Abstract
Objective
To investigate the expression of Hepcidin and Neogenin in tissue from patients with colorectal cancer, to evaluate the relationship between Hepcidin and Neogenin with clinical features, and to study their relationship with anemia.
Methods
Immuno- histochemical method was used to detect the expression of Hepcidin and Neogenin in 62 cases of colorectal cancer. At the same time, the relationship between them and their relationship with clinical characteristics and anemia were analyzed.
Results
The expression of Hepcidin was related to T stage (P<0.05), but not with age, gender, lymph node metastasis and distant metastasis. The expression of Neogenin was not correlated with T stage and lymph node metastasis, age, gender, and distant metastasis (P>0.05). There was no significant difference in the expression of Hepcidin and Neogenin between anemia group and non-anemia group. There was no correlation between Hepcidin and Neogenin (r =-0.04, P>0.05).
Conclusion
The expression of Hepcidin in colorectal cancer was related to the T stage, and had no correlation with Neogenin. The expression of Neogenin could not be used as an objective index to reflect the biological behavior of colorectal cancer.
Keywords: Hepcidin, Neogenin, Colorectal cancer, Anemia
1. Introduction
At present, iron may play an important role in promoting the proliferation and differentiation of tumors. So, there is an increased demand for iron in tumors. The disorder of iron metabolism can obviously affect the proliferation and metastasis of tumor. In addition, there are many tumor patients with anemia; in addition to blood loss, radiotherapy and chemotherapy, tumor bone marrow involvement, but also may be related to the disorder of iron metabolism. Recent studies have shown that hepcidin was highly expressed upon stimulation of inflammatory mediators, especially. interleukin 6 in patients with cancer [1,2]. Additionally, hepcidin has been shown to cause iron metabolism disorders which results in anemia in patients [3, 4]. However, recent reports have suggested that iron may function as a cofactor that contributes to the proliferation and differentiation of tumors [5, 6]. So iron metabolism disorders caused by hepcidin may play an important role in the development of cancer. Neogenin, a deleted in colorectal cancer (DCC) family member, has been identified as a receptor for the neuronal axon guidance cues netrins and repulsive guidance molecules repulsive guidance molecules (RGM). RGMc, also called hemojuvelin (HJV), is essential for iron homeostasis. Recent studies shown neogenin can inhibit hemojuvelin (HJV) secretion and regulates BMP-induced hepcidin expression and iron homeostasis [7]. Therefore, this study used immunohistochemical methods to determine the expression of Hepcidin and Neogenin in the pathological tissues of 62 cases of colorectal cancer patients, and analyzed the relationship between Hepcidin and Neogenin and their relationship with clinical characteristics and anemia.
2. Methods
2.1. Data collection and population
62 cases of colorectal cancer patients who hospitalized between January 2009 and January 2014 in our hospital were included in this study, along with their surgical specimens embedded by paraffin. All cases were confirmed by pathological examination, and none of them received chemotherapy or radiotherapy before surgery. There were 39 males and 23 females, the median age was 66 years. Criteria for the diagnosis of anemia in men were Hb less than 120.0 g/L and women with Hb less than 110.0 g/L, and there were 21 cases of anemia and 41 cases without anemia. Hb levels were110.0±12.9g/L(range: from 56.3g/L to 155.8g/L).TNM staging was consistent with the criteria for the pathology group of Chinese National Colorectal Cancer Cooperative Group. Among them, there were 16 cases in stage T1 and T2, and 46 cases in stage T3 and T4. There were 31 cases with and 31 cases without lymph node metastasis, 7 cases with and 55 cases without distant metastasis. During the same period, we randomly selected 15 cases of normal intestinal mucosa of patients with colorectal cancer as a control group. This study was approved by the Ethics Committee of Taicang Hospital of Suzhou University. Informed consent has been obtained from all individuals included in this study.
2.2. Experimental methods
Immunohistochemistry was performed by using the StreptAvidin-Biotin Complex (SABC) staining system. Four consecutive sections, each with a thickness of 5 μm, were cut from the paraffin blocks and then incubated at 60°C for 30 minutes. Dimethylbenzene, anhydrous alcohol, and different concentrations of alcohol were used to dewax the sections. They were then washed with water for two minutes. One section from each set was stained with hematoxylin and eosin to facilitate the pathological diagnosis according to the manufacturer’s protocol (Wuhan Boshide Biological Engineering Co., Ltd., China. Produced by R&D Co., Ltd., German). The remaining three sections were stained by the SABC method according to the manufacturer’s protocol (Fuzhou Maixin Biotech. Co, Ltd, primary antibodies was from Abcam Hong Kong, Ltd.). Phosphate-buffered saline instead of the primary antibody was used as a negative control.
2.3. Result Evaluation
The cytoplasm and nucleus stained as pale or yellow brown were evaluated as positive. At high magnification, the five fields were randomly selected, positive was defined as the staining color was yellow or deeper color and the number of positive cells was larger than 10%.
2.4. Statistical Analysis
Using the SPSS statistical software (version 16.0), the data were calculated by Chi square test.we compared groups with the SNK method, Person test, and using Spearman rank test to grade correlation analysis, P < 0.05 for the difference was statistically significant.
3. Results
3.1. Expression of Hepcidin in colorectal cancer
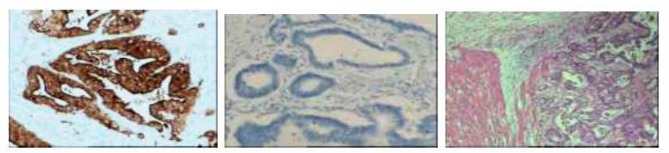
The positive expression rate of Hepcidin in normal intestinal mucosa was 20% (3/15), and the positive rate was 66.1% (41/62) in colorectal cancer tissues, and the difference was significant (P= 0.003). The expression of Hepcidin in cancer tissues in stage T3 and T4 was significantly higher than that in stage T1 and T2 (P<0.05). But there was no significant difference among the patients with or without lymph node metastasis and distant metastasis (P>0.05). (for detail See Figure 1 and Table 1).
Figure 1.
A. Hepcidin Positive (×100); B. Negative stain control (×100) C H&E (×100)
Table 1.
Expressions of Hepcidin and Neogenin in colorectal cancer and their association with clinical characteristics.
| Charact-eristics | Hepcidin Negative |
Positive | Chi Square | Neogenin Negative |
Positive | Chi Square | |
|---|---|---|---|---|---|---|---|
| Age | ≤60 | 5 | 14 | 0.70 | 1 | 18 | 2.60 |
| >60 | 16 | 27 | 9 | 34 | |||
| Gengder | male | 15 | 24 | 0.99 | 5 | 34 | 0.85 |
| female | 6 | 17 | 5 | 18 | |||
| T-stage | TI, T2 | 9 | 7 | 4.82* | 3 | 13 | 0.11 |
| T3, T4 | 12 | 34 | 7 | 39 | |||
| LNTa | Yes | 8 | 23 | 1.80 | 4 | 27 | 0.48 |
| Not | 13 | 18 | 6 | 25 | |||
| DMb | Yes | 1 | 6 | 1.52 | 1 | 6 | 0.02 |
| No | 20 | 35 | 1.52 | 9 | 46 |
P <0.05, others P >0.05. 2
LNT=LvmDh node metastasis.
DM= Distant metastasis
3.2. Expression of Neogenin in colorectal cancer
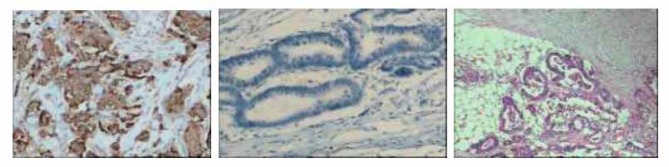
The positive expression rate of Neogenin in normal intestinal mucosa was 100% (15/15), the positive expression rate in cancer tissue was 83.8% (52/62), and the difference was not significant (P =0.45). There showed no significant difference in different stages (P>0.05), with or without lymph node metastasis (P>0.05), and with or without distant metastasis(P>0.05)(For details, see Figure 2 and Table 1).
Figure 2.
A. Neogenin Positive (×100); B Negative stain control (×100) C H&E (×100)
3.3. Correlation between anemia and the expression of Hepcidin and Neogenin
There was no correlation between the expression of Hepcidin and Neogenin in tumor tissues with anemia. Chi Square were 0.40 and 2.38 respectively,all P>0.05 (for detail, see Table 2).
Table 2.
Correlation between anemia and Hepcidin and Neogenin Expression in colorectal cancer tissue.
| Anemia | Neogenin Positive |
Negative | Hepcidin Positive |
Negative |
|---|---|---|---|---|
| Yes | 15 | 6 | 15 | 6 |
| NO | 37 | 4 | 26 | 15 |
At the same time, we also applied the Spearman rank correlation test method to analysis the relationship between hemoglobin levels and the expression of hepcidin and Neogenin in tumor tissues, the results were indicated that there also no correlation(r = 0.08 and r =-0.04 respectively, all P > 0.05.
3.4. Correlation between the expression of Neogenin and Hepcidin
32 of 52 Neogenin positive patients expressed Hepcidin, and 7 cases of 10 Neogenin negative patients expressed Hepcidin (χ2 =0.01, P >0.05).
4. Discussion
Iron might serve as the synergistic factor to induce the cellular proliferation and differentiation in cancer. Metabolism of iron in tumor cells has changed a lot, many proteins which participated in the regulation of iron metabolism showed highly expressed, and they could apparently affect the proliferation and metastasis in cancer [8-11]. Patients with cancer often show a high level of serum Hepcidin [12].Studies showed that the increased intracellular iron could promote the development of cancer mediated by the stimulation of Wnt signaling pathway [13,14] .We found that patients with high T staging (T3, 4) had high level of hepcidin expression(χ2 =4.82, P<0.05), which had no relevant with patients’ age, gender, lymph node metastasis or distant metastasis. And this result was consistent with Wards’ study [15,16] which showed that hepcidin expression of patients with colorectal cancer was related to T staging .We found the expression of neogenin was not correlated with T stage and lymph node metastasis, age, gender, and distant metastasis (P>0.05).The result showed neogenin was not correlated with tumor severity, and neogenin could not be used as an objective index to reflect the biological behavior of colorectal cancer.
At the same time, the study results showed that there was no correlation between neogenin expression and T stage, patients age, gender, lymph node metastasis and distant metastasis(all P>0.05), suggesting that the expression of Neogenin may have no association with hepcidin in colorectal cancer.
The correlation analysis between Hepcidin and Neogenin was carried out. The results confirmed that Hepcidin and Neogenin had no correlation with in colorectal cancer (χ2 =0.01,P > 0.05), which showed that the abnormal expression of Hepcidin in colorectal carcinoma was not related to Neogenin.
Of course, it is possible that less cases of general cases and case with distant metastasis before surgery (only 8 cases) led to the negative results, which certainly has to be further studied by expanding the clinical sample size.
Anemia is one of the common symptoms in patients with intestinal tumor. It is generally considered that the anemia is a chronic disease in chronic inflammation and malignant tumors (ACD), Current research [17-19] has confirmed that Hepcidin plays a key role in the occurrence and development of ACD [17]. Elevated expression of Hepcidin can inhibit the intestinal iron absorption and inhibit the release of iron from the mononuclear macrophage system, which makes the iron storage in the cells, iron reduction in bone marrow, and ultimately lead to iron loss and anemia [18,20-22]. Our previous studies [19] also showed that Hepcidin plays an important role in the treatment of postoperative radiotherapy and chemotherapy in patients with advanced tumor anemia, the expression of hepcidin in patients with anemia was significantly higher compared with that of patients without anemia (P<0.05), which was negatively correlated with Hb (r =-0.2597,P<0.01).
But there has not report research about the relationship between the expression of Hepcidin and anemia in tumor tissue yet. Our study showed that there was no difference between the Neogenin and the Hepcidin in the tissues of the patients with anemia and anemia, and there was no correlation between the level of Hb and Hepcidin and Neogenin in the patients. The local level of the iron in the tumor tissue does not represent the level of the whole body of the patient. In another research, we [23] analyzed the relationships between expression of Hepcidin in Breast cancer tissues and the levels of those factors in serum. No statistically significant correlations were observed(r =0.1419, P>0.05). So we speculated the serum level of hepcidin in colorectal cancer has not any relation with the hepcidin expression level of tumor tissue, and it was consistence with the result that there were no relationship between the expression of hepcidin in colorectal cancer tissue and anemia.
In conclusion, our study showed that the elevation expression of Hepcidin in colorectal cancer was related to the T staging of cancer patients, and the expression level of Neogenin was not related to the biological behavior of colorectal cancer. Also the expression of Hepcidin and Neogenin in tumor tissue was not related to anemia.
Footnotes
Conflict of interests: No authors report any conflict of interest.
References
- [1].Rodriguez R, Jung CL, Gabayan V. et al. Hepcidin induction by pathogens and pathogen-derived molecules is strongly dependent on interleukin-6. Infect Immun. 2014;82:745–752. doi: 10.1128/IAI.00983-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gardenghi S, Renaud TM, Meloni A. et al. Distinct roles for hepcidin and interleukin-6 in the recovery from anemia in mice injected with heat-killed Bruiellu abortus. Blood. 2014;123:1137–1145. doi: 10.1182/blood-2013-08-521625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93:1721–1741. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- [4].Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823:1434–1443. doi: 10.1016/j.bbamcr.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tirnitz-Parker JE, Glanfield A, Olynyk JK, Ramm GA. Iorn and hepatic carcinogenesis. Crit Rev Oncog. 2013;18:391–340. doi: 10.1615/critrevoncog.2013007759. [DOI] [PubMed] [Google Scholar]
- [6].Durrenberger F, Abbate V, Ma Y. et al. Functional characterization of fluorescent hepcidin. Bioconjug Chem. 2013;24:1527–1532. doi: 10.1021/bc400121x. [DOI] [PubMed] [Google Scholar]
- [7].Lee DH, Zhou LJ, Zhou Z. et al. Neogenin inhibits HJV secretion and regulates BMP-induced hepcidin expression and homeostasis. Blood. 2010;115:3136–3145. doi: 10.1182/blood-2009-11-251199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alkhateeb AA, Connor JR. The significance of ferritin in cancer: anti-oxidation, inflammation and tumorigenesis. Biochim Biophys Acta. 2013;1836(2):245–254. doi: 10.1016/j.bbcan.2013.07.002. [DOI] [PubMed] [Google Scholar]
- [9].Vander Wall K, Daniels-Wells TR, Penichet M. et al. Iron in multiple myeloma. Crit Rev Oncog. 2013;18(5):449–461. doi: 10.1615/critrevoncog.2013007934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tirnitz-Parker JE, Glanfield A, Olynyk JK. et al. Iron and hepatic carcinogenesis. Crit Rev Oncog. 2013;18(5):391–407. doi: 10.1615/critrevoncog.2013007759. [DOI] [PubMed] [Google Scholar]
- [11].Bookes MJ, Boult J, Roberts K.. et al. A role for iron in WNT signaling. Oncogene[J] 2008;27:966–975. doi: 10.1038/sj.onc.1210711. [DOI] [PubMed] [Google Scholar]
- [12].Ukarma L, Johannes H, Beyer U. et al. Hepcidin as a predictor of response to epoetin therapy in anem ic cancer patients [J] Clin Chem. 2009;55(7):1354–1360. doi: 10.1373/clinchem.2008.121285. [DOI] [PubMed] [Google Scholar]
- [13].Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13(5):342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell[J] 2000;244:76–78. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- [15].Ward DG, Roberts K, Brookes MJ. et al. Increased hepcidin expression in colorectal carcinogenesis [J] WorldJ Gastroenterol. 2008;14(9):1339–1345. doi: 10.3748/wjg.14.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Atanasiu YL, Manolescu B, Stoian I. Hepcidin-central regulator of iron metabolism. Eur J Haem. 2007;78(1):1–10. doi: 10.1111/j.1600-0609.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- [17].Gangat N, Wolanskyj AP. Anemia of chronic disease. Semin Hematol. 2013 Jul;50(3):232–238. doi: 10.1053/j.seminhematol.2013.06.006. [DOI] [PubMed] [Google Scholar]
- [18].Beguin Y, Aapro M, Ludwig H. et al. Epidemiological and nonclinical studies investigating effects of iron in carcinogenesis - A critical review. Crit Rev Oncol Hematol. 2014;89(1):1–15. doi: 10.1016/j.critrevonc.2013.10.008. [DOI] [PubMed] [Google Scholar]
- [19].Shi YJ, Pan XT.. BMP6 and BMP4 expression in patients with cancer-related anemia and its relationship with hepcidin and s-HJV. Genetics and molecular. Research[J] 2016;15(1):1–5. doi: 10.4238/gmr.15017130. [DOI] [PubMed] [Google Scholar]
- [20].Means RT. Hepcidin and cytokines in anemia. Hematology[J] 2004;9(56):357–362. doi: 10.1080/10245330400018540. [DOI] [PubMed] [Google Scholar]
- [21].Nemeth E, Tuttle MS, Powelson J. et al. Hepcidin regulates iron efflux by binding to ferroportin and inducing its internalization. Science [J] 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- [22].Fleming RE, Sly WS. Hepcidin: a putative iron-regulatory hormone relevant to hereditary hemochromatosis and the anemia of chronic disease. Proc Natl Acd Sci. USA [J] 2001;98(15):8160–8162. doi: 10.1073/pnas.161296298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pan XT, Lu y, Cheng X. et al. Hepcidin and forroportin expression in breast cancer tissue and serum and their relationship with anemia [J] 2016;23(1):24–26. doi: 10.3747/co.23.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]