.0 | |
| Class III obesity | 26.7 | 0.0 | |
| Comorbidities | |||
| GERD | 56.0 | 38.5 | 0.5530 |
| Hypertension | 60.0 | 69.2 | 0.2620 |
| Hyperlipidemia | 48.0 | 38.5 | 0.5630 |
| Atrial fibrillation | 24.0 | 15.4 | 0.4760 |
| DM | 48.0 | 7.7 | 0.1090 |
| CAD | 24.0 | 23.1 | 0.2770 |
| Asthma | 24.0 | 0.0 | 0.3130 |
| Hypothyroid | 28.0 | 0.0 | 0.2240 |
| Arthritis | 20.0 | 7.7 | 0.5270 |
| Other | 40.0 | 38.5 | 0.4250 |
BMI, body mass index.
The majority of patients were Caucasian (n=24, 66%), had some history of tobacco use (n=19, 53%), 2 or more comorbidities (n=27, 75%), and an ASA class of 3 (n=23, 64%). Most patients underwent total gastrectomy (n=26, 72%), while the remainder of the cohort underwent subtotal gastrectomy (n=10, 28%). All patients had confirmed diagnosis of adenocarcinoma, with the majority (n=28, 78%) having poorly differentiated disease (n=28, 78%); the mean tumor size was 5.6 cm, with a median of 2.5 cm. The majority of tumors were located in the body (n=18, 50%). The average lymph node harvest was 24, with a median of 22 and range of 4–45. The average number of positive lymph nodes was 5.4, with a median of 1, and a range of 0–33. The majority of the cohort had negative surgical margins (n=30, 83%). Most patients received epirubicin-based NAC (n=24, 67%), with the most common regimen consisting of epirubicin, cisplatin and 5-fluorouracil (n=19, 53%).
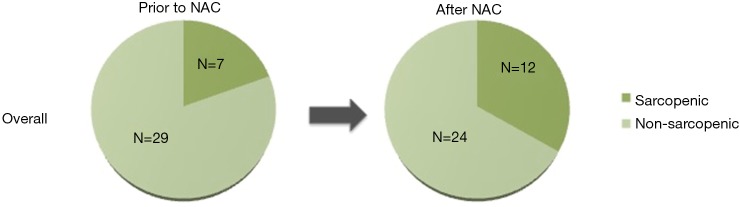
Prior to NAC, 7 patients (19.4%) were sarcopenic (Figure 1). Five patients (14%) became sarcopenic during NAC and one (3%) became non-sarcopenic. One third of the total study population (n=12) was sarcopenic after NAC, prior to surgical resection. There were no significant differences in patient, disease, or surgery characteristics between patients who were never sarcopenic and those who were ever sarcopenic.
Figure 1.
Sarcopenia.
Between patients who were never sarcopenic and those who were ever sarcopenic, there were no significant differences in type of surgical resection, surgical approach, or surgical margins (P>0.05). There was no significant difference in type of NAC administered (P=0.948). Additionally, there were no differences in tumor size, pre- and post-treatment AJCC T or N stage, number of positive lymph nodes, perineural invasion, and lymphovascular invasion (P>0.05). There were also no differences in pre- or post-operative weight, or weight change (P>0.05).
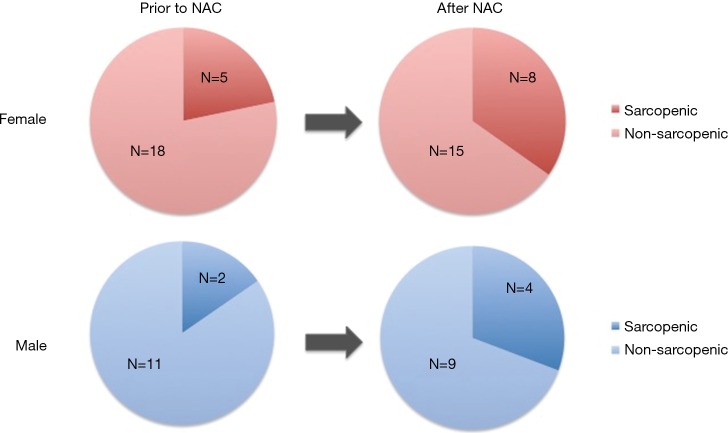
Of the 23 female patients, 5 (21.7%) were sarcopenic prior to NAC, and 8 (34.8%) were sarcopenic after NAC (Figure 2). Of the 13 male patients, 2 (15.4%) were sarcopenic prior to NAC, and 4 (30.8%) were sarcopenic after NAC.
Figure 2.
Sarcopenia by gender.
Peri-operative outcomes
Twelve (33%) patients developed post-operative complications, of which few (n=3, 25%) were Clavien-Dindo grade 3 (Table 2). There were no grade 4 or 5 complications. In total, 50% (n=3) of the sarcopenic patients who developed post-operative complications were overweight, and 17% (n=1) had class I obesity. Those who were sarcopenic prior to resection were more likely to develop post-operative complications than those who were never sarcopenic (58.3% vs. 25.0%, P=0.050). Patients with sarcopenic obesity (defined here as meeting our definition of sarcopenia with a BMI ≥30), were just as likely to develop a post-operative complication as patients who were sarcopenic but not obese (50.0% vs. 50.0%, P=1.000).
Table 2. Disease and surgery characteristics.
| Variable | Never sarcopenic (N=24) (%) | Ever sarcopenic (N=12) (%) | P value |
|---|---|---|---|
| Surgical resection | 0.565 | ||
| Subtotal gastrectomy | 26.7 | 23.1 | |
| Total gastrectomy | 56.7 | 76.9 | |
| Surgical approach | 0.728 | ||
| Robotic | 20.0 | 15.4 | |
| Tumor | |||
| Tumor size | 5.75 | 7.56 | 0.540 |
| Pre-treatment AJCC T | 2.61 | 2.78 | 0.484 |
| Post-treatment AJCC T | 2.60 | 2.69 | 0.853 |
| Change in AJCC T | −0.13 | −0.11 | 0.972 |
| Lymph nodes | |||
| Number of positive LN | 4.88 | 5.92 | 0.705 |
| Pre-treatment AJCC N | 1.00 | 1.46 | 0.193 |
| Post-treatment AJCC N | 1.32 | 1.23 | 0.843 |
| Perineural invasion | 40.0 | 36.4 | 0.842 |
| Lymphovascular invasion | 62.5 | 41.7 | 0.236 |
| Surgical margins | 0.068 | ||
| Positive | 6.7 | 30.8 | |
| Negative | 76.7 | 69.2 | |
| Post-operative complication | 25.0 | 58.3 | 0.050 |
| Weight (kg) | |||
| Pre-operative | 86.26 | 72.93 | 0.168 |
| Post-operative | 77.95 | 70.42 | 0.316 |
| Weight change | −8.49 | −2.51 | 0.139 |
| Neoadjuvant chemotherapy | 0.948 | ||
| DCF | 3.3 | 7.7 | |
| ECX | 10.0 | 15.4 | |
| ECF | 43.3 | 58.9 | |
| Other | 23.3 | 23.1 |
AJCC, American Joint Committee on Cancer.
There was no significant difference in hospital length of stay between those who were sarcopenic prior to resection and those who were never sarcopenic (9.0 days vs. 9.2 days, P = 0.125), data not shown. There was no significant difference in post-operative complications or hospital length of stay amongst those who were sarcopenic prior to NAC, after NAC, and sarcopenic both before and after NAC (P=0.100, P=0.385, respectively), data not shown. No significant difference was found in post-operative complications or hospital length of stay when comparing patients who were never sarcopenic, sarcopenic prior to NAC, sarcopenic after NAC, and sarcopenic both before and after NAC (Table 3).
Table 3. Perioperative outcomes.
| Outcomes | Sarcopenic | P value | |||
|---|---|---|---|---|---|
| Never sarcopenic (N=24) | Prior to NAC (N=1) | After NAC (N=5) | Both before and after NAC (N=6) | ||
| Post-operative complication | 26.1% | 0.0% | 20.0% | 83.3% | 0.054 |
| Length of stay (days) | 9.2 | 5 | 9.4 | 9.2 | 0.753 |
NAC, neoadjuvant chemotherapy.
Survival
There was no significant difference in survival in patients who were ever sarcopenic and those who were never sarcopenic (P=0.232) (Figure 3).
Figure 3.
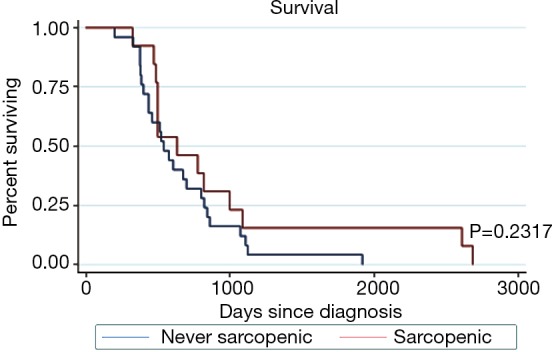
Kaplan Meier survival.
Discussion
Weight loss, early satiety, anorexia and dysphagia are some of the most common presenting symptoms of gastric cancer (12). Preoperative frailty and sub-optimal nutritional status are of particular concern in this patient population (13). In the US, it is possible to have patients who are simultaneously obese but also sarcopenic (14). The aim of this study was to investigate if sarcopenia impacted outcomes.
One third of our patient population was found to be sarcopenic prior to surgery, which is within the range of sarcopenia in gastric cancer patients reported in the literature (12.5–57.7%) (15-17). There is some controversy surrounding the association between sarcopenia and postoperative complications. In a study of 152 patients, sarcopenia was not associated with post-operative morbidity or mortality (17). In a large-scale Asian cohort of 937 patients, Zhuang et al. reported sarcopenia as an independent predictor of severe postoperative complications in patients who underwent radical gastrectomy for gastric cancer (16). Moreover, in another Asian study of 255 patients, Wang et al. reported sarcopenic patients were associated with a higher risk of postoperative complications, longer postoperative hospital stays and greater hospital costs (15). In our study, patients with sarcopenia were nearly twice as likely to experience post-operative complications.
There is evidence that sarcopenic obesity is a unique combination with compound burdens on outcomes, but there are conflicting reports regarding the association between sarcopenic obesity and postoperative outcomes (14). Peng et al. reported an increase in severe post-operative complications in patients with colorectal cancer with sarcopenic obesity who underwent hepatic resection (18). However, Lodewick et al. reported that sarcopenia and sarcopenic obesity did not impact complication rates in a similar study population (19). A meta-analysis of 14 studies linking sarcopenic obesity to clinical outcomes in cancer patients concluded that sarcopenic obesity was associated with increased surgical complications (14). This study found similar rates of complications between patients with sarcopenia and sarcopenic obesity, which was higher than non-sarcopenic patients.
Reports in the literature are varied regarding the impact of sarcopenia on mortality in patients with gastric cancer, however, there is a paucity of data from Western populations (16,17). In this small cohort, there was no significant difference in survival in patients who were never sarcopenic and those who were sarcopenic. This current study attempts to focus on determining the number of patients who become sarcopenic during NAC and determine whether sarcopenia has an impact on perioperative morbidity and mortality.
There are several limitations in this study that should be acknowledged. Our conclusions are limited by small sample size and the retrospective nature of this investigation. Regarding sarcopenia calculations, there is inherent variability in measuring skeletal muscle area on CT. A validation study with large sample size is necessary to further characterize the impact of preoperative sarcopenia on perioperative outcomes and survival.
Conclusions
During NAC, a significant number of patients with gastric cancer become sarcopenic, adding to a sizeable percentage of already sarcopenic patients presenting for surgery. In this small cohort, patients with sarcopenia were nearly twice as likely to develop post-operative complications; however, this did not appear to impact LOS or survival. Further study with a larger population is warranted to assess the impact of sarcopenia on gastric cancer outcomes in a Western population.
Acknowledgements
None.
Ethical Statement: The study was approved by the Institutional Scientific Review Committee of Penn State Hershey Cancer Institute (PSHCI 15-036).
Footnotes
Conflicts of Interest: SSAT Digestive Disease Week, Poster of Distinction, San Diego, CA, May 21–24, 2016.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. 10.3322/caac.21208 [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 3.Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 2010;28:5210-18. 10.1200/JCO.2009.26.6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang G, Li X, Sui C, et al. Incidence and risk factor analysis for sarcopenia in patients with cancer. Oncol Lett 2016;11:1230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889-96. 10.1046/j.1532-5415.2002.50216.x [DOI] [PubMed] [Google Scholar]
- 6.Metter EJ, Talbot LA, Schrager M, et al. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci 2002;57:B359-65. 10.1093/gerona/57.10.B359 [DOI] [PubMed] [Google Scholar]
- 7.Yip C, Goh V, Davies A, et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol 2014;24:998-1005. 10.1007/s00330-014-3110-4 [DOI] [PubMed] [Google Scholar]
- 8.Awad S, Tan BH, Cui H, et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr 2012;31:74-7. 10.1016/j.clnu.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 9.Tan BH, Brammer K, Randhawa N, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol 2015;41:333-8. 10.1016/j.ejso.2014.11.040 [DOI] [PubMed] [Google Scholar]
- 10.Del Fabbro E, Parsons H, Warneke CL, et al. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist 2012;17:1240-45. 10.1634/theoncologist.2012-0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. 10.1016/S1470-2045(10)70218-7 [DOI] [PubMed] [Google Scholar]
- 12.Wanebo HJ, Kennedy BJ, Chmiel J, et al. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg 1993;218:583-92. 10.1097/00000658-199321850-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen FF, Zhang FY, Zhou XY, et al. Role of frailty and nutritional status in predicting complications following total gastrectomy with D2 lymphadenectomy in patients with gastric cancer: a prospective study. Langenbecks Arch Surg 2016;401:813-22. 10.1007/s00423-016-1490-4 [DOI] [PubMed] [Google Scholar]
- 14.Carneiro IP, Mazurak VC, Prado CM. Clinical Implications of Sarcopenic Obesity in Cancer. Curr Oncol Rep 2016;18:62. 10.1007/s11912-016-0546-5 [DOI] [PubMed] [Google Scholar]
- 15.Wang SL, Zhuang CL, Huang DD, et al. Sarcopenia Adversely Impacts Postoperative Clinical Outcomes Following Gastrectomy in Patients with Gastric Cancer: A Prospective Study. Ann Surg Oncol 2016;23:556-64. 10.1245/s10434-015-4887-3 [DOI] [PubMed] [Google Scholar]
- 16.Zhuang CL, Huang DD, Pang WY, et al. Sarcopenia is an Independent Predictor of Severe Postoperative Complications and Long-Term Survival After Radical Gastrectomy for Gastric Cancer: Analysis from a Large-Scale Cohort. Medicine 2016;95:e3164. 10.1097/MD.0000000000003164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tegels JJ, van Vugt JL, Reisinger KW, et al. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J Surg Oncol 2015;112:403-7. 10.1002/jso.24015 [DOI] [PubMed] [Google Scholar]
- 18.Peng PD, van Vledder MG, Tsai S, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 2011;13:439-46. 10.1111/j.1477-2574.2011.00301.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lodewick TM, van Nijnatten TJ, van Dam RM, et al. Are sarcopenia, obesity and sarcopenic obesity predictive of outcome in patients with colorectal liver metastases? HPB (Oxford) 2015;17:438-46. 10.1111/hpb.12373 [DOI] [PMC free article] [PubMed] [Google Scholar]