Abstract
Blunt cerebrovascular injury (BCVI) encompasses two distinct clinical entities: traumatic carotid artery injury (TCAI) and traumatic vertebral artery injury (TVAI). The latter is the focus of our review. These are potentially devastating injuries which pose a diagnostic challenge in the acute trauma setting. There is still debate regarding the optimal screening criteria, diagnostic imaging modality and treatment methods. In 2012 the American College of Surgeons proposed criteria for investigating patients with suspected TVAI and subsequent treatment methods, caveated with the statement that evidence is limited and still evolving. Here we review the historical evidence and recent literature relating to these recommendations.
Keywords: Blunt, trauma, vertebral, vascular, injury, vertebral artery injury (VAI), blunt cerebrovascular injury (BCVI)
Introduction
Occlusion of the cerebrovascular circulation may occur spontaneously or as a result of trauma. Blunt cerebrovascular injury (BCVI) includes any form of non-penetrating injury to the internal carotid and vertebral arteries. The internal carotid artery commences at the bifurcation of the common carotid artery and enters that carotid canal in the petrous temporal bone at the base of the skull. The vertebral artery, a branch of the subclavian artery, most frequently travels in the foramen transversarium of the C6–C1 vertebrae piercing the dura mater at the foramen magnum (Figure 1). Ultimately, both internal carotid and vertebral arteries are tributaries of the eponymous Circle of Willis (Figure 2).
Figure 1.
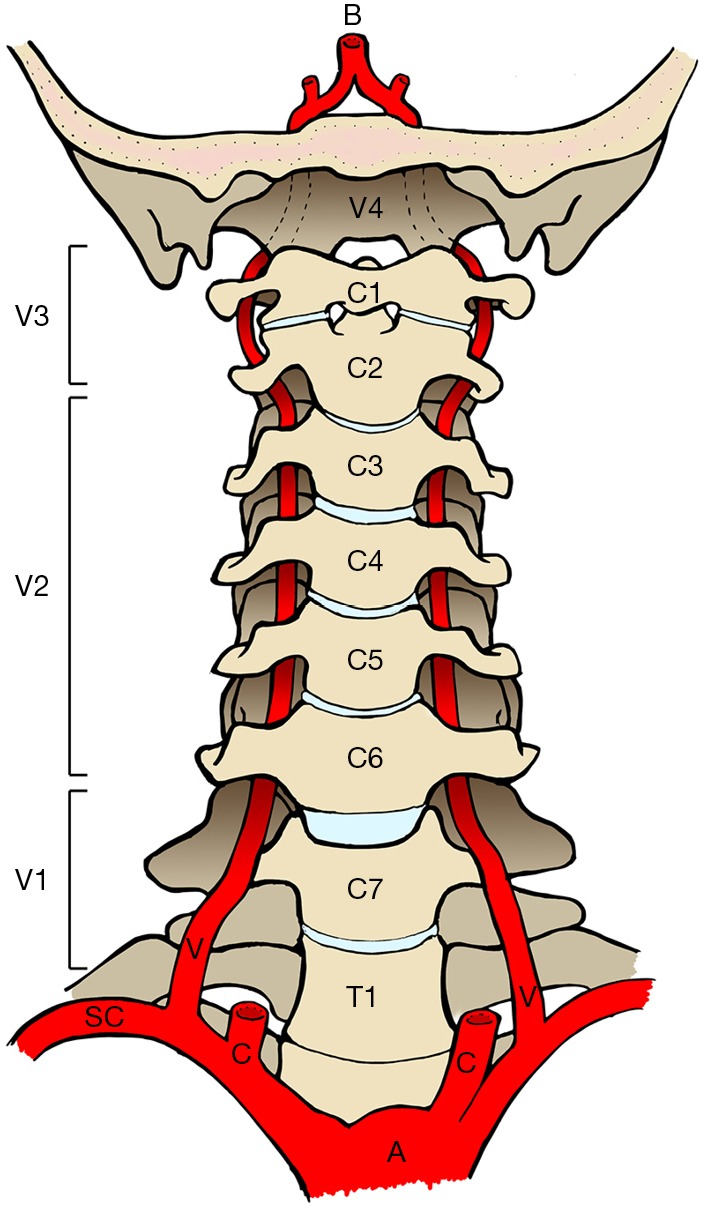
Vertebral Artery Anatomy. A, aorta; B, basilar artery; C, common carotid artery; SC, subclavian artery; V, vertebral artery. Segments of the path of the vertebral artery; V1, preforaminal; V2, foraminal; V3, atlantoaxial; V4, intradural.
Figure 2.
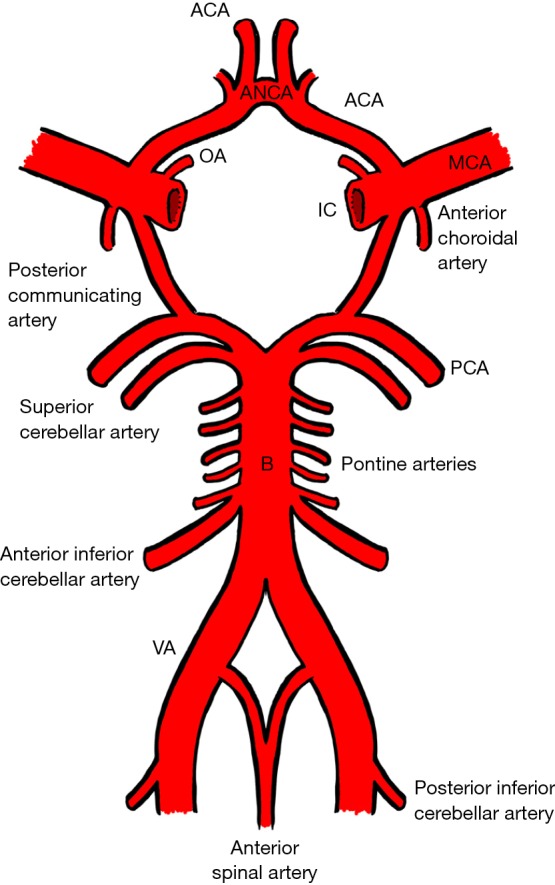
The Circle of Willis. VA, vertebral artery; B, basilar artery; PCA, posterior cerebral artery; IC, internal carotid artery; MCA, middle cerebral artery; ACA, anterior cerebral artery; ANCA, anterior communicating artery; OA, ophthalmic artery.
Although often described as a single clinical entity, BCVI encompasses two distinct clinical entities: traumatic vertebral artery injury (TVAI) and traumatic carotid artery injury (TCAI). The focus of our review is TVAI, which classically presents in the setting of cervical spine trauma. TVAI is a potentially devastating injury. First described by Matas in 1893 (1), TVAI often affects the young following road traffic accidents, hanging and sporting injuries and the mortality rate may be as high as 100% (2). Despite this, TVAI is notoriously difficult to diagnose (3). It is often occult or may occur in the presence of distracting injuries. As such, it has been suggested that a screening protocol should be established in at risk patients. However, there remains significant debate regarding who is at risk of TVAI and the imaging modality to be used as a screening tool. These issues are compounded by controversies regarding the treatment of TVAI.
These controversies were acknowledged by the American College of Surgeons. In 2012 the American College of Surgeons published the 9th edition of the Advanced Trauma Life Support (ATLS®) manual which proposed criteria for investigating patients with suspected TVAI and subsequent treatment (4). The authors suggest that screening should be performed in patients with fractures of the first three cervical vertebra, cervical fracture subluxations and fractures through the foramen transversarium. Further to this, recommended treatments include anticoagulant or antiplatelet therapy in the absence of any contraindications. These suggestions are caveated with the statement that evidence remains limited and is still evolving (5-11).
Our goal is to evaluate the existing evidence base and literature. Ultimately we aim to raise awareness of TVAI, identify those patients at risk and suggest possible screening and treatment modalities.
Methods
We conducted a review of the literature to identify articles relating to VAI using the PubMed, Web of Knowledge, Medline and Cochrane Database of Systematic Reviews. Only studies using human subjects and those written in English were included. A broad search strategy was employed using the terms: “cerebrovascular” OR “BCVI” OR “vertebral artery” AND (injury OR trauma OR occlusion OR dissection). Two reviewers (RS and SS) evaluated the abstract of each article to determine the value of those articles to our review. The bibliographies of relevant articles were also evaluated to obtain further pertinent articles.
Mechanisms & pathophysiology
Reported mechanisms of BCVI from prospective studies and assumptions based on the pathogenesis of injury include cervical spine flexion-distraction, flexion-compression, hyperextension, rotation and direct impact (12-15). Hyperextension injury has been found to be significantly associated with TVAI in particular (15). The most common cause of injury is major trauma sustained during road traffic accidents (16,17). Trivial trauma and spinal manipulation are also recognised modes of injury (16). Such trauma can culminate in numerous pathologies including dissection, thrombosis, aneurysms, pseudoaneurysms, arteriovenous fistula formation, transection or vasospasm (18,19).
Risk factors & radiological predictors
In a study of 249 blunt trauma patients, who underwent screening arteriography, Biffl et al. performed a linear regression analysis, identifying the risk factors for BCVI (Table 1). Interestingly, numerous independent predictors of carotid artery injury (CAI) were identified. Cervical spine fracture was found to be the only independent predictor of vertebral artery injury (VAI). It has been estimated that 70% to 78% of VAI’s occur in the presence of cervical spine fracture (5,8,20) and conversely VAI may occur in up to 39% of cervical spine fractures (20,21).
Table 1. Probability of BCVI in the presence of risk factors reported by Biffl et al. (12).
| Factors | Risk (%) |
|---|---|
| VAI risk factors (cervical spine fracture) | |
| No fracture | 3 |
| Cervical spine fracture | 33 |
| CAI risk factors (Glasgow coma scale <6, petrous bone fracture, diffuse axonal injury and LeFort II/III fracture) | |
| No risk factors | 20 |
| 1 risk factor | 33–48 |
| 2 risk factors | 56–74 |
| 3 risk factors | 80–88 |
| 4 risk factors | 93 |
Over nine years Cothren et al. undertook a screening program for BCVI on over 17,000 blunt trauma admissions (22). Patients were screened if they either had the signs of symptoms of a BCVI or if they had a risk factor for a BCVI (Table 2).
Table 2. Screening criteria for BCVI used by Cothren et al. (20).
| Signs & symptoms |
| Arterial hemorrhage |
| Cervical bruit |
| Expanding haematoma |
| Focal neurological deficit |
| Neurological examination incongruous with CT head findings |
| Stroke on secondary CT scan |
| Risk factors |
| High-energy mechanism with: |
| Cervical-spine fracture patterns |
| LeForte II or III fracture |
| Basilar skull fracture with carotid canal involvement |
| Diffuse axonal injury with GCS <6 |
| Near-hanging with anoxic brain injury |
Only 23 (0.1%) patients presented with signs or symptoms of BCVI. A total of 766 (4.5%) patients underwent screening with angiography, which identified 258 (34%) cases of BCVI. Of these patients 125 had sustained a fracture of the cervical spine, the majority associated with VAI (n=84) rather than carotid artery injury (n=18) or combined injuries (n=23).
Of the patients with cervical spine fracture and BCVI, 117 (93%) had fractures, with either subluxation (48%), C1 to C3 fractures (36%) or extension into the foramen transversarium (19%). Fractures of C1 to C3 involved the C1 arch, C2/C3 body or C1/2 subluxations. Fracture-subluxations associated with BCVI were not limited to a specific level and could be single or multilevel. Eight patients with BCVI had minor fractures of the cervical spine and were screened for other criteria. Importantly, of the total 17,007 trauma admissions 317 patients underwent screening angiography on the basis of the three specific cervical fracture patterns above and 37% were identified to have BCVI. The association between VAI and cervical spine fracture/dislocations is supported by more recent studies (23,24).
A multivariate analysis by Lebl et al. corroborated the need to screen patients with the above injury patterns but also suggested basilar skull fracture, occipitocervical dissociation, and cervical spine fracture in patients with ankylosing spondylitis/ diffuse idiopathic skeletal hyperostosis should be considered higher-risk injuries for VAI and associated neurological events (25). Vilela et al. demonstrated a 50% incidence of BCVI in patients with craniocervical injuries (C0–C1) (26). As such these patients may also need to be considered for screening.
Screening & imaging
Clinical symptoms of VAI may be attributed to ischaemic complications of the posterior circulation and include headache, dizziness, nausea, vomiting, reduced GCS, vision or speech disturbance and abnormalities in gait (27). That being said, a significant proportion of patients are asymptomatic, as demonstrated by Biffl and Cothren et a.l (20,22) and more recently by Jacobson et al. (3). Moreover, some may present with delayed neurological symptoms (13) or have a concomitant head injury. Löhrer et al. highlighted the rationale for a screening protocol, to identify VAI in asymptomatic patients, allowing early treatment before the onset of neurological sequelae (28).
The recommendations of the ATLS® subcommittee do not extend to the choice of imaging modality for the screening of BCVI but do allude to the use of computed tomography angiography (CTA). Numerous modalities have been described in the literature apart from CTA.
Digital subtraction angiography (DSA)
DSA has previously been considered the gold standard by the many groups supporting the introduction of screening protocols (5,29). DSA may detect BCVI in up to 34% of asymptomatic patients with blunt trauma (13,29). However, this modality is invasive, carries the risk of iatrogenic injury, a 1–2% risk of stroke as well as being comparatively expensive. Cothren et al. argued that when utilised as part of a screening protocol, DSA may reduce long term rehabilitation and treatment costs associated with neurological events (29). The possible risk of iatrogenic complications, along with the lack of availability of DSA in some institutions has led to increasing use of non-invasive imaging modalities.
Duplex doppler ultrasound (US)
Duplex Doppler US, although readily available and non-invasive, is associated with poor sensitivity (38%) when detecting BCVI (30). Other problems include operator dependency and poor visualisation of the vessels at the skull base. As a result US is not recommended as a screening modality for BCVI (5,30,31).
Magnetic resonance angiography (MRA)
MRA has gained favour as it is noninvasive and does not require the administration of contrast. Furthermore, diffusion weighted sequences may allow for rapid identification of cerebral ischaemia (27). Unfortunately studies investigating the use of MRA compared to DSA for the diagnosis of BCVI have demonstrated low sensitivity of 47–75% (21,32). It was felt MRA failed to detect low grade injuries. This, coupled with the lack of rapid availability at many institutions, long scan times and potential artefact with orthopaedic implants makes MRA a suboptimal recommended screening modality for BCVI.
Computed tomography angiography (CTA)
Historically early generation CTA was associated with poor sensitivity and specificity when screening for BCVI (21,32). Sensitivity and specificity has improved with the advent of higher resolution CT scanners (33). Berne et al. screened 435 patients for BCVI injury and reported an incidence of 1.2% (34) which is comparable with studies involving screening with DSA. No patients with negative scans subsequently developed adverse neurological symptoms suggestive of BCVI. Similarly Biffl et al. found that sixteen slice CTA did not miss any clinically relevant cases of BCVI in a screened group of 331 patients (5).
A comparative study by Eastman et al. utilised sixteen slice CTA and DSA to screen a group of 162 patients deemed at risk of BCVI (9). The results were concordant in 98% of cases. CTA produced a single false negative in a low grade VAI that ultimately required no intervention. In their study the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of CTA were 97.7%, 100%, 100% and 99.3% respectively. In their prospective study of 158 patients Goodwin et al. reported a lower sensitivity of 41% but a comparable specificity of 97% when combining both 16 and 64-slice CTA (10). In this study CTA was performed close to the time of injury whereas DSA occurred anywhere from 24–48 h post trauma. The authors felt CTA was not as accurate as DSA but did acknowledge that the delay in performing DSA may have allowed for evolving injuries not present at the time of injury to be missed by CTA.
Interpretation of CTA may also rely on accurate radiological interpretation. In their comparative study of DSA and CTA, Malhotra et al. found a specificity of 84% when using CTA to diagnose BCVI (35). The authors alluded to a high false negative rate in the first half of the study which they attributed to the learning curve of reporting radiologists. The specificity and NPV was found to be 100% in the second half of the study.
A meta-analysis by Roberts et al. suggests the variability in the diagnostic performance of CTA across studies is most likely due to variation in diagnostic thresholds set by individual trauma centers (36).
Although DSA remains the gold standard imaging modality for screening patients with suspected BCVI, CTA is a more accessible option in many institutions and may be more cost effective (23). In addition CTA is non-invasive, may allow for concurrent imaging of other injuries, and has benefited from improved accuracy with the advancement of CT technology. As such CTA should be considered as a suitable screening modality for BCVI.
Treatment
Once a diagnosis of BCVI is made, determining the grade of injury will guide treatment and prognosis. Biffl et al. developed the most commonly used grading system for these injuries based on angiographic appearances (Table 3) (37).
Table 3. Cerebrovascular injury grading scale [Biffl et al. (30)].
| Injury grade | Descriptions | Stroke rate (%) | Mortality rate (%) |
|---|---|---|---|
| Grade I | Luminal irregularity with <25% narrowing | 3 | 11 |
| Grade II | Dissection or intramural haematoma with 25% or greater narrowing, intraluminal thrombus or raised intimal flap | 11 | 11 |
| Grade III | Pseudoaneurysm | 33 | 11 |
| Grade IV | Occlusion | 44 | 22 |
| Grade V | Transection with extravasation | 100 | 100 |
Untreated CAI and VAI have a stroke rate of up to 68% (21,38) and 100% in Grade IV injuries (39). The increasing rate is associated with increasing grade of injury (38). Treatment of BCVI is aimed at reducing the risk of neurological sequelae and death (40).
There is currently no level I study comparing treatment modalities in BCVI. Optimal treatment is therefore a source of controversy. Treatment should be decided upon according to the patient’s clinical symptoms, site and grade of injury and the presence/absence of contraindications.
The options include observation, anti- thrombotic therapy, endovascular therapy or open surgery. The ATLS recommendations touch upon treatment with anticoagulants or antiplatelet agents in the absence of contraindications. Suggested relative contraindications include traumatic brain injury or haemorrhage, solid organ injury and pelvic fracture (41). A retrospective study by Callcut et al. has suggested early pharmacological treatment of BCVI is safe in the presence of hemorrhagic neurological injury (42).
Absolute contraindications may include those with uncontrolled haemorrhage of any source. Caution must be advised in the presence of poly-trauma patients without haemorrhage who may develop coagulopathy as a physiological response to their injuries.
Anticoagulant versus antiplatelet therapy
Medical therapy is aimed at hindering thrombus formation and the propagation of an embolus. Anticoagulation may be with Heparin initially, followed by a prolonged course of Warfarin. Antiplatelet therapy is typically with Aspirin or Clopidogrel. A number of studies have demonstrated antithrombotic therapy reduces both mortality and neurologic morbidity after BCVI (6,7,11,21,29). Although Scott et al. suggest that the rate of stroke in low grade injuries (I/II) was unaffected by pharmacological treatment (43).
An optimal regimen has yet to be determined. Cothren et al. compared anticoagulant and antiplatelet therapy in their retrospective review of 18,431 blunt trauma patients (6). They identified 422 BCVIs in 301 patients. Patients were started on either unfractionated Heparin or an antiplatelet agent (Aspirin and or Clopidogrel) according to surgeon discretion as soon as a diagnosis was BCVI was made. Patients treated with Heparin were later converted to Warfarin therapy. There was a 0.5% stroke rate in BCVIs treated with antithrombotic therapy. Untreated patients with BCVI had a stroke rate of 21%. Anticoagulant and antiplatelet agents were equally effective in reducing stroke. Follow-up imaging did not reveal any difference in healing or progression rates of radiological lesions. The authors did not comment on the duration of treatment. A prospective randomized study of anticoagulant vs. antiplatelet therapy for BCVI is currently being undertaken by these authors.
Stein et al. observed a lower incidence of stroke in their study but similarly observed no difference in the efficacy of anticoagulants or antiplatelet agents in preventing stroke following BCVI (11). A multicenter randomised trial of atraumatic cervical artery (carotid or vertebral) dissection has also failed to demonstrate any difference in the efficacy of anticoagulant or antiplatelet agents in preventing stroke and death (44). Ultimately the choice of agent may be made according to surgical and patient factors. Intravenous Heparin infusions may be useful in the acute setting where acute reversal of the agent may be required whereas long term antiplatelet therapy will be more convenient for the patient. The optimal duration of treatment is yet to be determined and therefore local hematological input is advised to direct treatment on a case by cases basis.
Endovascular therapy
Endovascular treatment may be warranted when antithrombotic treatment is contraindicated particularly in the presence of high grade or surgically inaccessible injuries. The presence of collateral circulation, site and grade of injury will determine the endovascular treatment utilised. Options include stenting, occlusion of the vertebral artery or coil embolisation of a pseudoaneurysm (27,45). Some authors have reported significant complication rates associated with endovascular BCVI treatment such as iatrogenic injury, ischaemic neurological injury and vessel occlusion (46,47).
There is little evidence regarding the outcomes of VAI treated with endovascular techniques and no studies exist directly comparing endovascular with medical therapy. Burlew et al. only performed endovascular intervention on 2% of high grade BCVIs in their study. They felt that antithrombotic therapy alone was effective in preventing stroke and negated the risks of endovascular treatment, and the latter should be reserved for symptomatic patients or those with enlarging pseudoaneurysms (47). Consequently, we would suggest that decision making for treatment of these injuries should follow a multidisciplinary approach and involve early consultation with a spinal surgeon, stroke physician, vascular surgeon and interventional radiologist.
Surgery
Surgical access to the VA is technically challenging but may have a role as a last resort for those patients whom medical and endovascular treatment is not possible or has failed. Uncontrollable haemorrhage from VA transection has been suggested as a definite indication for surgical treatment (27). Outcome data for open surgery for VAI is lacking but is likely to be associated with high morbidity and mortality.
Serial imaging
Vascular lesions may evolve over time with or without treatment and therefore follow-up imaging may be warranted. Biffl et al. used follow up arteriography at 7–10 days post injury and determined treatment changed 61% of the time as a result of evolving Grade I and II injuries (38), either increasing in grade or healing. Conversely follow-up arteriography of high-grade injuries did not alter management in a large proportion of patients and the group did not recommend early reimaging for these injuries (48). Further studies concur with the notion that higher grade injuries do not warrant intensive radiological follow up (49). Serial imaging has also been advocated for the presence of indeterminate BCVI on initial screening which is thought progress to true BCVI in 25.4% of imaged vessels with 5% of patients developing neurological symptoms (50).
We would suggest that early re-imaging, most likely a CTA, may be considered as an adjunct to determine the evolution of low grade lesions; monitor indeterminate lesions and investigate patients experiencing a clinical deterioration. It may also be useful in monitoring vascular lesions in patients in whom antithrombotic treatment is contraindicated.
Discussion
In light of the above evidence, patients presenting with a history of high-energy trauma and or concomitant head and neck trauma (including hanging) should be approached with a high index of suspicion for BCVI. Clinical signs of vascular trauma such haematoma, bruits and haemorrhage as well as neurological symptoms of posterior circulation insufficiency mandate further imaging. All patients presenting with cervical spine fractures at the C1–C3 levels, cervical fracture subluxations and those involving the foramen transversarium warrant screening for VAI. We suggest that CTA is the imaging modality of choice due its non-invasive nature, high sensitivity and wide availability. That being said, where available, units may opt for DSA.
When a VAI is detected, early discussion with a spinal surgeon, stroke physician or neurologist is advised. Medical treatment with anticoagulation (Heparin/Warfarin) or antiplatelets (Aspirin/Clopidogrel) should be considered in the absence of contraindications. Endovascular and surgical treatments should be reserved for high grade or progressing injuries and require early involvement of an interventional radiologist or vascular surgeon.
Conclusions
All physicians managing trauma patients should be aware of TVAI and have a low threshold for screening in the presence of risk factors. Patients with symptoms of posterior circulation ischaemia, high-energy injury mechanisms and specific cervical spine fracture patterns should raise the suspicion of TVAI and warrant further investigation in the form of radiological screening. Once TVAI is diagnosed, early treatment is recommended to avoid neurological complications with early involvement of the regional spinal unit. A multidisciplinary approach may be required with involvement of spinal surgeons, vascular surgeons, interventional radiologists, stroke physicians and hematologists. We recommend all trauma units should implement a local screening protocol.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Matas R. Traumatisms and Traumatic Aneurisms of the Vertebral Artery and Their Surgical Treatment with the Report of a Cured Case. Ann Surg 1893;18:477-521. 10.1097/00000658-189307000-00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott WW, Sharp S, Figueroa SA, et al. Clinical and radiological outcomes following traumatic Grade 3 and 4 vertebral artery injuries: a 10-year retrospective analysis from a Level I trauma center. The Parkland Carotid and Vertebral Artery Injury Survey. J Neurosurg 2015;122:1202-7. 10.3171/2014.9.JNS1461 [DOI] [PubMed] [Google Scholar]
- 3.Jacobson LE, Ziemba-Davis M, Herrera AJ. The limitations of using risk factors to screen for blunt cerebrovascular injuries: the harder you look, the more you find. World J Emerg Surg 2015;10:46. 10.1186/s13017-015-0040-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subcommittee A, Trauma ACoSCo, group IAw. Advanced trauma life support (ATLS®): the ninth edition. J Trauma Acute Care Surg 2013;74:1363-6. [DOI] [PubMed] [Google Scholar]
- 5.Biffl WL, Egglin T, Benedetto B, et al. Sixteen-slice computed tomographic angiography is a reliable noninvasive screening test for clinically significant blunt cerebrovascular injuries. J Trauma 2006;60:745-52; discussion 751-2. 10.1097/01.ta.0000204034.94034.c4 [DOI] [PubMed] [Google Scholar]
- 6.Cothren CC, Biffl WL, Moore EE, et al. Treatment for blunt cerebrovascular injuries: equivalence of anticoagulation and antiplatelet agents. Arch Surg 2009;144:685-90. 10.1001/archsurg.2009.111 [DOI] [PubMed] [Google Scholar]
- 7.Cothren CC, Moore EE, Biffl WL, et al. Anticoagulation is the gold standard therapy for blunt carotid injuries to reduce stroke rate. Arch Surg 2004;139:540-5; discussion 5-6. 10.1001/archsurg.139.5.540 [DOI] [PubMed] [Google Scholar]
- 8.Cothren CC, Moore EE, Biffl WL, et al. Cervical spine fracture patterns predictive of blunt vertebral artery injury. J Trauma 2003;55:811-3. 10.1097/01.TA.0000092700.92587.32 [DOI] [PubMed] [Google Scholar]
- 9.Eastman AL, Chason DP, Perez CL, et al. Computed tomographic angiography for the diagnosis of blunt cervical vascular injury: is it ready for primetime? J Trauma 2006;60:925-9; discussion 9. 10.1097/01.ta.0000197479.28714.62 [DOI] [PubMed] [Google Scholar]
- 10.Goodwin RB, Beery PR, 2nd, Dorbish RJ, et al. Computed tomographic angiography versus conventional angiography for the diagnosis of blunt cerebrovascular injury in trauma patients. J Trauma 2009;67:1046-50. 10.1097/TA.0b013e3181b83b63 [DOI] [PubMed] [Google Scholar]
- 11.Stein DM, Boswell S, Sliker CW, et al. Blunt cerebrovascular injuries: does treatment always matter? J Trauma 2009;66:132-44; discussion 143-4. 10.1097/TA.0b013e318142d146 [DOI] [PubMed] [Google Scholar]
- 12.Giacobetti FB, Vaccaro AR, Bos-Giacobetti MA, et al. Vertebral artery occlusion associated with cervical spine trauma. A prospective analysis. Spine (Phila Pa 1976) 1997;22:188-92. 10.1097/00007632-199701150-00011 [DOI] [PubMed] [Google Scholar]
- 13.Biffl WL, Moore EE, Offner PJ, et al. Optimizing screening for blunt cerebrovascular injuries. Am J Surg 1999;178:517-22. 10.1016/S0002-9610(99)00245-7 [DOI] [PubMed] [Google Scholar]
- 14.Vaccaro AR, Klein GR, Flanders AE, et al. Long-term evaluation of vertebral artery injuries following cervical spine trauma using magnetic resonance angiography. Spine (Phila Pa 1976) 1998;23:789-94; discussion 95. 10.1097/00007632-199804010-00009 [DOI] [PubMed] [Google Scholar]
- 15.Nakajima H, Nemoto M, Torio T, et al. Factors associated with blunt cerebrovascular injury in patients with cervical spine injury. Neurol Med Chir (Tokyo) 2014;54:379-86. 10.2176/nmc.oa.2013-0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haldeman S, Kohlbeck FJ, McGregor M. Risk factors and precipitating neck movements causing vertebrobasilar artery dissection after cervical trauma and spinal manipulation. Spine (Phila Pa 1976) 1999;24:785-94. 10.1097/00007632-199904150-00010 [DOI] [PubMed] [Google Scholar]
- 17.Biffl WL, Moore EE, Offner PJ, et al. Blunt carotid and vertebral arterial injuries. World J Surg 2001;25:1036-43. 10.1007/s00268-001-0056-x [DOI] [PubMed] [Google Scholar]
- 18.Fassett DR, Dailey AT, Vaccaro AR. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech 2008;21:252-8. 10.1097/BSD.0b013e3180cab162 [DOI] [PubMed] [Google Scholar]
- 19.Taneichi H, Suda K, Kajino T, et al. Traumatically induced vertebral artery occlusion associated with cervical spine injuries: prospective study using magnetic resonance angiography. Spine (Phila Pa 1976) 2005;30:1955-62. 10.1097/01.brs.0000176186.64276.d4 [DOI] [PubMed] [Google Scholar]
- 20.Biffl WL, Moore EE, Elliott JP, et al. The devastating potential of blunt vertebral arterial injuries. Ann Surg 2000;231:672-81. 10.1097/00000658-200005000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller PR, Fabian TC, Croce MA, et al. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg 2002;236:386-93; discussion 393-5. 10.1097/00000658-200209000-00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cothren CC, Moore EE, Ray CE, Jr, et al. Cervical spine fracture patterns mandating screening to rule out blunt cerebrovascular injury. Surgery 2007;141:76-82. 10.1016/j.surg.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 23.Wang AC, Charters MA, Thawani JP, et al. Evaluating the use and utility of noninvasive angiography in diagnosing traumatic blunt cerebrovascular injury. J Trauma Acute Care Surg 2012;72:1601-10. 10.1097/TA.0b013e318246ead4 [DOI] [PubMed] [Google Scholar]
- 24.Franz RW, Willette PA, Wood MJ, et al. A systematic review and meta-analysis of diagnostic screening criteria for blunt cerebrovascular injuries. J Am Coll Surg 2012;214:313-27. 10.1016/j.jamcollsurg.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 25.Lebl DR, Bono CM, Velmahos G, et al. Vertebral artery injury associated with blunt cervical spine trauma: a multivariate regression analysis. Spine (Phila Pa 1976) 2013;38:1352-61. 10.1097/BRS.0b013e318294bacb [DOI] [PubMed] [Google Scholar]
- 26.Vilela MD, Kim LJ, Bellabarba C, et al. Blunt cerebrovascular injuries in association with craniocervical distraction injuries: a retrospective review of consecutive cases. Spine J 2015;15:499-505. 10.1016/j.spinee.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 27.deSouza RM, Crocker MJ, Haliasos N, et al. Blunt traumatic vertebral artery injury: a clinical review. Eur Spine J 2011;20:1405-16. 10.1007/s00586-011-1862-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Löhrer L, Vieth V, Nassenstein I, et al. Blunt cerebrovascular injuries in acute trauma care: a screening protocol. Eur Spine J 2012;21:837-43. 10.1007/s00586-011-2009-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cothren CC, Moore EE, Ray CE, Jr, et al. Screening for blunt cerebrovascular injuries is cost-effective. Am J Surg 2005;190:845-9. 10.1016/j.amjsurg.2005.08.007 [DOI] [PubMed] [Google Scholar]
- 30.Mutze S, Rademacher G, Matthes G, et al. Blunt cerebrovascular injury in patients with blunt multiple trauma: diagnostic accuracy of duplex Doppler US and early CT angiography. Radiology 2005;237:884-92. 10.1148/radiol.2373042189 [DOI] [PubMed] [Google Scholar]
- 31.Bromberg WJ, Collier BC, Diebel LN, et al. Blunt cerebrovascular injury practice management guidelines: the Eastern Association for the Surgery of Trauma. J Trauma 2010;68:471-7. [DOI] [PubMed] [Google Scholar]
- 32.Biffl WL, Ray CE, Jr, Moore EE, et al. Noninvasive diagnosis of blunt cerebrovascular injuries: a preliminary report. J Trauma 2002;53:850-6. 10.1097/00005373-200211000-00008 [DOI] [PubMed] [Google Scholar]
- 33.Paulus EM, Fabian TC, Savage SA, et al. Blunt cerebrovascular injury screening with 64-channel multidetector computed tomography: more slices finally cut it. J Trauma Acute Care Surg 2014;76:279-83; discussion 284-5. 10.1097/TA.0000000000000101 [DOI] [PubMed] [Google Scholar]
- 34.Berne JD, Reuland KS, Villarreal DH, et al. Sixteen-slice multi-detector computed tomographic angiography improves the accuracy of screening for blunt cerebrovascular injury. J Trauma 2006;60:1204-9; discussion 1209-10. 10.1097/01.ta.0000220435.55791.ce [DOI] [PubMed] [Google Scholar]
- 35.Malhotra AK, Camacho M, Ivatury RR, et al. Computed tomographic angiography for the diagnosis of blunt carotid/vertebral artery injury: a note of caution. Ann Surg 2007;246:632-42; discussion 642-3. 10.1097/SLA.0b013e3181568cab [DOI] [PubMed] [Google Scholar]
- 36.Roberts DJ, Chaubey VP, Zygun DA, et al. Diagnostic accuracy of computed tomographic angiography for blunt cerebrovascular injury detection in trauma patients: a systematic review and meta-analysis. Ann Surg 2013;257:621-32. 10.1097/SLA.0b013e318288c514 [DOI] [PubMed] [Google Scholar]
- 37.Biffl WL, Moore EE, Offner PJ, et al. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma 1999;47:845-53. 10.1097/00005373-199911000-00004 [DOI] [PubMed] [Google Scholar]
- 38.Biffl WL, Ray CE, Jr., Moore EE, et al. Treatment-related outcomes from blunt cerebrovascular injuries: importance of routine follow-up arteriography. Ann Surg 2002;235:699-706; discussion -7. [DOI] [PMC free article] [PubMed]
- 39.Lauerman MH, Feeney T, Sliker CW, et al. Lethal now or lethal later: The natural history of Grade 4 blunt cerebrovascular injury. J Trauma Acute Care Surg 2015;78:1071-4; discussion 1074-5. 10.1097/TA.0000000000000654 [DOI] [PubMed] [Google Scholar]
- 40.DiCocco JM, Fabian TC, Emmett KP, et al. Functional outcomes following blunt cerebrovascular injury. J Trauma Acute Care Surg 2013;74:955-60. 10.1097/TA.0b013e318287800f [DOI] [PubMed] [Google Scholar]
- 41.Burlew CC, Biffl WL. Blunt cerebrovascular trauma. Curr Opin Crit Care 2010;16:587-95. 10.1097/MCC.0b013e32833ee8b4 [DOI] [PubMed] [Google Scholar]
- 42.Callcut RA, Hanseman DJ, Solan PD, et al. Early treatment of blunt cerebrovascular injury with concomitant hemorrhagic neurologic injury is safe and effective. J Trauma Acute Care Surg 2012;72:338-45; discussion 345-6. 10.1097/TA.0b013e318243d978 [DOI] [PubMed] [Google Scholar]
- 43.Scott WW, Sharp S, Figueroa SA, et al. Clinical and radiological outcomes following traumatic Grade 1 and 2 vertebral artery injuries: a 10-year retrospective analysis from a Level 1 trauma center. J Neurosurg 2014;121:450-6. 10.3171/2014.4.JNS132235 [DOI] [PubMed] [Google Scholar]
- 44.CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol 2015;14:361-7. 10.1016/S1474-4422(15)70018-9 [DOI] [PubMed] [Google Scholar]
- 45.Cohen JE, Gomori JM, Rajz G, et al. Vertebral artery pseudoaneurysms secondary to blunt trauma: Endovascular management by means of neurostents and flow diverters. J Clin Neurosci 2016;32:77-82. 10.1016/j.jocn.2016.03.023 [DOI] [PubMed] [Google Scholar]
- 46.Cothren CC, Moore EE, Ray CE, et al. Carotid artery stents for blunt cerebrovascular injury: risks exceed benefits. Arch Surg 2005;140:480-5; discussion 5-6. 10.1001/archsurg.140.5.480 [DOI] [PubMed] [Google Scholar]
- 47.Burlew CC, Biffl WL, Moore EE, et al. Endovascular stenting is rarely necessary for the management of blunt cerebrovascular injuries. J Am Coll Surg 2014;218:1012-7. 10.1016/j.jamcollsurg.2014.01.042 [DOI] [PubMed] [Google Scholar]
- 48.Wagenaar AE, Burlew CC, Biffl WL, et al. Early repeat imaging is not warranted for high-grade blunt cerebrovascular injuries. J Trauma Acute Care Surg 2014;77:540-5; quiz 650. 10.1097/TA.0000000000000418 [DOI] [PubMed] [Google Scholar]
- 49.Scott WW, Sharp S, Figueroa SA, et al. Clinical and radiographic outcomes following traumatic Grade 3 and 4 carotid artery injuries: a 10-year retrospective analysis from a Level 1 trauma center. The Parkland Carotid and Vertebral Artery Injury Survey. J Neurosurg 2015;122:610-5. 10.3171/2014.10.JNS14875 [DOI] [PubMed] [Google Scholar]
- 50.Crawford JD, Allan KM, Patel KU, et al. The Natural History of Indeterminate Blunt Cerebrovascular Injury. JAMA Surg 2015;150:841-7. 10.1001/jamasurg.2015.1692 [DOI] [PubMed] [Google Scholar]


