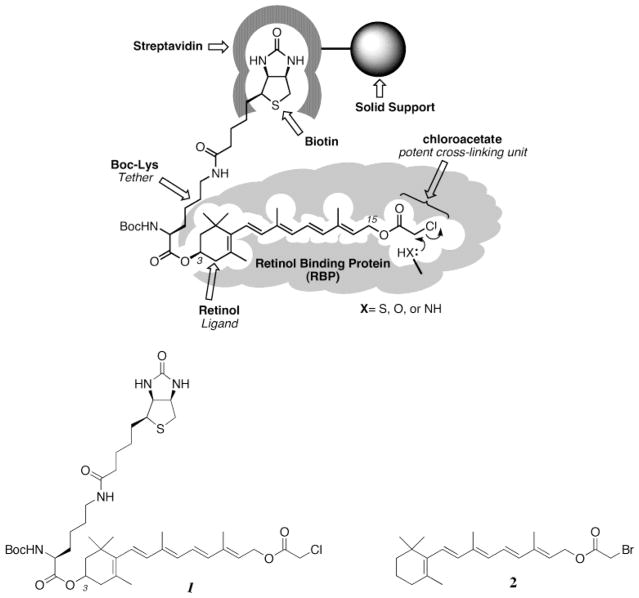
Abstract
Retinal pigment epithelial (RPE) membranes contain the full biochemical apparatus capable of processing all-trans-retinol (vitamin A) into 11-cis-retinal, the visual chromophore. As many of these proteins are integral membrane proteins and resistant to traditional methods of identification, alternate methods of identifying these proteins are sought. The approach described here involves affinity biotinylation with alkali cleavable linkers. A vitamin A containing affinity-labeling haloacetate is described which facilitates the identification of retinoid binding proteins (RBPs). Treatment of crude bovine RPE membranes with (3R)-3-[boc-lys(biotinyl)-O]-all-trans-retinol chloroacetate 1 in the low micromolar range led to the specific labeling of RPE65 and lecithin retinol acyltransferase (LRAT). Only RPE65 is labeled at 5 μM 1 at 4 °C. Labeled RPE65 was readily isolated by binding the labeled protein to avidin-containing beads, followed by cleavage of the protein from the beads at pH 11. Trypsin digestion of RPE65 modified by 1, followed by mass spectrometry, demonstrates that C231 and C448 are alkylated by 1. These studies validate the approach that was used, and furthermore demonstrate that RPE65, a major membrane-associated protein of the RPE, is a RBP.
Affinity biotinylation can be a powerful tool in proteomics for the identification of novel proteins (1–6). In this procedure, an irreversible affinity-labeling agent is modified to contain a biotinyl moiety. The extraordinary affinity of the protein avidin for biotin (KA ~ 1014) (7) allows for the detection and, in theory, the purification of the labeled proteins. Detection is readily managed through the specific binding of avidin fluorescent probes or enzyme conjugates to the modified proteins with concomitant gel electrophoresis (1–6). In principle, purification is also facilitated by avidin–biotin interactions. Immobilized tetrameric avidin is used to bind the modified proteins. The difficulty here lies in the elution step. The high affinity of tetrameric avidin for biotinylated proteins makes elution exceedingly difficult. Elution is usually attempted under strongly protein denaturing conditions (e.g., 8 M guanidinium hydrochloride, low pH, 70% formamide, and biotin). However, elution under these harsh conditions has not proven to be successful (8). While biotinylated proteins are easily eluted from monomeric avidin, the weak affinity of monomeric avidin for biotin decreases the extent of its use, especially when minor proteins are being targeted. The solution concentrations of these proteins may be substantially less than the KD for binding.
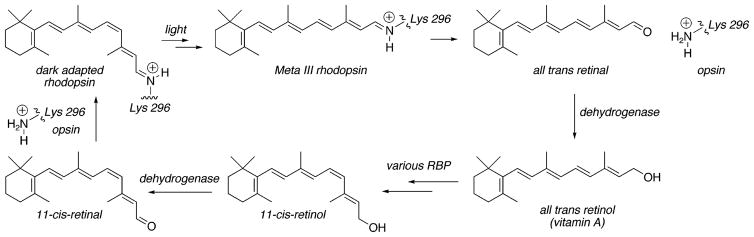
One solution to the elution problem caused by high-affinity tetrameric avidin–ligand interactions is to use alkali sensitive linking groups. This approach is reduced to practice in the current work. An alkali cleavable biotin-containing affinity reagent (Scheme 1) is used to specifically label retinoid binding proteins (RBPs)1 that are part of the mammalian visual cycle (9–11). This cycle is comprised of all of the biochemical reactions required for the biosynthesis of 11-cis-retinal, the visual chromophore (Scheme 2) (9–11). The visual cycle is still poorly understood, and key elements remain unidentified.
Scheme 1.
A Biotinylated Affinity-Labeling Agent for RBPs
Scheme 2.
Biochemical Reactions of the Visual Cycle
The visual cycle is comprised of the sum of biochemical processes that are essential for the processing of 11-cis-retinal. The absorption of light by the retinal photoreceptor rhodopsin leads to the photoisomerization of its 11-cis-retinal Schiff base chromophore into all-trans-retinal (12). Enzyme-mediated reduction of the dissociated all-trans-retinal to all-trans-retinol (vitamin A) occurs in the photoreceptors (13,14). The remaining biochemistry of the mammalian visual cycle occurs in the RPE (9–11). The vitamin A liberated from the photoreceptors is transported to the RPE where it is processed into 11-cis-retinal, before being recycled to the RPE (9–11).
Several RBPs of the mammalian visual cycle have been characterized. These include lecithin retinol acyl transferase (LRAT) (3), 11-cis-retinol dehydrogenase (15), all-trans-retinol dehydrogenase (14), cellular retinal binding protein (CRALBP) (16, 17), and the retinal G protein-coupled receptor (RGR) (18, 19). RPE65 (20, 21), a major membrane-associated RPE protein, is another protein which may be of interest in the operation of the visual cycle, inasmuch as a mouse knockout of this protein disrupts 11-cis-retinal biosynthesis (22). A RBP function for this protein has not been reported. Finally, of course, the enzyme(s) responsible for the trans to cis double-bond isomerization/hydrolysis has yet to be identified (Scheme 2).
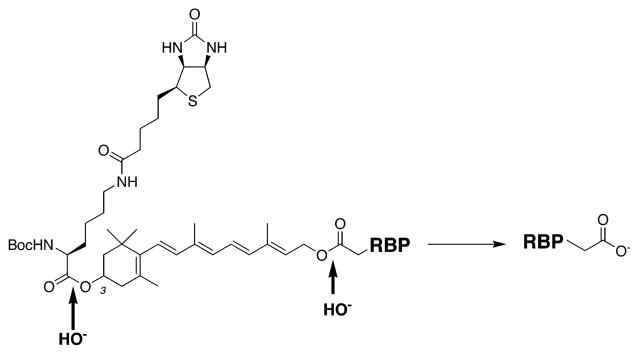
To initiate the establishment of an inventory of the RBPs of the mammalian visual system, a cleavable biotinylated retinoid affinity-labeling agent is described here. The vitamin A-containing reagent is shown in Scheme 3. The all-trans-retinyl ester 1 is a biotinylated analogue of all-trans-retinyl bromoacetate (RBA) 2, which had been used to affinity label lecithin retinol acyltransferase (LRAT), a key component of the visual cycle (23). The chloroacetate analogue 1 (RCA) of RBA inactivates LRAT as well (unpublished experiments). The chloroacetate analogue 1 was chosen for study here because of its anticipated higher chemical reactivity. This analogue contains a biotin moiety linked via the 3-hydroxyl group of the vitamin A analogue. Initial experiments are presented here which establish the utility of 1 in the specific labeling of both RPE65, revealed here to be a RBP, and LRAT, a known RBP (23).
Scheme 3.
Alkali Cleavable Sites of RBPs Labeled with 1
EXPERIMENTAL PROCEDURES
Materials
The synthesis of (3R)-3-[boc-lys(biotinyl)-O]-all-trans-retinol chloroacetate 1 is described elsewhere (24). Frozen bovine eye cups were obtained from W. L. Lawson Co. (Lincoln, NE). Dithiothreitol (DTT), all-trans-retinol, all-trans-retinyl acetate, oleyl acetate, gelatin, and the protease inhibitor cocktail (AEBSF, aprotinin, bestatin, EDTA, E-64, leupeptin, and pepstatin A) were from Sigma. Triton X-100 was from Fluka, and Tween 20 was from Bio-Rad. Centriprep, Centricon 10, and an electroeluter were from Amicon. Western blot blocking buffer, Gel-code blue, neutravidin, Tris-buffered saline pack [25 mM Tris and 150 mM NaCl (pH 7.2)] were from Pierce. HPLC grade solvents were from J. T. Baker. Coomassie brilliant blue R-250 was from Bio-Rad. The silver staining kit, polyvinylidene fluoride membrane, anti-rabbit Ig-conjugated horseradish peroxidase, and the ECL-Western blotting kit were from Amersham Pharmacia Biotech. Precast mini/two-dimensional (2D) gels (4 to 12%, 18%, 4 to 20%, 8 cm × 8 cm) for SDS–PAGE were from Invitrogen Life Technologies, and the preparative precast gel (4 to 20%, 16 cm × 18 cm) was from Jule Inc. (New Haven, CT). Precast medium size gels (4 to 20%, 8 to 16%, 2D gel, 9 cm × 13 cm) were from Bio-Rad. Dalton VI molecular mass markers for 14, 18, 24, 35, 45, and 66 kDa were from Sigma, and Benchmark prestained markers for 8, 15, 20, 26, 37, 50, 64, 81, 114, and 177 kDa were from GibcoBRL Life Technologies. Biotinylated molecular mass markers (7, 14, 22, 31, 45, 66, 97, 116, and 200 kDa), two-color prestained markers (10, 15, 20, 25, 37, 50, 75, 100, 150, and 200 kDa), avidin-conjugated horseradish peroxidase, and a 10× Tris-buffered saline solution [20 mM Tris and 500 mM NaCl (pH 7.5)] were from Bio-Rad. All other reagents were analytical grade.
Methods
Preparation of the Bovine Pigment Epithelium (RPE) Membranes
The procedure for the preparation of bovine RPE membranes was described elsewhere (25). Prior to being used, the membranes were irradiated with UV light (365 nm) on ice for 5 min so endogenous retinoids would be destroyed. The RPE stock solution contains 2–4 mg of proteins/mL, as determined by the Bradford assay.
The solubilization of RPE in detergents was reported previously (25). Briefly, RPE membranes were solubilized in 10 mM Tris-HCl (pH 9.0), 2 mM DTT, 1 mM EDTA, and 1% Triton X-100. After thorough mixing for 1 h at 4 °C, the material was centrifuged at 10500g for 1 h. Dialysis was performed at 4 °C using 10 mM Tris-HCl (pH 8.0), 1 mM EDTA, 2 mM DTT, and 0.1% Triton X-100 after concentrating the sample with a Centriprep.
Specific RPE Protein Labeling by 1
All labeling experiments using retinoids were performed in a dark room under dim red light. Generally, 100 μL of RPE (200–300 μg of total proteins) was incubated with compound 1 (10 μM) for 1 h at 4 °C to label the specific retinoid binding proteins. At the end of the incubation periods, excess reagent was removed by centrifugation (174000g for 20 min at 4 °C), dialysis (MW cutoff of 8 kDa), or acetone precipitation (1 mL, −20 °C). The labeled sample was dissolved in 2% SDS and subjected to SDS–PAGE. In the kinetic experiments, the RPE with 1 (5 μM) was successively quenched by the Laemmli sample buffer (26) at 20 s, 2 min, 10 min, 30 min, 1 h, and 3 h and then analyzed by SDS–PAGE. To study the binding pocket competition by retinoids and thiol reactive molecules, active site preblocking experiments were performed. RPE was preincubated with all-trans-retinol (1 mM), all-trans-retinyl acetate (1 mM), oleyl acetate (1 mM), iodoacetamide (1 or 55 mM), NEM (1 or 55 mM), and RBA (90 μM or 1 mM) for 1 h at 4 °C, and then 1 (5 or 10 μM) was added to the preincubated solution for 10–60 min at 4 °C, followed by SDS–PAGE analysis and biotin detection blotting.
One-Dimensional (1D) SDS-PAGE Analysis
Tris-glycine polyacrylamide gel (4 to 12%, 4 to 20%, or 18%) electrophoresis was carried out using the buffer system (25 mM Tris, 192 mM glycine, and 0.1% SDS) described by Laemmli (26). Denaturing of proteins was performed by heating samples (100 °C for 2 min) in sample buffer (2×) containing 4% SDS, 10% 2-mercaptoethanol, 20% glycerol, 0.004% bromophenol blue, and 125 mM Tris-HCl (pH 6.8). Proteins were visualized by Coomassie staining (0.1%) or silver staining. For mini gels and medium size gels (8 cm × 8 cm × 1 mm and 9 cm × 13 cm × 1 mm), 20 μL of the protein solution (20–30 μg of total proteins, as determined by the Bradford assay) was loaded in each well, and for preparative gels (16 cm × 18 cm × 0.75 mm), 500 μL of the sample (1–1.5 mg of protein based on the Bradford assay) was loaded along with the molecular mass markers.
2D SDS–PAGE Analysis
2D gel electrophoresis was performed by isoelectric focusing (IEF) using Immobiline Dry Strips with a pH gradient from 3 to 10 (7, 11, and 13 cm, Amersham Pharmacia Biotech) in the first dimension and 8 to 16% (9 cm × 13 cm, Bio-Rad) or 4 to 20% (8 cm × 8 cm, Invitrogen, and 16 cm × 18 cm, Jule Inc.) SDS–PAGE in the second dimension. Before rehydration of the immobilized pH gradient (IPG) strip, 125 μL of rehydration buffer for 7 cm (7 M urea, 2 M thiourea, 4 μL of Triton X-100, 2% CHAPS, 0.5% IPG buffer, a few grains of bromophenol blue, and 0.7 mg of DTT in doubly distilled water; 200 μL for 11 cm and 250 μL for 13 cm) were mixed with the sample. Iso-electrofocusing in the IPGphor system (Amersham Pharmacia Biotech) was conducted at successive voltages of 500 (3 h), 1000 (3 h), 4000 (1 h), 6000 (1 h), and 8000 V (11 h) with very low currents (less than 50 μA per IPG strip). After IEF, strips were equilibrated with 10 mL of SDS equilibration buffer (50 mM Tris-HCl, 6 M urea, 30% glycerol, 2% SDS, 100 mg of DTT, and a few grains of bromophenol blue). Prestained molecular mass markers (177, 114, 81, 64, 50, 37, 26, 20, 15, and 8 kDa, Invitrogen) or biotinylated markers (200, 116, 97, 66, 45, 31, 22, 14, and 7 kDa) were run in the side well. Proteins were visualized by biotin detection blots and silver staining.
General Purification of Biotinylated Proteins Using Neutravidin–Agarose Beads
After dialysis, the solution labeled with 1 (20 μM) and Triton X-100-solubilized proteins (final protein concentration of 1–2 mg/mL) was incubated for 3 h at 4 °C with a slurry of neutravidin–agarose beads. An approximate ratio of 3 mg of protein/mL of beads is sufficient for near-quantitative uptake of the biotinylated proteins onto the beads. The beads were washed five times in PBS [20 mM sodium phosphate and 150 mM NaCl (pH 7.5)] and 0.1% Triton X-100, and then were left rotating overnight in this solution. After 12 h, the PBS solution was removed and replaced with a 200 mM sodium carbonate/bicarbonate buffered solution (pH 11 in 0.1% Triton X-100). The beads were rotated in 2 volumes of this solution at room temperature, for 6 h. This solution was concentrated using an Ultrafree centrifugal filter device (5 kDa MW cutoff, Millipore) and then run on a 4 to 20% Tris-glycine gel. Proteins were visualized using either a modified, mass spectrometry-compatible silver staining procedure (27) or Coomassie blue staining. Bands visualized in this manner were excised and analyzed by mass spectrometry.
Active Site Peptide Purification
After RPE had been labeled (0.5 mL, 1–1.5 mg of total proteins) with compound 1 (100 μM, 1 h at 4 °C), followed by preparative 1D SDS–PAGE (16 cm × 18 cm), the 60 kDa band was excised and proteins were eluted using an electroeluter (Amicon) at 100 V for 3 h. Proteins were digested with trypsin (20–50 μg) in 50 mM ammonium bicarbonate buffer overnight at 37 °C. Digested peptides were incubated with neutravidin–agarose beads overnight at room temperature, followed by washing five times with sodium phosphate buffer [20 mM, 150 mM NaCl (pH 7.5)] before elution. Biotinylated proteins were eluted from the beads by incubation in sodium carbonate/bicarbonate buffer (200 mM, pH 11) overnight. Eluted peptides were purified with a C18 column (Waters) twice using an acetonitrile gradient elution buffer (0 to 90% MeCN and 0.1% TFA) prior to mass spectrometric analysis.
Western Blot Analysis
The preparation of polyclonal anti-LRAT peptide antibodies was reported previously (3). The polyclonal anti-LRAT protein antibody was obtained from D. Bok (University of California, Los Angeles, CA). An anti-RPE65 peptide (NFITKVNPETLETIK) antibody was obtained from Genemed (San Francisco, CA). After protein separation by SDS–PAGE, proteins were transferred to a polyvinylidene fluoride (PVDF) membrane for 30 min at 15 V using Tris-glycine buffer (25 mM Tris and 192 mM glycine) and ethanol (20%) on a semidry transfer apparatus (Bio-Rad). Alternatively, biotin-detected PVDF membrane was washed with erasure buffer [1% SDS, 20 mM Tris, and 500 mM NaCl (pH 7.5)] at 60 °C for 1 h, followed by washing four times (30 min) with TTBS buffer [0.05% Tween 20, 20 mM Tris, and 500 mM NaCl (pH 7.5)]. The membrane was blocked with 5% nonfat dried milk or super block blocking buffer (Pierce, 3% BSA) for 2 h at room temperature. Anti-LRAT antibody (1:4000 dilution, 2 h), anti-rabbit Ig-linked horseradish peroxidase (1:8000 dilution, 1 h) from donkey, and the enhanced chemiluminescence (ECL) system were used to detect the LRAT band. For the RPE65 Western blot, the same protocol was applied except that an anti-RPE65 peptide antibody was used.
Biotin Detection Blot Analysis
After protein separation by SDS–PAGE, proteins were transferred to a polyvinylidene fluoride (PVDF) membrane for 30 min at 15 V using Tris-glycine buffer (25 mM Tris and 192 mM glycine) and ethanol (20%) on a semidry transfer apparatus (Bio-Rad). The membrane was blocked in 100 mL of the blocking solution [3% gelatin, 25 mM Tris, and 150 mM NaCl (pH 7.2)] using a shaker platform for 1 h. The blocking solution was removed, and the membrane was washed twice with TTBS solution [0.05% Tween 20, 25 mM Tris, and 150 mM NaCl (pH 7.2)] for 5 min. After removal of TTBS buffer, avidin-conjugated horseradish peroxidase (33 μL) in 100 mL of antibody buffer [1% gelatin, 0.05% Tween 20, 25 mM Tris, and 150 mM NaCl (pH 7.2)] was incubated for 1 h. The membrane was washed twice with 100 mL of TTBS buffer [0.05% Tween 20, 25 mM Tris, and 150 mM NaCl (pH 7.2)] for 5 min and twice with 100 mL of TBS [25 mM Tris and 150 mM NaCl (pH 7.2)] for 5 min. The ECL solution (6 mL) was added to the membrane for 1 min before X-ray film exposure. Using a higher concentration of saline TTBS buffer [20 mM Tris, 0.05% Tween 20, and 500 mM NaCl (pH 7.5)] reduced the background signal and the magnitude of the endogenously biotinylated protein band.
Mass Spectrometry Analysis
Protein labeled with 1 was processed for mass spectrometry as follows. The protein was purified by neutravidin–agarose chromatography and separated by SDS–PAGE, and the mass spectrometry compatible silver-stained protein band (60–65 kDa) was cut and dehydrated in MeCN for 10 min. Gel pieces were covered with DTT (10 mM) in NH4HCO3 (100 mM) to reduce proteins for 1 h at 56 °C. After the mixture had cooled to room temperature, the reducing buffer was removed. The gel washing/dehydration cycle was repeated three times with the NH4HCO3/MeCN mixture before trypsin (12.5 ng/μL, 5 μL/mm2 gel, overnight) digestion at 37 °C. Gel pieces were centrifuged, and the supernatant was collected. Peptides were further extracted by one change of 20 mM NH4HCO3 and three changes of 5% formic acid in 50% CH3CN (20 min between changes) at 25 °C. Trypsin digestion of the gel band and the mass spectrometric analysis were performed at the Taplin Biological Mass Spectrometry Facility at Harvard Medical School. When peptides were analyzed by ion-trap mass spectrometry, the amino acid sequence was determined by tandem mass spectrometry (MS/MS) and a database search. LCQ DECA ion-trap mass spectrometers (Thermo-Finnigan) were used. For microcapillary LC elution, a linear gradient of 100% buffer A (5% MeCN, 95% H2O, 0.1% formic acid, and 0.005% heptafluorobutyric acid) through 100% buffer B (95% MeCN, 5% H2O, 0.1% formic acid, and 0.005% heptafluorobutyric acid) was used. The capillary column was 75 μm (inside diameter) × 12 cm (bed length). The flow rate was split down from the pumps to 200 nL/min for the separation. The bovine database was extracted and downloaded from the NCBI website (http://www.ncbi.nlm.nih.gov/). The same procedure was followed for the 20–25 kDa band.
MALDI-TOF mass analysis was performed using Voyager-DE STR from Applied Biosystems at the Dana-Farber Cancer Institute Core Facility (Boston, MA). α-Cyano-4-hydroxy-cinnamic acid (0.5 μL, for peptide) or sinapinic acid (0.5 μ L, for protein) was used as the matrix for each sample (0.5 μL). Reflector mode, an accelerating voltage of 20 000 V, and an extraction delay time of 200 ns were applied. The laser intensity was 1900–2300, and 100–200 laser shots were collected for each spectrum. The acquisition mass range was 750–4500 Da with a low-mass gate of 600 Da.
RESULTS
Labeling of RPE65 and LRAT by 1
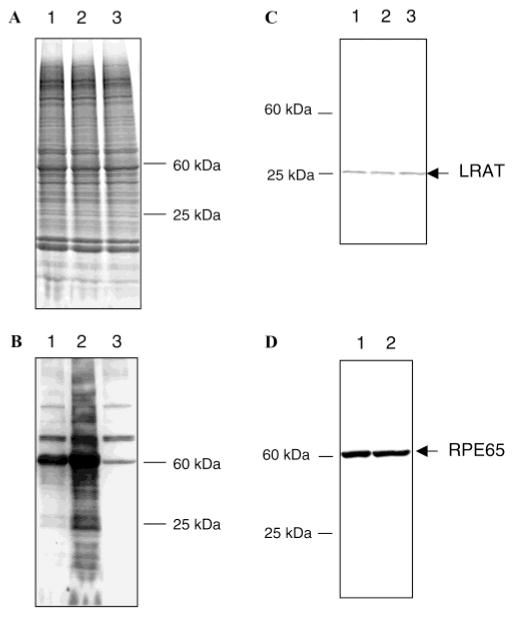
At the end of the incubation periods, excess reagent was removed by centrifugation or cold acetone precipitation. The labeled sample was dissolved in 2% SDS and subjected to SDS–PAGE. Detection of the biotinylated protein bands affinity labeled with 1 was with avidin-bound HRP (Figure 1B), and total protein staining was with Coomassie blue (Figure 1A). Figure 1A shows the Coomassie-stained gel revealing the high degree of complexity consistent with a crude RPE membrane preparation. At 10 μM 1 (Figure 1B, lane 1), clear-cut preferential labeling of an ~60 kDa protein is observed. The labeled protein is demonstrated below to be RPE65. The observed specific labeling is in stark contrast to the complexity observed in the Coomassie-stained gel (Figure 1A). At higher concentrations of 1, additional labeled biotinylated species are observed (Figure 1B, lane 2). The data shown in Figure 1 demonstrate that the labeling of RPE membranes by 1 at low concentrations is highly selective. Figure 1B (lane 3) shows three groups of endogenous proteins with molecular masses of approximately 114, 74, and 60 kDa which either cross react with avidin or are in fact biotinylated proteins. Interestingly, these proteins do not appear on an 18% gel (data not shown), but only when running 4 to 20% gradient gels, probably due to the higher resolution of the latter gel. In any case, these proteins are lost upon avidin chromatography and do not interfere with the identification of the proteins biotinylated with 1. When the membranes were incubated at 10 μM 1 at 25 °C for 1 h again, the strong labeling of a protein at approximately 60 kDa was observed. In addition, the appearance of several weaker bands was also noted, including the labeling of proteins at approximately 25 kDa. When labeling with 10 μM 1 was carried out at 4 °C (Figure 1B, lane 1), the same pattern of bands was observed, but their intensity was somewhat lower than at the higher temperature. Labeling with 1 at 100 μM at 4 °C (Figure 1B, lane 2) produced more general labeling, but the labeling of the approximately 60 kDa protein was still major. Clear bands in the 25 kDa region can be discerned here. When the labeling was carried out with 10 μM 1 for 5 min at 4 °C, the labeling was simplified further, and clearly, the labeling of the 60 kDa protein predominated.
Figure 1.
Retinoid affinity biotinylation of RPE. (A) SDS–PAGE gradient gel (4 to 20%). Proteins were visualized by Coomassie blue staining: lane 1, RPE labeled with 1, 10 μM, 1 h at 4 °C; lane 2, RPE labeled with 1, 100 μM, 1 h at 4 °C; and lane 3, control RPE. (B) Biotin detection of labeled proteins. Proteins were transferred to a PVDF membrane and visualized by avidin-HRP/ECL. Lanes are the same as those for panel A. (C) LRAT Western blot. Proteins were transferred to a PVDF membrane, and LRAT was visualized by anti-LRAT antibody/anti-rabbit Ig-HRP/ECL. Lanes are the same as those for panel A. (D) RPE65 Western blot. Proteins were transferred to a PVDF membrane, and RPE65 was visualized by anti-RPE65 antibody/anti-rabbit Ig-HRP/ECL: lane 1, RPE labeled with 1, 50 μM, 1 h at 4 °C; and lane 2, control RPE.
While it is clear that labeling of the 60 kDa protein predominates, it is also clear that other proteins are labeled when labeling is carried out under more forcing conditions. Western blotting experiments suggest that at least two well-known proteins are labeled. Figure 1C shows a Western blot using an anti-LRAT antibody, and Figure 1D shows RPE65 Western blot analysis using an anti-RPE65 polyclonal antibody. The approximately 25 kDa band labeled by 1 (Figure 1B) is also stained by the anti-LRAT antibody (Figure 1C), and the labeling of this band by 1 is blocked by added RBA (see below; data not shown), the specific affinity-labeling agent for LRAT (23).
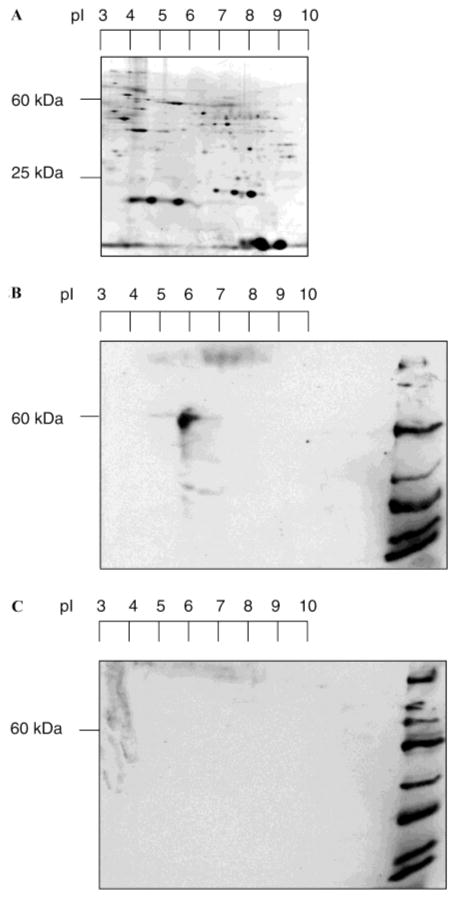
A 2D gel was run to further investigate possible complexity in the labeling process (Figure 2). In Figure 2A, the silver-stained gel again shows the high degree of proteomic diversity in RPE membranes. As can be seen in this 2D gel, the 60 kDa band (RPE65) (pI = 5.5–5.8) is by far the predominantly labeled protein (Figure 2B), and there are no observable avidin cross-reactive proteins in the unlabeled control (Figure 2C). Thus, the labeling of RPE membranes with 1 provides a remarkably simple profile, and is dominated by the labeling of the 60 kDa protein. The calculated pI for RPE65 of 6.0 (http://us.expasy.org/tools/pi_tool.html) is in good agreement with the experimental value of 5.5–5.8 determined here.
Figure 2.
2D SDS–PAGE analysis of labeled RPE. (A) RPE proteome in 2D electrophoresis. Proteins were separated by isoelectric focusing (first dimension) and SDS–PAGE (second dimension). An immobilized pH gradient strip (pH 3 to 10, 13 cm) for IEF and a gradient gel (4 to 20%, 16 cm × 18 cm) for SDS–PAGE were used. Proteins were visualized by silver staining. (B) Biotin detection of labeled proteins. RPE was incubated with 1, at 10 μM, for 1 h at 4 °C. Proteins were transferred to a PVDF membrane and visualized by avidin-HRP/ECL. Biotinylated molecular mass markers (200, 116, 97, 66, 45, 31, 22, 14, and 7 kDa) were loaded in the right-most lane. (C) Unlabeled control RPE. Proteins were transferred to a PVDF membrane and visualized by avidin-HRP/ECL.
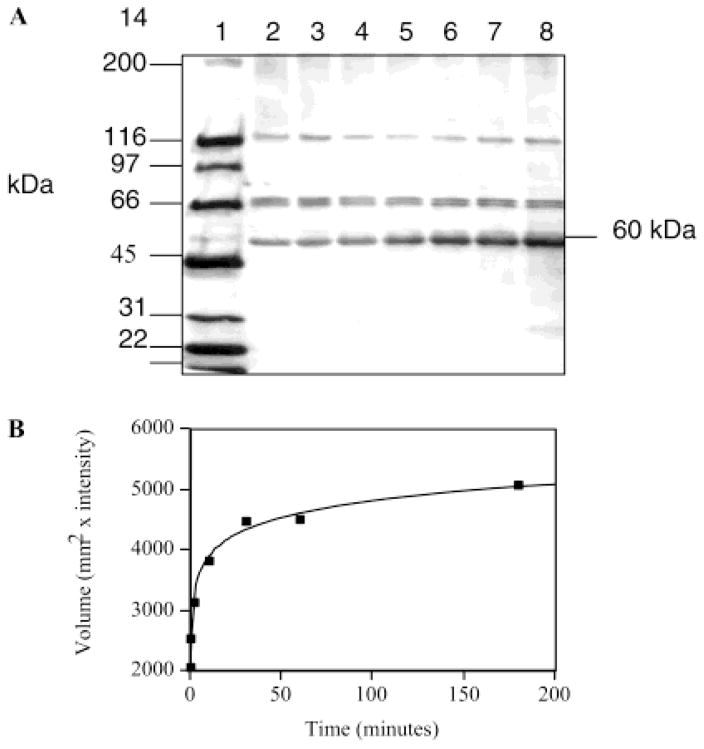
The specificity of labeling of the approximately 60 kDa species was further explored by determining a time course for the labeling of this protein with 5 μM 1 (Figure 3). As shown here, a hyperbolic time course for labeling is observed. At 4 °C, the half-time for labeling is approximately 10 min.
Figure 3.
Time-dependent RPE labeling. (A) Biotin detection analysis. Proteins were visualized by using avidin-HRP/ECL after transferring proteins to the PVDF from the 4 to 12% SDS–PAGE gel: lane 1, biotinylated markers (200, 116, 97, 66, 45, 31, 22, and 14 kDa); lane 2, RPE control; lane 3, RPE incubated with 1, at 5 μM, for 20 s at 4 °C; lane 4, RPE incubated with 1, at 5 μM, for 2 min at 4 °C; lane 5, RPE incubated with 1, at 5 μM, for 10 min at 4 °C; lane 6, RPE incubated with 1, at 5 μM, for 30 min at 4 °C; lane 7, RPE incubated with 1, at 5 μM, for 1 h at 4 °C; and lane 8, RPE incubated with 1, at 5 μM, for 3 h at 4 °C. (B) Time-dependent RPE labeling. The biotin signal is represented by a plot of volume vs time.
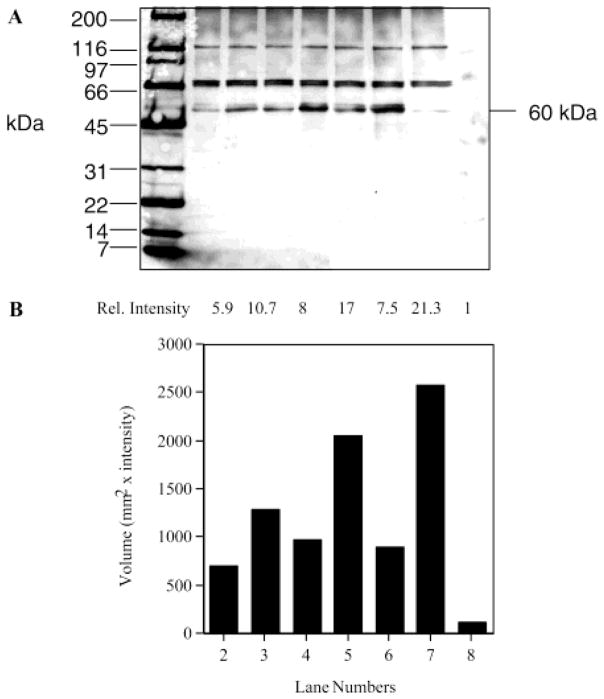
The inhibition of labeling of the 60 kDa protein by 1 with added retinoids was studied to further reveal the specificity of labeling (Figure 4). The extent of this labeling was strongly diminished by preincubating RPE membranes with the high concentrations of the thiol reactive reagents iodoacetamide (lane 2) and NEM (data not shown). Thus, under varying labeling conditions of crude RPE membranes with 1, it is clear that the approximately 60 kDa band is the predominantly labeled species. The extent of labeling of the 60 kDa band by 1 was diminished by preincubation with all-trans-retinol (Figure 4B, lane 3) and all-trans-retinyl acetate (Figure 4B, lane 4). Preincubation with the non-retinoid oleyl acetate (Figure 4B, lane 5) was less effective at preventing biotinylation. The extent of labeling was also strongly diminished by adding all-trans-retinyl bromoacetate (RBA) 3 (Figure 4B, lane 6), an affinity-labeling agent for the vitamin A esterifying enzyme LRAT (23). These data are consistent with the specific labeling of the 60 kDa protein.
Figure 4.
Competition analysis of RBPs by preblocking and labeling. (A) Biotin detection analysis. Proteins were visualized by using avidin-HRP/ECL after SDS–PAGE on a 4 to 20% gradient gel. Proteins were transferred to a PVDF membrane: lane 1, biotinylated markers (200, 116, 97, 66, 45, 31, 22, 14, and 7 kDa); lane 2, RPE preblocked with 55 mM iodoacetamide for 1 h and then incubated with 1, at 5 μM, for 10 min at 4 °C; lane 3, RPE preblocked with 1 mM retinol for 1 h and then incubated with 1, at 5 μM, for 10 min at 4 °C; lane 4, RPE preincubated with 1 mM retinyl acetate for 1 h and then incubated with 1, at 5 μM, for 10 min at 4 °C; lane 5, RPE preincubated with 1 mM oleyl acetate and then incubated with 1, at 5 μM, for 10 min at 4 °C; lane 6, RPE preincubated with RBA (90 μM, 1 h) and then 1, at 5 μM, for 10 min at 4 °C; lane 7, RPE labeled with 1, at 5 μM, for 10 min at 4 °C; lane 8, control RPE; and lane 9, prestained molecular mass markers (177, 114, 81, 64, 50, 37, 26, 20, 15, and 8 kDa). (B) Relative intensity of RPE labeling compared to the control. The biotin signal is represented by volume in the graph.
Identification of RPE65 and LRAT as Species Labeled by 1
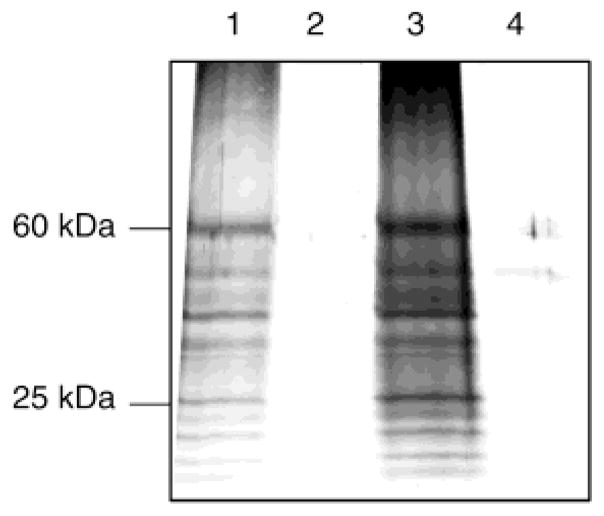
The next series of experiments were focused on methods for specifically binding the biotinylated proteins to neutravidin beads and then cleaving the proteins from the beads with alkali. RPE membranes were labeled with 20 μM 1, solubilized in Triton X-100, and then dialyzed to remove excess reagent. The addition of neutravidin beads to this solution showed that the biotinylated proteins were almost completely taken up by the beads by 3 h. Treatment of the beads at pH 11 (Figure 5, lanes 1 and 3), but not at pH 7.5 (Figure 5, lanes 2 and 4), led to the release of the proteins into solution. After incubation for 6 h at pH 11, more than 90% of the biotinylated proteins were removed from the column. There are two alkali labile ester linkages in compound 1, and cleavage of either releases protein from the beads.
Figure 5.
Elution of biotinylated proteins from neutravidin–agarose beads. The neutravidin–agarose beads which contain the labeled RPE were treated with buffer at either pH 7.5 or 11: lane 1, pH 11 for 6 h; lane 2, pH 7.5 for 6 h; lane 3, pH 11 overnight; and lane 4, pH 7.5 overnight.
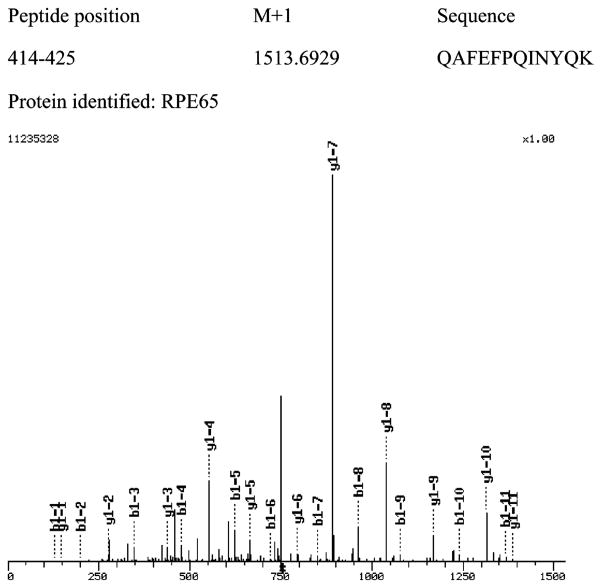
SDS–PAGE was performed on proteins released at pH 11. The approximately 60 kDa band was excised, and after proteolysis with trypsin, mass spectrometry was performed on the peptide products (Table 1). As shown in Table 1A and Figure 5, RPE65 was identified as the predominant product and 13 peptides from RPE65 were identified. Bovine serum precursor protein (two or three peptides) was also identified, possibly because bovine serum albumin also has an affinity for retinoids. However, this abundant protein was also found in nonlabeled controls which were run through the same protocol. No RPE65 was observed in nonlabeled control samples. For MALDI-TOF analysis, precise molecular mass determinations of the tryptic fragments of the proteins allow for their identification because these fragments are unique for a particular protein. In the ESI-MS/MS analyzer, a mixture of peptide ions is generated by electrospray ionization. The tandem MS selects a single m/z species for collision-induced dissociation of the peptide into ions and neutral fragments. The sequence of the peptide is deduced from this MS/MS spectrum. One representative peptide mass spectrum of the 13 identified RPE65 peptides is shown in Figure 6. B1-x and y1-x represent N-terminal and C-terminal fragments, respectively. In Table 1A are shown data for the 25–35 and 60–65 kDa regions. The same analysis was performed on the 25 kDa band. LRAT was identified here (Table 1B), verifying the labeling approach described here. Moreover, as shown above, anti-LRAT antibodies also stained the 25–35 kDa region of the gel. In separate experiments, we were able to demonstrate that C161 of cloned LRAT is specifically labeled by 1 (W. J. Jahng and R. R. Rando, unpublished experiments). This cysteine residue is the catalytically relevant active site nucleophile of LRAT (28).
Table 1.
Mass Spectrometric Identification of RPE Proteins Labeled with 1a
| potein X corr | positions | MW (MH+) | sequence |
|---|---|---|---|
| (A) Labeled 60 kDa Region | |||
| RPE65 | 60945 | ||
| 2.2152 | 509–515 | 789.8637 | DLSEVAR |
| 1.6138 | 119–124 | 866.9948 | FFSYFR |
| 2.3747 | 348–355 | 1062.1674 | ENWEEVKK |
| 2.3770 | 368–376 | 1075.2932 | YVLPLNIDK |
| 2.5204 | 155–164 | 1144.3104 | VNPETLETIK |
| 2.4300 | 199–208 | 1169.3660 | NFSIAYNIVK |
| 3.2525 | 367–376 | 1231.4807 | RYVLPLNIDK |
| 2.7699 | 34–44 | 1269.5728 | IPLWLTGSLLR |
| 3.2833 | 414–425 | 1513.6929 | QAFEFPQINYQK |
| 4.0892 | 209–222 | 1551.7802 | IPPLQADKEDPISK |
| 4.8063 | 15–33 | 2087.3374 | LFETVEELSSPLTAHVTGR |
| 2.7135 | 426–446 | 2389.6750 | YGGKPYTYAYGLGLNHFVPDR |
| 2.3806 | 235–258 | 2771.2310 | FKPSYVHSFGLTPNYIVFVETPVK |
| (B) Labeled 25 kDa Region | |||
| LRAT | 25701 | ||
| 4.6637 | 56–69 | 1660.8295 | THLTHYGIYLGDNR |
RPE membranes (5–6 mg) in 1 mL of 100 mM phosphate buffer (pH 7.5) were labeled with 20 μM 1 for 1 h at 25 °C. Proteins were solubilized with 1% Triton X-100, followed by dialysis. To the neutravidin beads (0.5 mL) was added 1 mL of the solubilized, labeled RPE, and beads were resuspended at 4 °C for 3 h until complete uptake of the biotinylated proteins occurred. After the beads had been washed five times, the proteins were eluted at pH 11. The supernatant was concentrated to ~40 μL, and the proteins were subjected to SDS–PAGE. The silver-stained bands at approximately 60 (A) and 25 kDa (B) were excised and submitted for tandem mass spectrometric analysis. The sequences of the peptides were determined by electrospray MS/MS. In separate experiments, peptide molecular masses were also verified by MALDI-TOF.
Figure 6.
Representative mass spectrum from the 60 kDa band. A representative mass spectrum is shown from the 13 identified RPE65 peptides. B1-x and y1-x represent N-terminal and C-terminal fragments, respectively. The X-axis is the mass over charge (m/z), and the Y-axis is the relative intensity.
To identify the sites of labeling of RPE65 by 1, the protein was first labeled with 1 and the 60 kDa protein was separated by SDS–PAGE and electroelution. The eluted biotinylated protein was trypsinized, and the peptides were bound to a neutravidin column. Mass spectrometry of the fraction that eluted at pH 11 showed that two cysteine residues were uniquely modified by the addition of a fragment with a molecular mass of 58 Da (Table 2). Note that after hydrolysis of 1 attached to RPE65, the protein is labeled with a methylene carboxylate moiety (CH2CO2H) because the remainder of the retinoid is cleaved. Thus, the labeled peptide would have an increment of 58 Da over the unlabeled species.
Table 2.
Active Site Labeling of RPE65a
| X corr | position | MH+ | sequenceb |
|---|---|---|---|
| Cys231 | |||
| 2.4480 | 231–261 | 3459.987 | C#SDRFKPSYVHSFGLTPNYIVFVETPVKIN |
| 1.9998 | 209–237 | 3285.782 | IPPLQADKEDPISKSEIVVQFPC#SDRFKP |
| 1.4603 | 217–241 | 2909.286 | EDPISKSEIVVQFPC#SDRFKPSYVH |
| 1.6114 | 224–244 | 2443.782 | EIVVQFPC#SDRFKPSYVHSFG |
| Cys448 | |||
| 1.8152 | 405–451 | 5439.215 | PEVLFSGPRQAFEFPQINYQKYGGKPYT-YAYGLGLNHFVPDRLC#KLN |
| 1.4762 | 431–453 | 2686.146 | YTYAYGLGLNHFVPDRLC#KLNVK |
| 1.4409 | 443–464 | 2686.100 | VPDRLC#KLNVKTKETWVWQEPD |
RPE (1–1.5 mg of total proteins) was labeled with 100 μM 1 for 1 h at 4 °C. After SDS–PAGE, the 60 kDa band was excised for electroelution, followed by trypsin digestion in the ammonium bicarbonate buffer (50 mM). Digested peptides were purified with neutravidin–agarose beads followed by C18 column chromatography, and analyzed by tandem mass spectrometry. The sequences of the peptides were determined by electrospray MS/MS.
C# is the modified cysteine (MW + 58).
DISCUSSION
The experiments described herein were begun to establish a proteomic inventory of RBPs of the RPE. The membrane fraction was chosen for initial study because it possesses all of the known enzymatic machinery required for the conversion of vitamin A (all-trans-retinol) into 11-cis-retinal, the visual chromophore (9–11). Biotin affinity labeling is the approach used for the identification of RPE membrane RBPs. In this approach, a biotin moiety is adducted via a base cleavable linker to an affinity-labeling agent targeted to a specific enzyme, or family of enzymes (1–6).
In the study presented here, an affinity-labeling agent based on RBA is used at low concentrations to label proteins in RPE membranes. Incubating crude RPE membranes with low concentrations of 1 led to the specific labeling of RPE65 and LRAT. Importantly, the labeled proteins were readily eluted from neautravidin beads under very mild conditions (pH 11). This cleavage leaves the labeled protein tagged with a methylene carboxyl group (Scheme 3). Thus, it is a simple matter to determine which peptide and which amino acid in the peptide have been modified.
The labeling of LRAT is not surprising, since all-trans-retinyl bromoacetate (RBA) is a known affinity-labeling agent for LRAT (23), and is structurally very similar to 1. Indeed, the detection of LRAT by this labeling technique validates the potential of this labeling approach inasmuch as an expected target is identified in a mixture of thousands of proteins. Moreover, LRAT is also labeled at its active site cysteine residue (C161), further demonstrating selectivity in the labeling process (unpublished experiments). This particular residue is the catalytically active nucleophile involved in the acyl transfer reaction (28).
The specific labeling of RPE65 with 1 was unexpected, and is of substantial interest inasmuch as it reveals an RBP role for this protein. The labeling of RPE65 by 1 is robust and could hardly be due to nonspecific alkylation. While RPE65 is clearly a quantitatively significant protein in the RPE, perusal of the Coomassie-stained gels (Figures 1 and 2) shows that it is only one of many major proteins revealed in the gel. However, RPE65 is clearly labeled preferentially by 1 in the crude RPE preparation that was studied (Figure 1). Further evidence for the specific labeling of RPE65 comes from mass spectroscopic studies which revealed that only two cysteine residues out of a total of 12 (C231 and C448) are labeled by 1. This mass spectroscopic result was repeated on two occasions with exactly the same results. That cysteine residues are labeled is consistent with the observation that high concentrations of NEM and iodoacetate both strongly diminish the extent of labeling of RPE65 by 1. NEM and iodoacetate are both thiol-directed reagents.
The specific labeling of RPE65 by retinoid 1 means that RPE65 can bind retinoids. RPE65 is a major protein of the RPE (20, 21) of unknown function and, from knockout studies in mice, is clearly important, either directly or indirectly, for 11-cis-retinal biosynthesis in vivo (22). In the knockout mouse mentioned here, the biosynthesis of 11-cis-retinal is severely curtailed, while all-trans-retinyl esters inappropriately accumulate as oil droplets in the RPE (22), suggesting the possibility that RPE65 is involved in retinyl ester processing. However, in the years since its discovery, no function for RPE65 has been reported, enzymatic or otherwise.
Sequence homology comparisons of RPE65 with other known proteins show a rather strong homology to β-carotene 15,15′-dioxygenase (29). This enzyme oxidatively cleaves β-carotene centrically into two molecules of all-trans-retinal (29). Since β-carotene is essentially a head to head dimer of retinal molecules, β-carotene 15,15′-dioxygenase by definition can bind the retinoid moiety. By homology arguments, it is possible that RPE65 may bind retinoids as well. Interestingly, the observation that a stoichiometry of 2 is found for the binding of 1 to RPE65 is most simply understood in terms of the homology of RPE65 to β-carotene 15,15′-dioxygenase which essentially binds a dimer of retinal. At this stage, it is not known which retinoid shows the highest capacity for binding to RPE65. Moreover, it is not known how specific the binding is for retinoids, although it could be shown here that oleyl acetate is less effective in competing with 1 than vitamin A or all-trans-retinyl acetate.
There are several retinoid binding proteins already known to be important in visual function. The proteins in question, CRBP (30, 31), IRBP (32, 33), and CRALBP (34, 35), are all soluble proteins and shuttle retinoids inter- or intracellularly (9, 10). The extant retinoid binding proteins are known to bind retino(a)ls, but not retinyl esters. Some, like CRALBP (36), are specific for retinoids, while others, like IRBP, are less specific and bind to a wide assortment of hydrophobic alcohols and acids (37).s Retinyl esters pose a particularly challenging problem with respect to mobilization. While the retino(a)ls undergo rapid intermembranous transfer, retinyl esters are essentially inert to uncatalyzed transfer (38). This is because of the extreme hydrophobicity of these long chain fatty acid esters of the already hydrophobic retinols. Retinyl esters are key components in the visual cycle, and are processed into 11-cis-retinol in the RPE (3, 10). The assisted mobilization of retinyl esters in vivo is likely to be important in visual cycle function. It is reasonable to wonder whether RPE65 might bind and mobilize retinyl esters in vivo. Several lines of experimental evidence are at least consistent with this being a possibility. RPE65 is found in RPE membranes, which otherwise contain the enzymatic machinery essential for 11-cis-retinal biosynthesis (9, 10). RPE65 is also found in substantial quantities in RPE membranes, which is more consistent with its having a stoichiometric binding function, rather than a catalytic function. Importantly, esters accumulate atypically in RPE65 knockout mice (22). The inability to mobilize retinyl esters in vivo would, of course, severely affect visual cycle function because the substrate for isomerization would be rendered unavailable for further processing. Finally, the experiments reported here demonstrate that RPE65 binds retinyl esters. The possibility still exists that RPE65 possesses enzymatic activity. However, in the years since the discovery of RPE65, no enzymatic activity has ever been described for this protein. While the sequence of RPE65 is most homologous to that of β-carotene 15,15′-dioxygenase, it has been explicitly shown not to possess this enzymatic activity (29). A β-carotene processing activity for RPE65 would be obscure in any case inasmuch as there is virtually no β-carotene in the eye.
The experiments reported here also demonstrate that the cleavable biotinylated affinity-labeling agent described here can be used to selectively identify RBPs in crude RPE membranes. From perhaps thousands of RPE membrane proteins, labeling with 1 at low concentrations allowed for the specific labeling of RPE65 and LRAT. The data presented here demonstrate the validity of the approach used here in functional proteomics. Further experimentation on the use of 1, and similar analogues, at higher concentrations and for longer incubation periods is anticipated to reveal further RBPs using the methods already demonstrated to be effective here. In addition, 1 was only studied in the context of RPE labeling. Cleavable biotinylated analogues, including 1, will be studied on soluble fractions from the RPE as well as on photoreceptors.
Footnotes
The work described here was supported by U.S. Public Health Service NIH Grant EY-04096.
Abbreviations: BSA, bovine serum albumin; HRP, horseradish peroxidase; IEF, isoelectric focusing; LRAT, lecithin retinol acyltransferase; NEN, N-ethylmaleimide; OA, oleyl acetate; RBA, all-trans-retinyl bromoacetate; RBP, retinoid binding protein; RPE, retinal pigment epithelium.
References
- 1.Orr GA. J Biol Chem. 1981;256:761–766. [PubMed] [Google Scholar]
- 2.Gilbert BA, Rando RR. J Am Chem Soc. 1995;117:8061–8066. [Google Scholar]
- 3.Ruiz A, Winston A, Lim YH, Gilbert BA, Rando RR, Bok D. J Biol Chem. 1998;274:3834–3841. doi: 10.1074/jbc.274.6.3834. [DOI] [PubMed] [Google Scholar]
- 4.Cheng H, Parish CA, Gilbert BA, Rando RR. Biochemistry. 1995;34:1662–1667. doi: 10.1021/bi00051a014. [DOI] [PubMed] [Google Scholar]
- 5.Kidd D, Liu Y, Cravatt BF. Biochemistry. 2001;40:4005–4015. doi: 10.1021/bi002579j. [DOI] [PubMed] [Google Scholar]
- 6.Kam CH, Abuelyaman AS, Li Z, Hudig D, Powers JC. Bioconjugate Chem. 1993;4:560–567. doi: 10.1021/bc00024a021. [DOI] [PubMed] [Google Scholar]
- 7.Green NM. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed ARH, Olivier GWJ, Adams G, Erskine ME, Kinsman RG, Branch SK, Moss SH, Notarianni LJ, Pouton CW. Biochem J. 1992;286:377–382. doi: 10.1042/bj2860377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bok D. J Cell Sci. 1993;S17:189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- 10.Rando RR. Chem Rev. 2001;101:1881–1896. doi: 10.1021/cr960141c. [DOI] [PubMed] [Google Scholar]
- 11.Saari JC. Invest Ophthalmol Visual Sci. 2000;41:337–348. [PubMed] [Google Scholar]
- 12.Wald G. Annu Rev Biochem. 1953;22:497–526. doi: 10.1146/annurev.bi.22.070153.002433. [DOI] [PubMed] [Google Scholar]
- 13.Groenendijk GW, Jacobs CW, Bonting SL, Daemen F. J Eur Biochem. 1980;106:119–128. doi: 10.1111/j.1432-1033.1980.tb06002.x. [DOI] [PubMed] [Google Scholar]
- 14.Rattner A, Smallwood PM, Nathans JJ. J Biol Chem. 2000;275:11034–11043. doi: 10.1074/jbc.275.15.11034. [DOI] [PubMed] [Google Scholar]
- 15.Simon A, Hellman U, Wernstedt C, Eriksson U. J Biol Chem. 1995;270:1107–1112. [PubMed] [Google Scholar]
- 16.Crabb JW, Nie Z, Chen Y, Hulmes JD, West KA, Kapron JT, Ruuska SE, Noy N, Saari JC. J Biol Chem. 1998;273:20712–20720. doi: 10.1074/jbc.273.33.20712. [DOI] [PubMed] [Google Scholar]
- 17.Crabb JW, Carlson A, Chen Y, Goldflam S, Intres R, West KA, Hulmes JD, Kapron JT, Luck LA, Horwitz J, Bok D. Protein Sci. 1998;7:746–757. doi: 10.1002/pro.5560070324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen P, Hao W, Rife L, Wang XP, Shen D, Chen J, Ogden T, Van Boemel GB, Wu L, Yang M, Fong HKW. Nat Genet. 2001;28:256–260. doi: 10.1038/90089. [DOI] [PubMed] [Google Scholar]
- 19.Hao W, Fong HKW. Biochemistry. 1996;35:6251–6256. doi: 10.1021/bi952420k. [DOI] [PubMed] [Google Scholar]
- 20.Bavik CO, Levy F, Hellman U, Wernstedt C, Eriksson U. J Biol Chem. 1993;268:20540–20546. [PubMed] [Google Scholar]
- 21.Hamel CP, Tsilou E, Pfeffer BA, Hooks JJ, Detrick B, Redmond TM. J Biol Chem. 1993;268:15751–15757. [PubMed] [Google Scholar]
- 22.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 23.Shi YQ, Furuyoshi S, Hubacek I, Rando RR. Biochemistry. 1993;32:3077–3080. doi: 10.1021/bi00063a019. [DOI] [PubMed] [Google Scholar]
- 24.Nesnas N, Rando RR, Nakanishi K. Tetrahedron. 2002;58:6577–6584. [Google Scholar]
- 25.Barry RJ, Cañada FJ, Rando RR. J Biol Chem. 1989;264:9231–9238. [PubMed] [Google Scholar]
- 26.Laemmli UK. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Shevchenko A, Wilm M, Vorm O, Mann M. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 28.Mondal MS, Ruiz R, Bok D, Rando RR. Biochemistry. 2000;39:5215–5220. doi: 10.1021/bi9929554. [DOI] [PubMed] [Google Scholar]
- 29.Von Lintig J, Vogt K. J Biol Chem. 2000;275:11915–11920. doi: 10.1074/jbc.275.16.11915. [DOI] [PubMed] [Google Scholar]
- 30.Bridges CD, Alvarez RA, Fong SL, Gonzalez-Fernandez F, Lam DM, Liou GI. Vision Res. 1984;24:1581–1594. doi: 10.1016/0042-6989(84)90316-x. [DOI] [PubMed] [Google Scholar]
- 31.Saari JC, Nawrot M, Garwin GG, Kennedy MJ, Hurley JB, Ghyselinck NB, Chambon P. Invest Ophthalmol Visual Sci. 2002;43:1730–1735. [PubMed] [Google Scholar]
- 32.Adler AJ, Evans CD, Stafford WF., III J Biol Chem. 1985;260:4850–4855. [PubMed] [Google Scholar]
- 33.Chader GJ, Wiggert B, Lai YL, Lee L, Fletcher RT. In: Progress in Retinal Research. Osborne NN, Chodes GJ, editors. Vol. 2. Pergamon Press; Oxford, England: 1983. p. 163. [Google Scholar]
- 34.Intres R, Goldflam S, Cook JR, Crabb JW. J Biol Chem. 1994;269:25411–25418. [PubMed] [Google Scholar]
- 35.Shaw NS, Noy N. Exp Eye Res. 2001;72:183–190. doi: 10.1006/exer.2000.0945. [DOI] [PubMed] [Google Scholar]
- 36.Livrea MA, Bongiorno A, Tesoriere L, Nicotra C, Bono A. Experientia. 1987;43:582–586. doi: 10.1007/BF02143594. [DOI] [PubMed] [Google Scholar]
- 37.Noy N, Chen Y. Biochemistry. 1994;33:10658–10665. doi: 10.1021/bi00201a013. [DOI] [PubMed] [Google Scholar]
- 38.Rando RR, Bangerter FW. Biochem Biophys Res Commun. 1982;104:430–436. doi: 10.1016/0006-291x(82)90655-6. [DOI] [PubMed] [Google Scholar]