Abstract
Arpin is an Arp2/3 inhibitory protein, which decreases the protrusion lifetime and hence directional persistence in the migration of diverse cells. Arpin is activated by the small GTPase Rac, which controls cell protrusion, thus closing a negative feedback loop that renders the protrusion intrinsically unstable. Because of these properties, it was proposed that Arpin might play a role in directed migration, where directional persistence has to be fine-tuned. We report here, however, that Arpin-depleted tumour cells and Arpin knock-out Dictyostelium amoeba display no obvious defect in chemotaxis. These results do not rule out a potential role of Arpin in other systems, but argue against a general role of Arpin in chemotaxis.
Keywords: Actin, Cell migration, Cytoskeleton
Graphical abstract
In the field of cell migration, chemotaxis represents a particular challenge. Indeed, despite numerous successes in the identification of core machineries that power cell migration, how these machineries are controlled in space and time to guide cell migration is not yet elucidated. In most cases, cell migration depends on membrane protrusions called lamellipodia that are driven by actin polymerisation. After random initiation of lamellipodia, chemotactic molecules promote a longer lifetime of correctly oriented lamellipodia (Andrew and Insall, 2007). Mechanistically, chemotactic signalling appears to transiently activate a cytoskeletal module that generates oscillatory protrusions at idle (Huang et al., 2013). Lateral propagation of protrusions also gradually reorients lamellipodia in the direction of the steeper gradient (Arrieumerlou and Meyer, 2005). These critical phenomena of control of protrusion duration, of membrane oscillations, of lateral propagation of protrusions can be accounted for by the combination of a positive feedback loop that maintains actin polymerisation where it was previously polymerised and a negative feedback loop that antagonises the positive one after a delay.
The Arp2/3 complex, which generates branched actin networks, is a major machinery for the induction of membrane protrusions (Wu et al., 2012; Suraneni et al., 2012). For protrusions to occur, this machinery must be activated by the small GTPase Rac and its downstream effector, the WAVE complex. We have reported the identification of an Arp2/3 inhibitory molecule, Arpin, that controls the lifetime of lamellipodia and the ability of cells to turn (Dang et al., 2013). In random migration assays, that is, assays in which migration is not biased by external cues, we reported that Arpin-depleted cells migrate along straighter trajectories than controls. Arpin allows turning by inducing idling phases, when cells slow down (Gorelik and Gautreau, 2015). Arpin is a pure regulator of cell migration, since it is dispensable for efficient migration. When injected in cells displaying continuous protrusions, Arpin creates pronounced oscillatory protrusions of the plasma membrane, suggesting that Arpin is responsible for the cyclical inactivation of actin polymerisation. These various properties can be explained by the fact that the Arp2/3 inhibitory protein, Arpin is under the control of Rac, just like the Arp2/3 activatory complex, WAVE. Together they create a so-called ’incoherent feedforward loop’ from Rac to Arp2/3, that is a circuit where two pathways deliver opposite signals. The incoherent feedforward loop modulates the response to the feedback that senses actin polymerisation and subsequently activates Rac (Dang et al., 2013). These properties raised the idea that Arpin might be important for chemotaxis (Veltman, 2014; Schmick and Bastiaens, 2014). Arpin could be in particular the inhibitory protein postulated and required by several models of chemotaxis (Neilson et al., 2011; Huang et al., 2013; Schmick and Bastiaens, 2014). Here we tested this hypothesis.
We used stable Arpin-depleted clones from the human breast carcinoma cell line, MDA-MB-231, in customised microchannels testing chemotaxis by offering cells a choice between two paths of different lengths towards chemotactic molecules. These clones obtained by transfections of shRNAs were previously characterised (Dang et al., 2013; Gorelik and Gautreau, 2015). One chamber contains the chemoattractant containing medium, which diffuses through the microchannels towards the other chamber that contains the cells. Since the short path provides a steeper gradient than the long one, this system offers a simple way to quantify chemotactic behaviour by counting cells and choosing the shorter path at the bifurcation. Two symmetrical designs are simultaneously tested on the same microfluidic device (Figure 1): the top one displays the short path at a right angle, whereas the bottom one displays the short path straight ahead. Since Arpin depletion induces a higher directional persistence in random migration assays, one can expect that Arpin-depleted cells will make wrong decisions on the top one, where they should turn, but not on the bottom one.
Figure 1. Arpin depletion does not impair chemotaxis in MDA-MB-231 cells.
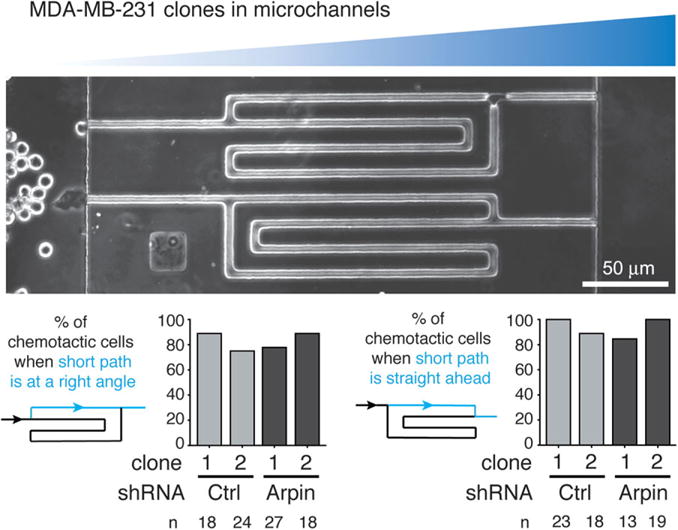
Control MDA-MB-231 clones (shRNA Ctrl #1 and #2) or Arpin-depleted clones (shRNA Arpin #1 and #2) were seeded in the left chamber of the microfluidic device, whereas chemoattractant containing medium was placed on the right chamber of the microfluidic device. Two symmetrical devices are shown: they both display a bifurcation, where cells choose either a short or a long path. The short path has a steeper gradient than the long one. A chemotactic cell thus chooses the short path. Depending on the device, the short path is straight ahead (right histogram) or at an angle (left histogram). In both cases, Arpin-depleted clones do not display a chemotaxis behaviour that differs from the one of control clones. A single experiment is displayed. The cells were starved for 5 h and chemotaxis was stimulated in the microfluidic device by 1% foetal calf serum and 20 ng/ml EGF. The number of cells counted in each condition is indicated below the histograms.
We explored a number of different culture conditions with the microchannels. As a source of chemoattractants, we used medium supplemented with 10% foetal calf serum, or with 1% foetal calf serum supplemented with 20 ng/ml EGF. MDA-MB-231 clones were starved for 5 h in serum-free medium before injection in the microfluidic device, or not. In all tested conditions, the majority of cells took the short path in both designs, indicating that MDA-MB-231 cells are chemotactic and that the microfluidic device accurately measures chemotaxis in these conditions. We also tested the condition where the two chambers were similarly filled with medium containing serum. As previously described by one of us, cells in the left chamber act as a sink for growth factors and also yield a chemotactic condition (Scherber et al., 2012). Without serum in both chambers, MDA-MB-231 cells are immobile and do not enter the microchannels.
The design of these microchannels is thus efficient to score chemotaxis. One of the difficulties, however, is that cells may obstruct the microchannel they occupy and distort the gradient for their followers. We thus took the precaution of counting only the first cells that enter microchannels to avoid this confounding effect. In all the situations explored, we observed that the chemotactic behaviour of Arpin-depleted clones was not significantly different from the control clones. In particular, Arpin-depleted clones were as efficient as the control cells at making a turn when the short path was at a right angle, indicating that, in this setting, Arpin does not play a critical role in chemotaxis.
To extend these observations to an evolutionary distant model system, we used the previously obtained Arpin knock-out (KO) Dictyostelium discoideum amoeba (Dang et al., 2013), which completely lacks Arpin, as opposed to RNAi-mediated depletion of mammalian cells. Chemotaxis was assessed by examining migration towards a micropipette releasing cAMP. In this classical assay, Arpin-depleted amoebae polarised and efficiently migrated towards the micropipette (Figure 2A). Quantification of speed and directionality did not reveal defects in chemotaxis of Arpin KO amoebae towards the cAMP micropipette compared with the wild-type (Figure 2B). These two experiments in different cell systems therefore indicate that Arpin is dispensable for gradient sensing and chemotaxis.
Figure 2. Arpin depletion does not impair chemotaxis in Dictyostelium amoeba.
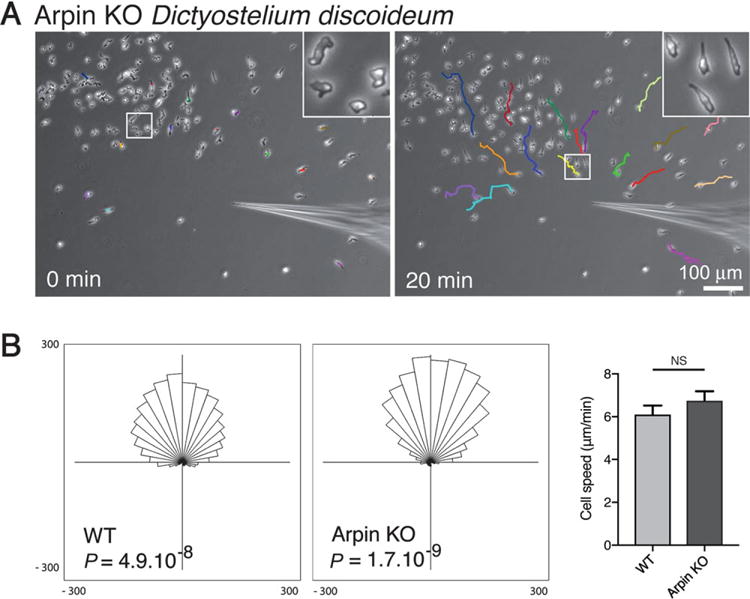
(A) Arpin knock-out (KO) amoebae polarise and migrate towards a micropipette releasing chemoattractant. Tracks of individual cells are represented in different colours. The inset displays how amoebae became polarised towards the micropipette. (B) Both wild-type and Arpin KO amoebae migrate efficiently towards the micropipette (top position of the rose plot; Rayleigh statistical test; n = 30 cells; Ibidi chemotaxis and migration plug-in). Speed of Arpin KO amoebae does not significantly differ from the one of the wild-type (NS: not significant, n = 30 cells).
There is a single Arpin gene in both Dictyostelium amoeba and mammalian cells, and so functional redundancy is an unlikely explanation for this lack of effect of Arpin depletion. However, these two experiments do not rule out a role for Arpin in more physiological situations, for example, when a neutrophil chases moving pathogens within the organism, a situation which requires numerous dynamic cytoskeletal reorganisations. In dendritic cells, the formin mDia1 is critical for chemotaxis in tissue explants, but not in simplified in vitro assays (Vargas et al., 2016). These experiments also do not rule out a potential role of Arpin in other types of guided migration, when cells detect and respond to matrix density, substrate rigidity or electric fields, for example. But our experiments clearly indicate that Arpin is not essential for gradient sensing during chemotaxis.
The discovery that Arpin, which controls directional persistence in random migration assays (Dang et al., 2013), is dispensable for chemotaxis, and should be discussed in light of the debate concerning the role of the Arp2/3 complex in chemotaxis. One report indicated that the Arp2/3 complex was dispensable for chemotaxis (Wu et al., 2012), whereas other ones reported a requirement for the Arp2/3 complex in directional migration towards gradients of EGF (Suraneni et al., 2012; 2015). It appears more logical that Arpin is dispensable for chemotaxis, if the Arp2/3 complex is also dispensable for chemotaxis. But a possibility is that the Arp2/3 complex is required for chemotaxis, while Arpin is not. These various studies agree, however, that the Arp2/3 complex is required for lamellipodium formation (Wu et al., 2012; Suraneni et al., 2012). Dendritic cells devoid of WAVE complexes are also unable to develop lamellipodia and develop an alternative mode of cell migration characterised by high directional persistence, but defective chemotaxis (Leithner et al., 2016), suggesting that the lamellipodium is indeed a structure associated with chemotaxis. If Arpin is not the molecule that plays the inhibitory role required by most chemotaxis models (Neilson et al., 2011; Huang et al., 2013; Schmick and Bastiaens, 2014), which molecule does? This work illustrates the difficulty in assigning molecules to postulated activities, in bridging the gap between theoretical models and molecular components.
Acknowledgments
Funding
This work was supported by grants from the Agence Nationale pour la Recherche (ANR ANR-15-CE13-0016-01) from the foundation ARC pour la Recherche sur le Cancer (PGA120140200831) and from Institut National du Cancer (INCA_6521).
Footnotes
Conflict of interest statement
The authors have declared no conflict of interest.
References
- Andrew N, Insall RH. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat Cell Biol. 2007;9:193–200. doi: 10.1038/ncb1536. [DOI] [PubMed] [Google Scholar]
- Arrieumerlou C, Meyer T. A local coupling model and compass parameter for eukaryotic chemotaxis. Dev Cell. 2005;8:215–227. doi: 10.1016/j.devcel.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Dang I, Gorelik R, Sousa-Blin C, Derivery E, Guérin C, Linkner J, Nemethova M, Dumortier JG, Giger FA, Chipysheva TA, Ermilova VD, Vacher S, Campanacci V, Herrada I, Planson AG, Fetics S, Henriot V, David V, Oguievetskaia K, Lakisic G, Pierre F, Steffen A, Boyreau A, Peyriéras N, Rottner K, Zinn-Justin S, Cherfils J, Bièche I, Alexandrova AY, David NB, Small JV, Faix J, Blanchoin L, Gautreau A. Inhibitory signalling to the Arp2/3 complex steers cell migration. Nature. 2013;503:281–284. doi: 10.1038/nature12611. [DOI] [PubMed] [Google Scholar]
- Gorelik R, Gautreau A. The Arp2/3 inhibitory protein arpin induces cell turning by pausing cell migration. Cytoskeleton (Hoboken) 2015;72:362–371. doi: 10.1002/cm.21233. [DOI] [PubMed] [Google Scholar]
- Huang CH, Tang M, Shi C, Iglesias PA, Devreotes PN. An excitable signal integrator couples to an idling cytoskeletal oscillator to drive cell migration. Nat Cell Biol. 2013;15:1307–1316. doi: 10.1038/ncb2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leithner A, Eichner A, Müller J, Reversat A, Brown M, Schwarz J, Merrin J, de Gorter DJJ, Schur F, Bayerl J, de Vries I, Wieser S, Hauschild R, Lai FPL, Moser M, Kerjaschki D, Rottner K, Small JV, Stradal TEB, Sixt M. Diversified actin protrusions promote environmental exploration but are dispensable for locomotion of leukocytes. Nat Cell Biol. 2016;18:1253–1259. doi: 10.1038/ncb3426. [DOI] [PubMed] [Google Scholar]
- Neilson MP, Veltman DM, van Haastert PJM, Webb SD, Mackenzie JA, Insall RH. Chemotaxis: a feedback-based computational model robustly predicts multiple aspects of real cell behavior. PLoS Biol. 2011;9:e1000618. doi: 10.1371/journal.pbio.1000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherber C, Aranyosi AJ, Kulemann B, Thayer SP, Toner M, Iliopoulos O, Irimia D. Epithelial cell guidance by self-generated EGF gradients. Integr Biol (Camb) 2012;4:259–269. doi: 10.1039/c2ib00106c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmick M, Bastiaens PIH. The interdependence of membrane shape and cellular signal processing. Cell. 2014;156:1132–1138. doi: 10.1016/j.cell.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Suraneni P, Fogelson B, Rubinstein B, Noguera P, Volkmann N, Hanein D, Mogilner A, Li R. A mechanism of leading-edge protrusion in the absence of Arp2/3 complex. Mol Biol Cell. 2015;26:901–912. doi: 10.1091/mbc.E14-07-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suraneni P, Rubinstein B, Unruh JR, Durnin M, Hanein D, Li R. The Arp2/3 complex is required for lamellipodia extension and directional fibroblast cell migration. J Cell Biol. 2012;197:239–251. doi: 10.1083/jcb.201112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas P, Maiuri P, Bretou M, Saez PJ, Pierobon P, Maurin M, Chabaud M, Lankar D, Obino D, Terriac E, Raab M, Thiam HW, Brocker T, Kitchen-Goosen SM, Alberts AS, Suraneni P, Xia S, Li R, Voituriez R, Piel M, Lennon-Dumenil AM. Innate control of actin nucleation determines two distinct migration behaviours in dendritic cells. Nat Cell Biol. 2016;18:43–53. doi: 10.1038/ncb3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltman D. Actin dynamics: cell migration takes a new turn with arpin. Curr Biol. 2014;24:R31–R33. doi: 10.1016/j.cub.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Wu C, Asokan SB, Berginski ME, Haynes EM, Sharpless NE, Griffith JD, Gomez SM, Bear JE. Arp2/3 is critical for lamellipodia and response to extracellular matrix cues but is dispensable for chemotaxis. Cell. 2012;148:973–987. doi: 10.1016/j.cell.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]


