Abstract
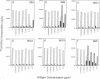
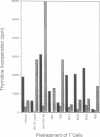
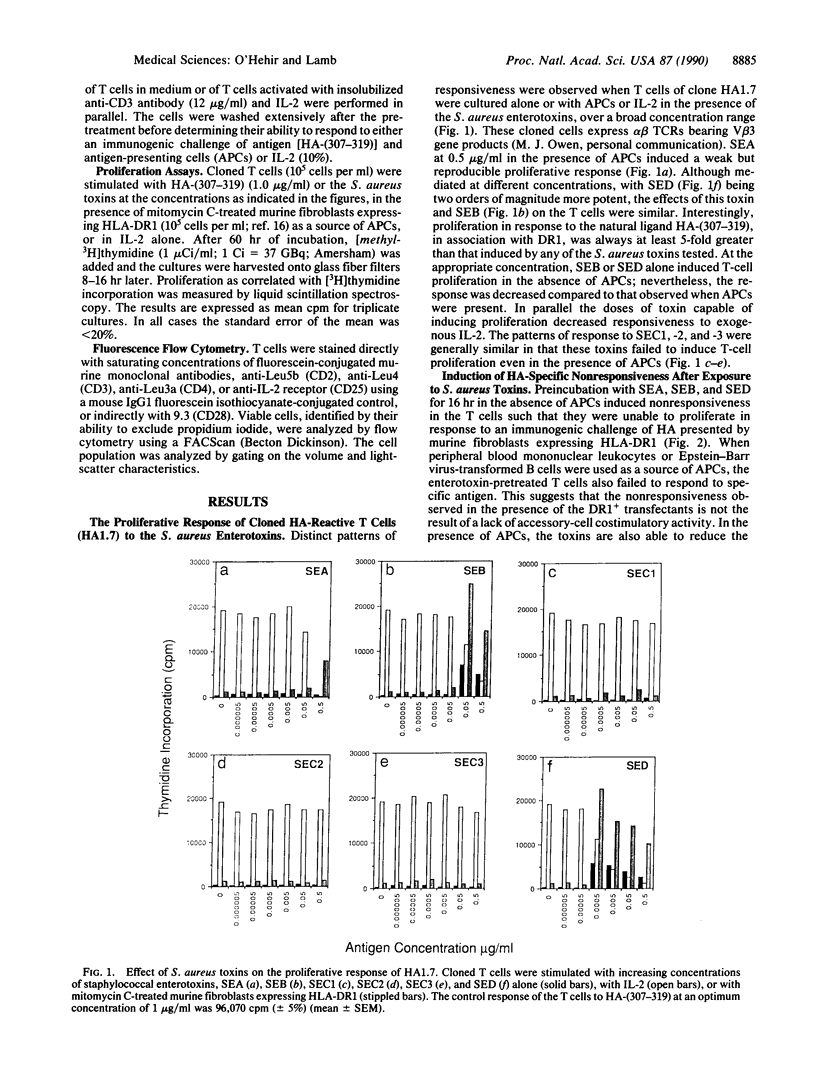
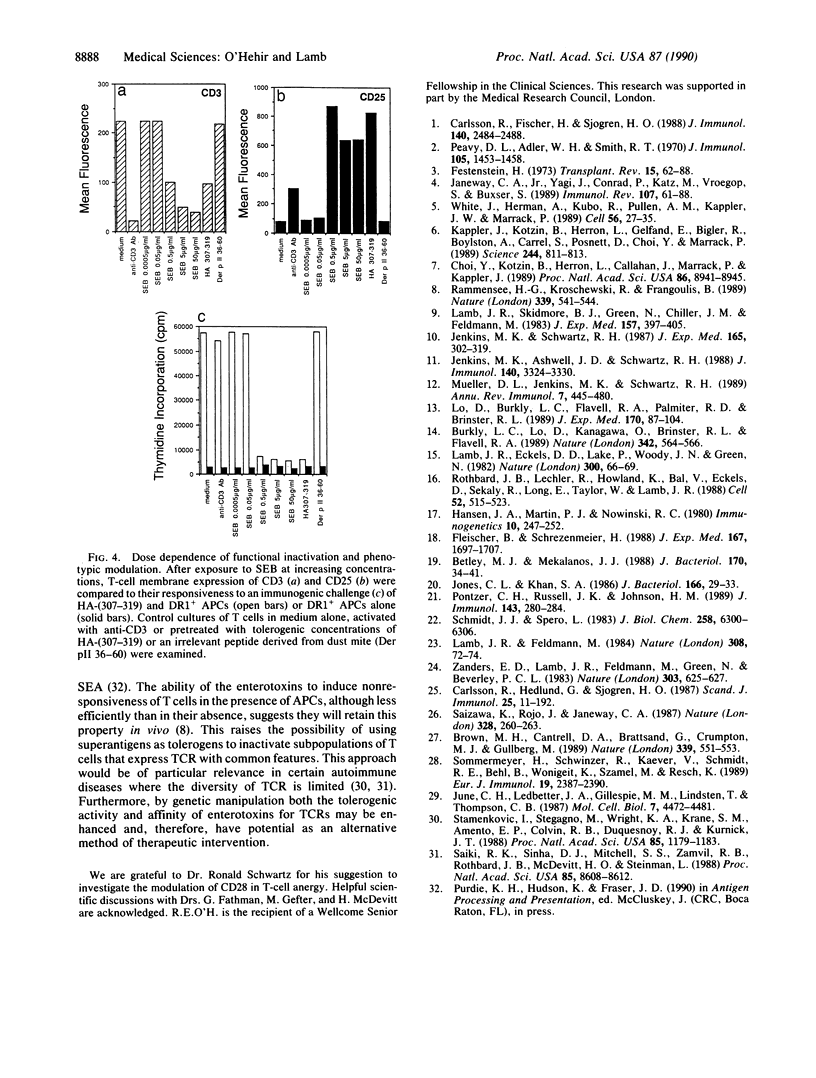
The exotoxins produced by certain strains of Staphylococcus aureus are able to stimulate powerful polyclonal proliferative responses and to induce nonresponsiveness by clonal deletion of T lymphocytes expressing the appropriate T-cell antigen receptor V beta gene products. This paper examines the ability of S. aureus enterotoxins to modulate the responsiveness of human CD4+ T lymphocytes with defined antigen specificity. It was observed that certain S. aureus toxins were able to activate and induce anergy in hemagglutinin-reactive T cells expressing V beta 3+ elements. After exposure to S. aureus enterotoxins A, B, and D in the absence of antigen-presenting cells, the T cells failed to respond to their natural ligand presented in an immunogenic form, despite enhanced proliferation to exogenous interleukin 2. The S. aureus toxin-induced anergy was associated with modulation of T-cell membrane receptors; down-regulation of the T-cell antigen receptor was concomitant with enhanced expression of CD2 and CD25. Interestingly, CD28 was increased only on stimulation, suggesting this protein may be differentially expressed by activated and anergic T cells. These results indicate that bacterial toxins are able to induce antigen-specific nonresponsiveness in human T cells, the application of which may be relevant in the regulation of T cells expressing a particular family of V beta gene products.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betley M. J., Mekalanos J. J. Nucleotide sequence of the type A staphylococcal enterotoxin gene. J Bacteriol. 1988 Jan;170(1):34–41. doi: 10.1128/jb.170.1.34-41.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. H., Cantrell D. A., Brattsand G., Crumpton M. J., Gullberg M. The CD2 antigen associates with the T-cell antigen receptor CD3 antigen complex on the surface of human T lymphocytes. Nature. 1989 Jun 15;339(6225):551–553. doi: 10.1038/339551a0. [DOI] [PubMed] [Google Scholar]
- Burkly L. C., Lo D., Kanagawa O., Brinster R. L., Flavell R. A. T-cell tolerance by clonal anergy in transgenic mice with nonlymphoid expression of MHC class II I-E. Nature. 1989 Nov 30;342(6249):564–566. doi: 10.1038/342564a0. [DOI] [PubMed] [Google Scholar]
- Carlsson R., Fischer H., Sjögren H. O. Binding of staphylococcal enterotoxin A to accessory cells is a requirement for its ability to activate human T cells. J Immunol. 1988 Apr 15;140(8):2484–2488. [PubMed] [Google Scholar]
- Carlsson R., Hedlund G., Sjögren H. O. Abrogation of staphylococcal enterotoxin A-induced suppressor cell activity by the anti-Tac monoclonal antibody. Scand J Immunol. 1987 Jan;25(1):11–19. doi: 10.1111/j.1365-3083.1987.tb01041.x. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festenstein H. Immunogenetic and biological aspects of in vitro lymphocyte allotransformation (MLR) in the mouse. Transplant Rev. 1973;15:62–88. doi: 10.1111/j.1600-065x.1973.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med. 1988 May 1;167(5):1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Yagi J., Conrad P. J., Katz M. E., Jones B., Vroegop S., Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989 Feb;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Ashwell J. D., Schwartz R. H. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol. 1988 May 15;140(10):3324–3330. [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987 Feb 1;165(2):302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. L., Khan S. A. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J Bacteriol. 1986 Apr;166(1):29–33. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Gillespie M. M., Lindsten T., Thompson C. B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987 Dec;7(12):4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J., Kotzin B., Herron L., Gelfand E. W., Bigler R. D., Boylston A., Carrel S., Posnett D. N., Choi Y., Marrack P. V beta-specific stimulation of human T cells by staphylococcal toxins. Science. 1989 May 19;244(4906):811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Eckels D. D., Lake P., Woody J. N., Green N. Human T-cell clones recognize chemically synthesized peptides of influenza haemagglutinin. Nature. 1982 Nov 4;300(5887):66–69. doi: 10.1038/300066a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Feldmann M. Essential requirement for major histocompatibility complex recognition in T-cell tolerance induction. Nature. 1984 Mar 1;308(5954):72–74. doi: 10.1038/308072a0. [DOI] [PubMed] [Google Scholar]
- Lo D., Burkly L. C., Flavell R. A., Palmiter R. D., Brinster R. L. Tolerance in transgenic mice expressing class II major histocompatibility complex on pancreatic acinar cells. J Exp Med. 1989 Jul 1;170(1):87–104. doi: 10.1084/jem.170.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Peavy D. L., Adler W. H., Smith R. T. The mitogenic effects of endotoxin and staphylococcal enterotoxin B on mouse spleen cells and human peripheral lymphocytes. J Immunol. 1970 Dec;105(6):1453–1458. [PubMed] [Google Scholar]
- Pontzer C. H., Russell J. K., Johnson H. M. Localization of an immune functional site on staphylococcal enterotoxin A using the synthetic peptide approach. J Immunol. 1989 Jul 1;143(1):280–284. [PubMed] [Google Scholar]
- Rammensee H. G., Kroschewski R., Frangoulis B. Clonal anergy induced in mature V beta 6+ T lymphocytes on immunizing Mls-1b mice with Mls-1a expressing cells. Nature. 1989 Jun 15;339(6225):541–544. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Lechler R. I., Howland K., Bal V., Eckels D. D., Sekaly R., Long E. O., Taylor W. R., Lamb J. R. Structural model of HLA-DR1 restricted T cell antigen recognition. Cell. 1988 Feb 26;52(4):515–523. doi: 10.1016/0092-8674(88)90464-3. [DOI] [PubMed] [Google Scholar]
- Saizawa K., Rojo J., Janeway C. A., Jr Evidence for a physical association of CD4 and the CD3:alpha:beta T-cell receptor. Nature. 1987 Jul 16;328(6127):260–263. doi: 10.1038/328260a0. [DOI] [PubMed] [Google Scholar]
- Sakai K., Sinha A. A., Mitchell D. J., Zamvil S. S., Rothbard J. B., McDevitt H. O., Steinman L. Involvement of distinct murine T-cell receptors in the autoimmune encephalitogenic response to nested epitopes of myelin basic protein. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8608–8612. doi: 10.1073/pnas.85.22.8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. J., Spero L. The complete amino acid sequence of staphylococcal enterotoxin C1. J Biol Chem. 1983 May 25;258(10):6300–6306. [PubMed] [Google Scholar]
- Sommermeyer H., Schwinzer R., Kaever V., Wessel K., Schmidt R. E., Behl B., Wonigeit K., Szamel M., Resch K. Cholera toxin modulates the T cell antigen receptor/CD3 complex but not the CD2 molecule and inhibits signaling via both receptor structures in the human T cell lymphoma Jurkat. Eur J Immunol. 1989 Dec;19(12):2387–2390. doi: 10.1002/eji.1830191232. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Stegagno M., Wright K. A., Krane S. M., Amento E. P., Colvin R. B., Duquesnoy R. J., Kurnick J. T. Clonal dominance among T-lymphocyte infiltrates in arthritis. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1179–1183. doi: 10.1073/pnas.85.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Zanders E. D., Lamb J. R., Feldmann M., Green N., Beverley P. C. Tolerance of T-cell clones is associated with membrane antigen changes. Nature. 1983 Jun 16;303(5918):625–627. doi: 10.1038/303625a0. [DOI] [PubMed] [Google Scholar]