Summary
Formation of neutrophil extracellular traps (NETs) is an important function of the innate immune system against infections. It has been proven that aging dysregulates immunity and impairs neutrophil function. However, the influence of aging on the ability to produce NETs has yet to be fully addressed. In this study, we tested the hypothesis that a lower level of autophagy in neutrophils from aged mice was responsible for the decrease in NET formation. We demonstrated that a broad range of Toll‐like receptor 2 (TLR2) ligands could efficiently induce reactive oxygen species (ROS) ‐dependent NET release in young mice, but not in aged ones. We further explored that the difference between young and aged mice in TLR2 ligand‐induced NETosis is the result of an Atg5 defect and subsequent impaired autophagy. Furthermore, we found that lower autophagy capacity led to not only reduced NET formation, but also increased apoptosis. Our results suggest an important role of Atg5 and autophagy in maintaining the function of NETs formation in response to infection and in regulating neutrophil death. Targeting autophagy‐promoted NETs may present a therapeutic strategy to improve infection defence in an aged population.
Keywords: aging, apoptosis, Atg5, autophagy, neutrophil extracellular traps
Abbreviations
- CLP
caecal ligation and puncture
- fMLP
N‐formylmethionyl‐leucyl‐phenylalanine
- GEO
Gene Expression Omnibus
- gp91 KO
gp91phox knockout
- IL‐8
interleukin‐8
- LM
lipomannans
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- mTOR
mammalian target of rapamycin
- NETs
neutrophil extracellular traps
- Pam2CSK4
synthetic diacylated lipoprotein
- Pam3CSK4
synthetic triacylated lipoprotein
- PGN
peptidoglycan
- PMNs
polymorphonuclear leucocytes
- ROS
reactive oxygen species
- TLR2
Toll‐like receptor 2
- WT
wild‐type
Introduction
Human aging is now a global trend, with an increasing relative and absolute number of elderly in the world.1 Currently, 23% of the disease burden is attributable to illness in the population aged 60 years and over,2 and one‐third of deaths in the elderly are due to infectious diseases.3 Health problems of aged people are associated with a progressive deterioration of the immune system, a process known as immunosenescence.
Cumulative evidence indicates that aging can cause a paradox in the immune system, characterized by an elevation of basal inflammation, yet an impaired ability to mount efficient innate and adaptive immune responses. As the most abundant immune effector cells, neutrophils are essential for innate immunity and resistance to pathogens. Neutrophil functions including migration, phagocytosis, as well as cytokine and chemokine production, have been reported to become abnormal with aging.4, 5 However, little is known about the effect of aging on formation of neutrophil extracellular traps (NETs) and its underlying mechanisms.
Neutrophil extracellular traps, released by activated neutrophils, are highly decondensed DNA structures, decorated with histones, proteases and antimicrobial peptides. Since their first discovery,6 NETs have been recognized as an important strategy of neutrophils to respond to infections, and have extended our understanding of how phagocytes kill pathogens. Research has indicated that NETs are usually formed in the context of dying neutrophils, making it a new form of cell death, NETosis,7 which differs from apoptosis or necroptosis morphologically and biochemically. The nuclear and granular membranes disintegrate during NETosis, whereas the plasma membrane remains integral with no phosphatidylserine exposure until the last moment when NETs are released.8 Caspase activity is not detected during PMA‐induced NETosis. Moreover, the addition of the pan‐caspase inhibitor carbobenzoxy‐valyl‐alanyl‐aspartyl‐[O‐methyl]‐fluoromethylketone (zVAD) or necroptosis inhibitor necrostatin‐1 does not affect PMA‐induced NET formation.9
Toll‐like receptor 2 (TLR2) is a member of the TLR family. It is crucial to the recognition of diverse microbial molecules from a broad group of microbes, such as Gram‐positive and Gram‐negative bacteria, mycoplasma and yeast. TLR2 forms heterodimers with TLR1 or TLR6, each dimer having different specificities for different ligands. For example, TLR2/TLR6 is required for responses to diacylated lipoproteins, whereas TLR2/TLR1 recognizes triacylated lipoproteins.10 Although a variety of different stimuli are capable of inducing NETosis, including live bacteria, fungi, bacterial lipopolysaccharide (LPS), cytokine interleukin 8 (IL‐8) and chemical compound PMA, there are only two studies testifying to the effect of TLR2 ligand Pam3CSK4 on NET formation with controversial results.11, 12
Here we systematically investigated whether diverse TLR2 ligands could induce NETs, and whether the ability of neutrophils to form NETs was compromised in aged mice. The effect of aging on neutrophil apoptosis was also examined. Furthermore, we explored the possible mechanisms through which aging affected NETosis and apoptosis.
Methods
Animals and in vivo treatments
Young wild‐type (WT) mice and gp91phox knockout (KO) mice (male, 8–12 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME). Aged mice (male, over 18 months old) were obtained from the National Institute of Aging (Bethesda, MD). TLR2 KO and MyD88 KO mice were bred at the University of Pittsburgh (T.R. Billiar). Mice used in the study were of the same genetic background (C57BL/6). Mice were raised and cared for in a pathogen‐free facility according to institutional guidelines. The in vivo experiments were approved by the institutional animal care and use committee, and were performed in compliance with the guidelines for animal experimentation.
Mice underwent caecal ligation puncture (CLP). They were anaesthetized by intraperitoneal injection of 50 mg/kg ketamine and 5 mg/kg xylazine. After disinfection, a midline incision was made in the abdomen. The caecum was then exteriorized, and the distal end was ligated and punctured once with a needle (21‐gauge) to achieve a sub‐lethal sepsis model as previously reported.13 Mice were resuscitated with (5 ml/100 g) saline, and killed 6 hr after surgery to retrieve the peritoneal lavage.
Polymorphonuclear leucocyte isolation and NET induction
Most assays in this study were performed with polymorphonuclear leucocytes (PMNs) enriched from the peritoneal cavity. Briefly, mice were injected intraperitoneally with 1 ml 9% casein solution twice overnight. The animals were killed 3 hr after the second injection to harvest peritoneal fluid. The lavage was subsequently centrifuged, and the resulting pellet was washed. The PMNs were isolated by discontinuous density gradient centrifugation with two commercially available solutions (Histopaque‐1077 and Histopaque‐1119) of differential density purchased from Sigma (St Louis, MO) (#11191 and #10771), according to the manufacturer's instructions.
To compare the ability of bone marrow to produce neutrophils in young and aged mice, PMNs were extracted and isolated from bone marrow by density gradient centrifugation, as reported elsewhere.14
Samples were measured by flow cytometry to detect the percentage of PMNs based on surface marker Ly6G (#11‐5931‐81; eBioscience, San Diego, CA) and CD11b (#12‐0112‐81; eBioscience).
Various TLR2 ligands as well as LPS (10 μg/ml) were used to induce NETs. PMNs treated with 100 nm PMA were used as positive control. Doses of TLR2 ligands used were as follows: peptidoglycan (PGN, 10 μg/ml; #tlrl‐pgns2; InvivoGen, San Diego, CA), lipomannans (10 ng/ml; #tlrl‐lmms1; InvivoGen), lipoteichoic acid (1 μg/ml; #tlrl‐slta; InvivoGen), synthetic triacylated lipoprotein (Pam3CSK4, 100 ng/ml; #tlrl‐pms; InvivoGen), synthetic diacylated lipoprotein (Pam2CSK4, 100 ng/ml; #tlrl‐pm2s‐1; InvivoGen) and zymosan (10 μg/ml; #tlrl‐zyn; InvivoGen). Inhibitors and enhancers used were rapamycin (100 nm; #R8781; Sigma), wortmannin (150 nm; #tlrl‐wtm; InvivoGen), and bafilomycin A1 (1 μm; #tlrl‐baf1; InvivoGen).
NETs quantification assay
The PMNs were plated and cultured in 96‐well plates. At the indicated time‐points after treatment, 1 U/ml micrococcal nuclease (#M0247S; New England Biolabs, Ipswich, MA) was added. PMNs were incubated at 37° for 15 min to allow the extruded DNA to detach from cell debris. Cells were then centrifuged at 1800 g for 10 min. Cell‐impermeable DNA‐binding dye SYTOX Green (# S7020, 1 μm; Thermo Fisher Scientific, Waltham, MA) was added to the extracted supernatants and incubated in the dark for 15 min. Extracellular DNA content is represented by the fluorescence intensity detected with SpectraMax M2 (excitation wavelength 485 nm, and emission wavelength 530 nm).
Confocal microscopy
The PMNs were allowed to settle on glass coverslips pre‐coated with poly‐l‐lysine (#354085; Corning, Corning, NY) for 30 min, before they were treated with different stimuli for a specific period of time. Cells were washed, fixed with 4% paraformaldehyde in PBS, and blocked for 1 hr before being incubated with anti‐neutrophil elastase antibody (#sc‐9521; Santa Cruz Biotechnology, Dallas, TX). After staining with Hoechst 33342, SYTOX Green or donkey anti‐goat IgG conjugated with Alex Fluor 488, cells were analysed by confocal microscopy.
Surface TLR2 expression and reactive oxygen species production
The expression of TLR2 on the cell surface was measured using flow cytometry with anti‐mouse CD282 FITC antibody (#11‐9021‐82; eBioscience). Similarly, the intracellular reactive oxygen species (ROS) generation was determined by flow cytometry with CM‐H2DCFDA (#C6827; Thermo Fisher Scientific). PMNs were removed from growth media, washed and then resuspended in pre‐warmed loading buffer containing freshly prepared antibody (1 : 100) or probe (10 μm). After a 30‐min incubation at room temperature, fluorescence intensity was examined by flow cytometry.
Measurement of cell death
Programmed cell death was analysed by flow cytometry with apoptosis detection kit (#559763; BD Biosciences, Franklin Lakes, NJ). Cells were centrifuged, washed twice with ice‐cold PBS, and resuspended in × 1 binding buffer. Cells were incubated with Annexin‐V and 7‐AAD for 15 min at room temperature in the dark, and then were analysed by flow cytometry. Cells stained positive for Annexin‐V and negative for 7‐AAD were considered to be apoptotic, according to the manufacturer's instruction.
Microarray data analysis
The NCBI Gene Expression Omnibus (GEO) database was searched to find and retrieve any appropriate data set. If raw data of the data set were provided by the authors, the r software (for free download at https://www.r-project.org) and the limma package in bioconductor would be used to standardize, analyse and annotate the data. If original data were not available, the GEO2R from the NCBI could be used to analyse the processed data uploaded by the authors to detect differentially expressed genes. Benjamini & Hochberg adjustment to P‐values was considered.
Western blot
Samples were lysed in the ice‐cold lysis buffer for 30 min. The supernatant after centrifugation was collected, and the total protein concentration was quantified. Cell lysates were fractionated by 8–15% SDS–PAGE, according to the molecular size of the target protein. Proteins from gels were transferred onto a PVDF membrane. After incubation with the appropriate primary antibodies overnight at 4°, the washed membrane was stained with IRDye® (LI‐COR Biosciences, Lincoln, NE) secondary antibodies and visualized with an LI‐COR Odyssey near‐infrared imaging system.
The primary antibodies used (ULK1 #8054, Atg13 #13273, LC3B #2775, Atg5 #12994, mTOR #2983, and GAPDH #5174) were all purchased from Cell Signaling Technology (Danvers, MA).
Statistical analysis
The unpaired, two‐tailed t‐test was used to compare the difference between two groups. One‐way analysis of variance was used to compare differences among groups, with Dunnett's post hoc multiple comparisons conducted. Factorial design analysis of variance was used to examine the effects of different treatments and different age groups in Western blot. A P‐value < 0·05 was considered statistically significant. Data were analysed using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL).
Results
TLR2 ligand‐induced NETosis is regulated by ROS production and autophagy
PMA has been extensively used for activating NETosis. Other treatments, such as LPS, IL‐8 and N‐formylmethionyl‐leucyl‐phenylalanine (fMLP) are also commonly used. We first examined whether NETosis can be effectively elicited by TLR2 ligands in vitro. Several representatives from diverse categories of cell wall components were selected to stimulate PMNs isolated from the peritoneal cavity. The ligands used were all able to induce NETosis, as demonstrated by fluorescence intensity assay and confocal microscopy. The formation of NETs increased gradually within a 6‐hr time frame (Fig. 1).
Figure 1.
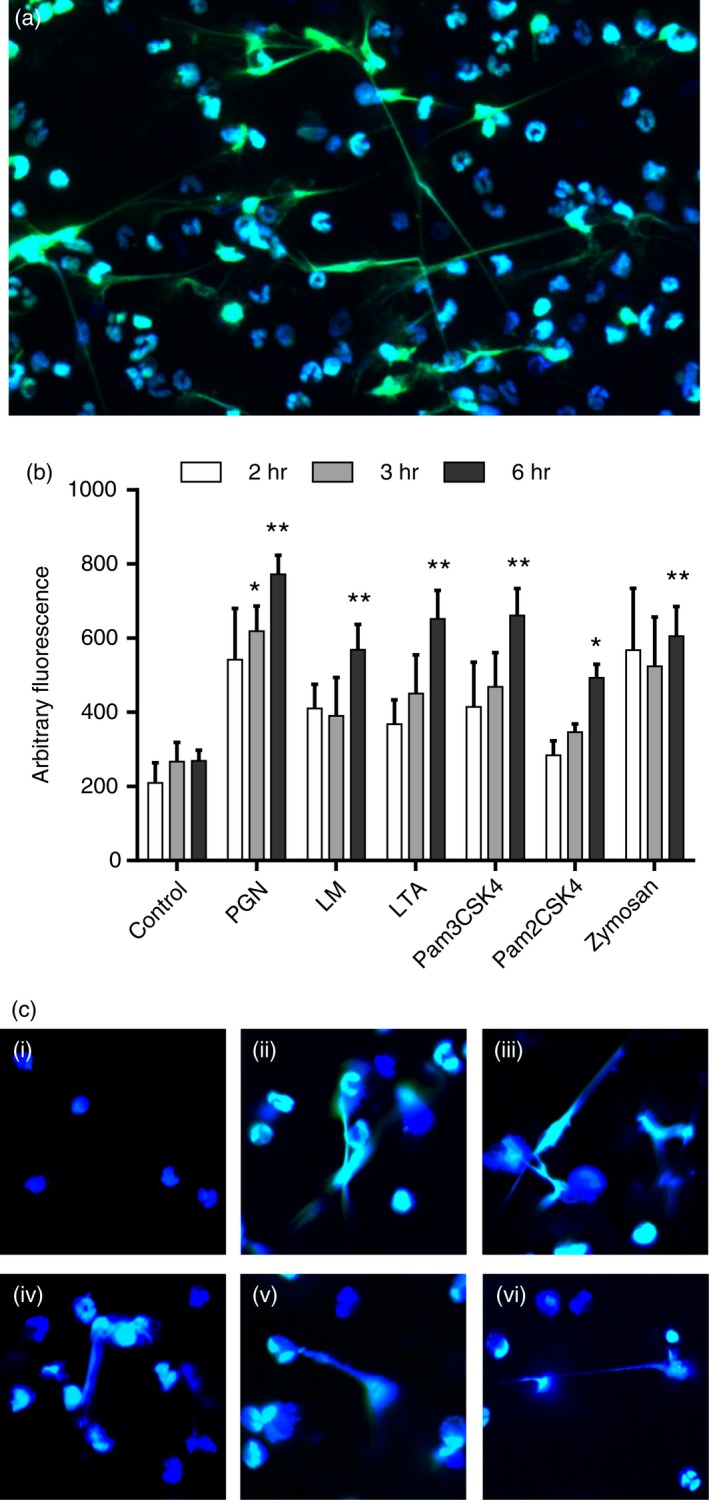
Toll‐like receptor 2 (TLR2) ligands induced NETosis. (a) A typical representation of peptidoglycan (PGN) ‐induced NETosis. (b) Quantification of neutrophil extracellular trap (NET) formation after 2, 3 and 6 hr of treatment with TLR2 ligands. (c) NETs induced by various TLR2 ligands after 6 hr of treatment. (i–vi): control, lipomannan (LM), lipoteichoic acid (LTA, Pam3CSK4, Pam2CSK4 and zymosan. Fluorescent dyes used in confocal microscopy: blue, Hoechst 33342; Green, SYTOX Green. Data are represented as mean ± SEM of at least triplicate samples. Comparisons were made with the control group: *P < 0·1; **P < 0·05. [Colour figure can be viewed at wileyonlinelibrary.com]
MyD88 has been regarded as the key adaptor molecule for TLR2 signalling. To clarify the role of TLR2 and MyD88 in NET formation, we repeated the experiments on neutrophils from TLR2 KO and MyD88 KO mice. The results showed that the ligands above could not induce NET formation in PMNs from these two mouse types (Fig. 2).
Figure 2.
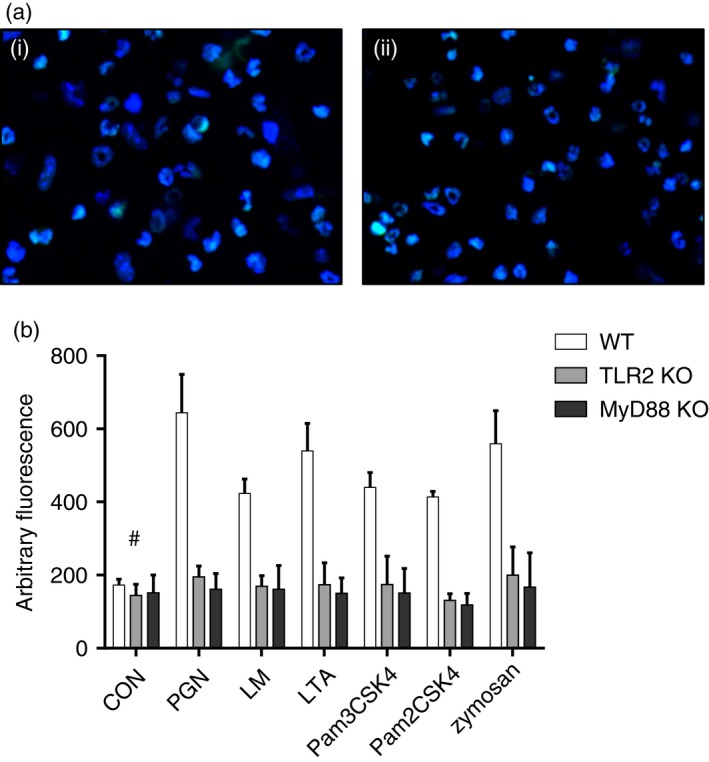
Toll‐like receptor 2 (TLR2) ligands induced neutrophil extracellular trap (NET) formation, which was dependent on MyD88. (a) NET formation after 6 hr treatment of peptidoglycan (PGN): (i) polymorphonuclear leucocytes (PMNs) from TLR2 knockout (KO) mice; (ii) PMNs from MyD88 KO mice. (b) Quantification of NET formation after 6 hr of treatment with TLR2 ligands. Fluorescent dyes used in confocal microscopy: blue, Hoechst 33342; Green, SYTOX Green. Data are represented as mean ± SD of at least triplicate samples. Comparisons were made between the two KO mice and WT mice. Differences were significant except for the control group marked with #. [Colour figure can be viewed at wileyonlinelibrary.com]
There is a synergistic effect between different TLRs on inflammatory cytokine release.15 To determine whether TLR2 and TLR4 play a synergistic role in inducing NET formation, low doses of LPS and PGN (both at 10 ng/ml) were used alone or in combination. The use of low‐dose LPS and PGN increased NET formation more than either one alone, indicating a synergistic effect (Fig. 3).
Figure 3.
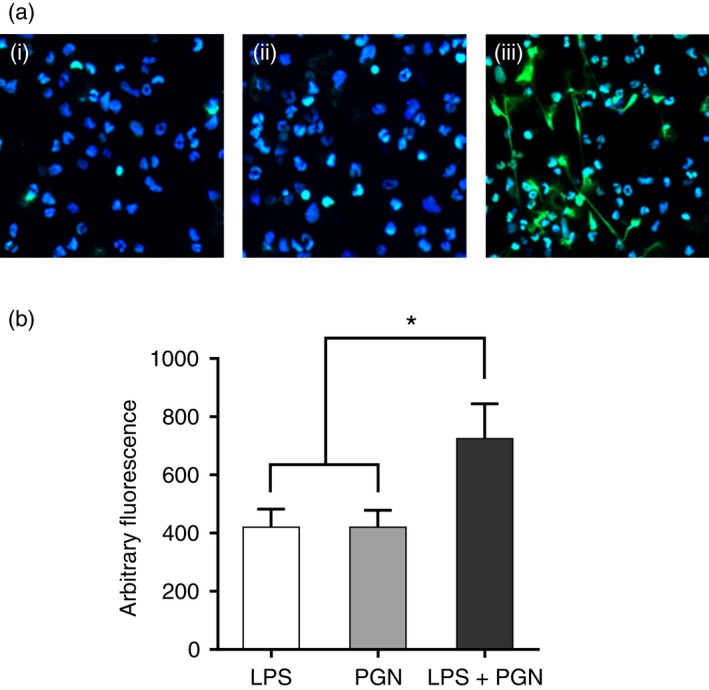
Synergistic effect between Toll‐like receptor 2 (TLR2) and TLR4 on neutrophil extracellular trap (NET) formation. (a) NET formation induced by 6 hr treatment of low‐dose lipopolysaccharide (LPS) or peptidoglycan (PGN) (10 ng/ml). (i–iii) LPS, PGN, and LPS/PGN combination. (b) Quantification of NET formation after 6 hr of treatment with low‐dose LPS or PGN (10 ng/ml). Fluorescent dyes used in confocal microscopy: blue, Hoechst 33342; Green, SYTOX Green. Data are represented as mean ± SD of at least triplicate samples. *P < 0·05. [Colour figure can be viewed at wileyonlinelibrary.com]
The mechanisms of NETosis are not fully understood. Research has revealed that cellular processes, such as ROS generation and autophagy,16 are required for the release of NETs induced by PMA or fMLP. Here we used gp91 KO mice, enhancer and inhibitor of autophagy to examine the role of ROS and autophagy in TLR2 ligand‐induced NETosis. Lower ROS production after stimulation was found in PMNs from gp91 KO mice than in those from WT mice, whereas the basal levels in the two groups were comparable (Fig. 4a). Similarly, there was also less NET production in gp91 KO mice than in WT mice (Fig. 4b,c). Inhibition of mammalian target of rapamycin (mTOR) to augment autophagic activity enhanced PGN‐induced NET formation (Fig. 4d). These results verified that ROS generated by NADPH oxidase and autophagy were both involved in TLR2 ligand‐induced NETosis.
Figure 4.
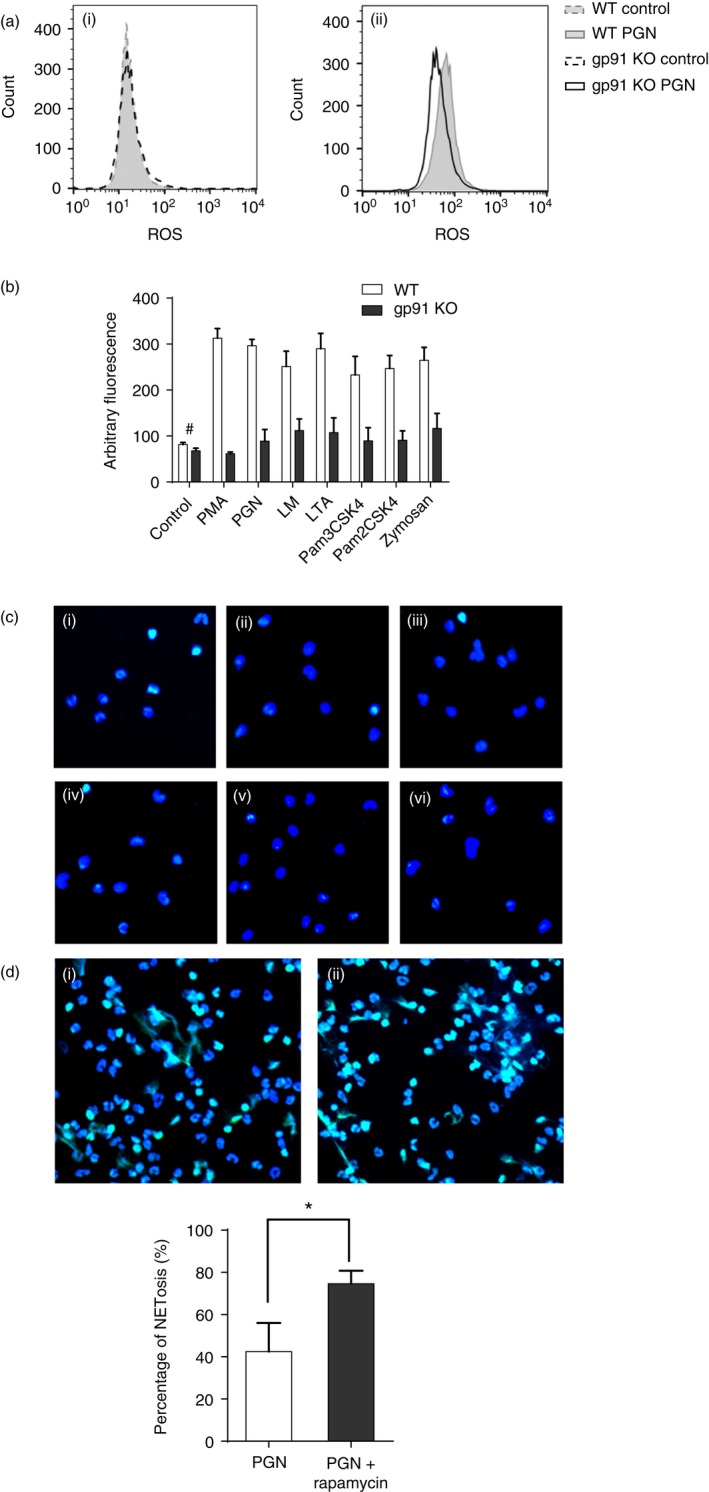
Toll‐like receptor 2 (TLR2) ligands‐induced NETosis is regulated by reactive oxygen species (ROS) production and autophagy. (a) ROS production before and after 40 min treatment with peptidoglycan (PGN) of polymorphonuclear leucocytes (PMNs) from gp91 knockout (KO) and wild‐type (WT) mice. (b) Quantification of neutrophil extracellular trap (NET) formation in PMNs from gp91 KO and WT mice after 6 hr of treatment with TLR2 ligands. Data are represented as mean ± SEM of at least triplicate samples. Statistically significant except for the control group marked with #. (c) NETs induced by various TLR2 ligands after 6 hr of treatment in PMNs from gp91 KO mice. (i–vi) PGN, lipomannan (LM), lipoteichoic acid (LTA), Pam3CSK4, Pam2CSK4 and zymosan. (d) NET formation after augmentation of autophagy by rapamycin: (i) PGN; (ii) PGN + rapamycin. NETotic cell counts are represented as mean ± SD *P < 0·05. Fluorescent dyes used in confocal microscopy: blue, Hoechst 33342; Green, SYTOX Green. [Colour figure can be viewed at wileyonlinelibrary.com]
Diminished NETosis in aged mice
The innate immune system becomes dysregulated in many aspects with aging. Cellular responses to pathogens, such as migration, signal transduction and cytokine production, are possibly impaired in aged individuals.17 To compare the differences in NETosis between aged and young mice in vivo, NETs formation in the peritoneal lavage was analysed 6 hr after CLP. There were significantly fewer NETs in aged mice compared with their young counterparts (Fig. 5a,b).
Figure 5.
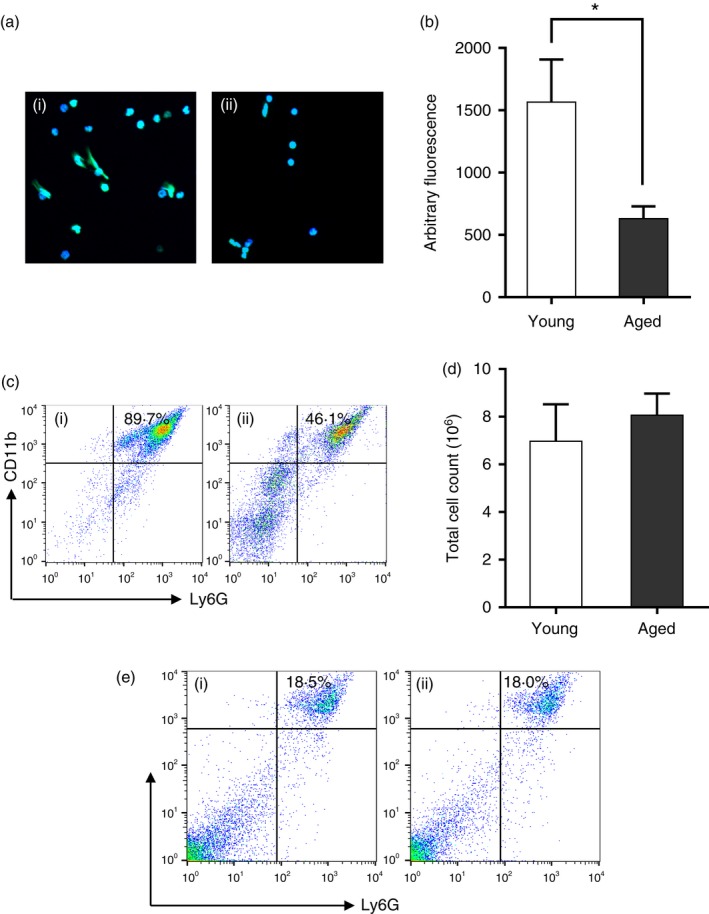
Diminished NETosis in aged mice in vivo. (a) Neutrophil extracellular trap (NET) formation in the peritoneal lavage 6 hr after caecal ligation and puncture (CLP): (i) young mice; (ii) aged mice. (b) Quantification of NET formation in the peritoneal lavage 6 hr after CLP. (c) Proportion of polymorphonuclear leucocytes (PMNs) in the peritoneal lavage 6 hr after CLP: (i) young mice; (ii) aged mice. (d) Total cell counts in the peritoneal lavage 6 hr after CLP. (e) Proportion of PMNs in the bone marrows of unstimulated mice: (i) young mice; (ii) aged mice. Fluorescent dyes used in confocal microscopy: blue, Hoechst 33342; Green, SYTOX Green. Data are represented as mean ± SD *, P < 0·05. [Colour figure can be viewed at wileyonlinelibrary.com]
To determine the relationship between chemotaxis and the diminished NET formation, the proportion of PMNs in the peritoneal lavage was detected at 6 hr after CLP. The percentage of Ly6G+ CD11b+ cells was lower in aged mice compared with in young mice (89·7% versus 46·1%, Fig. 5c); despite this, there was no difference in total cell counts in the peritoneal lavage after CLP (Fig. 5d). The percentage of PMNs in the bone marrow of unstimulated mice was also detected to rule out any difference in the ability to generate mature neutrophils. The proportion of neutrophils in the bone marrow was similar in young and aged mice (18·5% versus 18·0%, Fig. 5e).
In addition, the PMNs from aged mice generated much fewer NETs after in vitro stimulation with TLR2 ligands, compared with those from young mice (Fig. 6a,b). This difference could also be found when PMNs from aged mice were stimulated with LPS (Fig. 6c,d), indicating that the observed reduction in NET formation was not specific to TLR2 stimulation. Hazeldine et al.18 believe that reduced ROS production is the cause of LPS‐induced NETs in neutrophils from older subjects. Therefore, we detected TLR2 ligand‐induced ROS generation in PMNs from young and aged mice. Surface expression of TLR2 was measured by flow cytometry. Although the basal level of TLR2 expression on the surface of PMNs from aged mice was slightly lower, there was no significant difference after PGN stimulation (Fig. 6e). However, we found lower ROS generation in neutrophils from aged mice after challenge with various TLR2 ligands (Fig. 6f). This may serve as one of the reasons leading to the decreased NET formation following TLR2 stimulation in aged animals.
Figure 6.
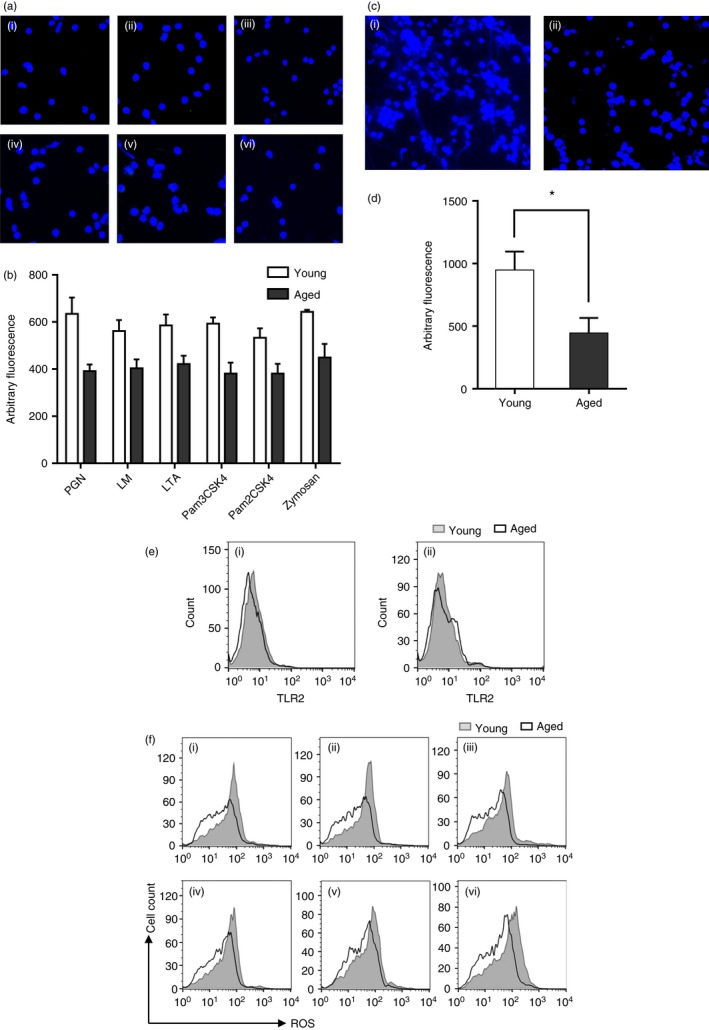
Diminished NETosis in aged mice in vitro and Toll‐like receptor 2 (TLR2) induced reactive oxygen species (ROS). (a) Neutrophil extracellular trap (NET) formation after 6 hr treatment of TLR2 ligands in aged mice: (i–vi) peptidoglycan (PGN), lipomannan (LM), lipoteichoic acid (LTA), Pam3CSK4, Pam2CSK4 and zymosan. (b) Quantification of NET formation after 6 hr of treatment with TLR2 ligands. Statistically significant in all treatment groups between young and aged mice. (c) NET formation after 6 hr treatment of lipopolysaccharide (LPS): (i) young mice; (ii) aged mice. (d) Quantification of NET formation after 6 hr of treatment with LPS. *P < 0·05. (e) Surface expression of TLR2 before and after PGN stimulation: (i) before stimulation; (ii) 4 hr after stimulation. (f) ROS generation after 3 hr treatment of TLR2 ligands: (i–vi): PGN, LM, LTA, Pam3CSK4, Pam2CSK4 and zymosan. Fluorescent dyes used in confocal microscopy: blue, Hoechst 33342. Data are represented as the mean ± SD of at least triplicate samples. [Colour figure can be viewed at wileyonlinelibrary.com]
Atg5‐related defect is responsible for the diminished NETs formation
We further investigated whether autophagic activity was associated with the decreased NET release in aged mice. PMNs from young and aged mice were stimulated with PGN for 2, 4 and 6 hr. The autophagic activity, represented by the expression of Light Chain 3B (LC3B), was monitored. The expression of LC3B in the young group increased with time, and reached its peak at 4 hr after stimulation. However, the level of LC3B in the aged group increased slightly, and was lower than that in the young group (Fig. 7a). Meanwhile, the use of rapamycin greatly restored the ability of PMNs from aged mice to release NETs after stimulation (Fig. 7b). Taken together, these results implied that the decreased capability of PMNs to form NETs after TLR2 stimulation might be associated with defective autophagic activity in aged mice.
Figure 7.
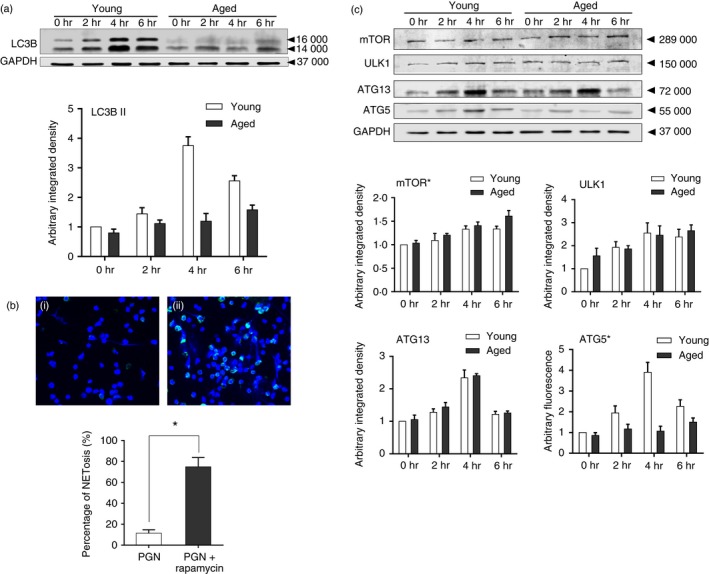
Atg5‐related defect in autophagy is responsible for reduced neutrophil extracellular trap (NET) formation in aged mice. (a) LC3B expression in polymorphonuclear leucocytes (PMNs) after peptidoglycan (PGN) stimulation. Factorial design analysis indicated a significant difference between young and aged mice. (b) NET formation in aged mice with the use of rapamycin: (i) PGN; (ii) PGN + rapamycin. *P < 0·05. (c) Expression of mammalian target of rapamycin (mTOR), ULK1, Atg13, and Atg5 in PGN‐stimulated PMNs. *Factorial design analysis indicated a significant difference between young and aged mice. Fluorescent dyes used in confocal microscopy: blue, Hoechst 33342; Green, neutrophil elastase‐ Alex Fluor 488. Data are represented as the mean ± SD. [Colour figure can be viewed at wileyonlinelibrary.com]
Autophagy is a self‐degradation process in response to extracellular or intracellular stress and signals, which involves several distinct steps, including induction, cargo recognition, phagophore and autophagosome formation, autophagsome–lysosome fusion and autolysosome breakdown.19 Different sets of autophagy‐related (Atg) proteins assemble into the core machinery during these steps. To find the key molecule responsible for defective autophagic activity in PMNs from aged mice, we used transcriptome data analysis together with verification at the protein level. The public data set used was Series GSE67652 from the NCBI GEO database. Transcriptional profiles of PMNs from 24 subjects were used to investigate the effect of aging on patients with sepsis in the original article.20 A two‐colour hybridization design was adopted. Here we used two group sets from the data series, e.g. ‘Adult with Sepsis’ and ‘Elder with Sepsis’, with six biological replicates per group. We analysed the expression of common ATG genes, based on the list of ATG family from the HUGO Gene Nomenclature Committee. In selected ATG genes, the difference of ULK1, ATG13 and ATG5 between the two groups was significant (Table 1, a logFC > 0 indicating an increased expression in the aged group compared with young participants). To confirm the differences of these three genes between aged and young mice, the expression after PGN stimulation was measured by Western blot. There was no significant difference in the expression of ULK1 or ATG13. However, the level of ATG5 in aged mice was significantly lower than that in young counterparts after PGN treatment (Fig. 7c). In addition, the expression of mTOR was also compared between the two groups. The relatively higher level of mTOR in aged mice may also contribute to the lower autophagic activity in the aged group (Fig. 7c).
Table 1.
Transcriptome analysis for seleted Atg genes between aged and adult subject
| ID | P value | LogFC | Gene_symbol |
|---|---|---|---|
| 29356 | 0·001414 | 1·93E‐01 | ULK1 |
| 34681 | 0·121761 | −1·29E‐01 | ULK2 |
| 28782 | 0·047251 | 0·11 | ATG2A |
| 2300 | 0·213405 | 0·0735 | ATG2A |
| 39616 | 0·224845 | 0·0697 | ATG2B |
| 36905 | 0·148097 | −0·0515 | ATG3 |
| 33866 | 0·064194 | −0·0765 | ATG4A |
| 56625 | 0·324826 | 0·039 | ATG4B |
| 24414 | 0·856333 | 0·00726 | ATG4B |
| 55495 | 0·070256 | −0·167 | ATG4C |
| 19751 | 0·164497 | −0·122 | ATG4C |
| 60077 | 0·459674 | 0·0472 | ATG4D |
| 5027 | 0·729321 | 0·0164 | ATG4D |
| 56833 | 0·965027 | 0·00345 | ATG4D |
| 19005 | 0·017444 | −0·0902 | ATG5 |
| 55329 | 0·018984 | −0·0986 | ATG5 |
| 55805 | 0·084749 | −0·0721 | ATG5 |
| 25617 | 0·167545 | −0·102 | ATG5 |
| 25138 | 0·123041 | 0·0445 | BECN1 |
| 46800 | 0·721665 | 0·0178 | ATG7 |
| 18191 | 0·197379 | 0·0505 | GABARAP |
| 35537 | 0·093617 | 0·0913 | GABARAPL1 |
| 28547 | 0·983039 | −0·001 | GABARAPL1 |
| 23325 | 0·453279 | −0·0375 | GABARAPL2 |
| 61873 | 0·288447 | −0·0562 | MAP1LC3B |
| 20786 | 0·394961 | −0·0325 | MAP1LC3B |
| 48560 | 0·396084 | 0·035 | ATG9A |
| 35519 | 0·414994 | −0·0303 | ATG12 |
| 10984 | 0·010188 | 0·16 | ATG13 |
| 1093 | 0·356698 | −0·0535 | ATG13 |
| 52226 | 0·192525 | 0·0584 | ATG16L1 |
| 38597 | 0·078559 | 0·137 | ATG16L2 |
| 39196 | 0·380682 | −0·036 | RB1CC1 |
| 21895 | 0·495782 | −0·0342 | WIPI1 |
| 1916 | 0·09436 | 0·07 | WIPI2 |
| 213 | 0·640536 | −0·0178 | WIPI2 |
| 20545 | 0·193245 | −0·0848 | SNX30 |
| 4180 | 0·644598 | −0·0218 | SNX4 |
The ID column is provided by the original supplier. P‐value is calculated as moderated t‐statistic by limma package. Log FC is the log2 fold changes of expression. The gene symbols in bold indicate that these genes' expression exhibits statistically significant difference between aged and young mice.
PMNs from aged mice are prone to undergoing apoptosis other than NETosis
NETosis is one death form that PMNs can undergo in an inflammatory environment. Other forms of neutrophil death include apoptosis, necroptosis and pyroptosis.21 Apoptosis has always been deemed as the final destiny for neutrophils no matter spontaneously or under stimulation, before NETosis is found.22 Therefore, we investigated whether apoptosis in PMNs from aged mice. The proportion of apoptotic PMNs (Annexin‐V+, 7‐AAD−) at 6 and 12 hr after PGN stimulation or left untreated was measured by flow cytometry. In the untreated group (6 hr), or after PGN stimulation, the percentage of apoptosis was higher in the aged group than in the young group (Fig. 8a).
Figure 8.
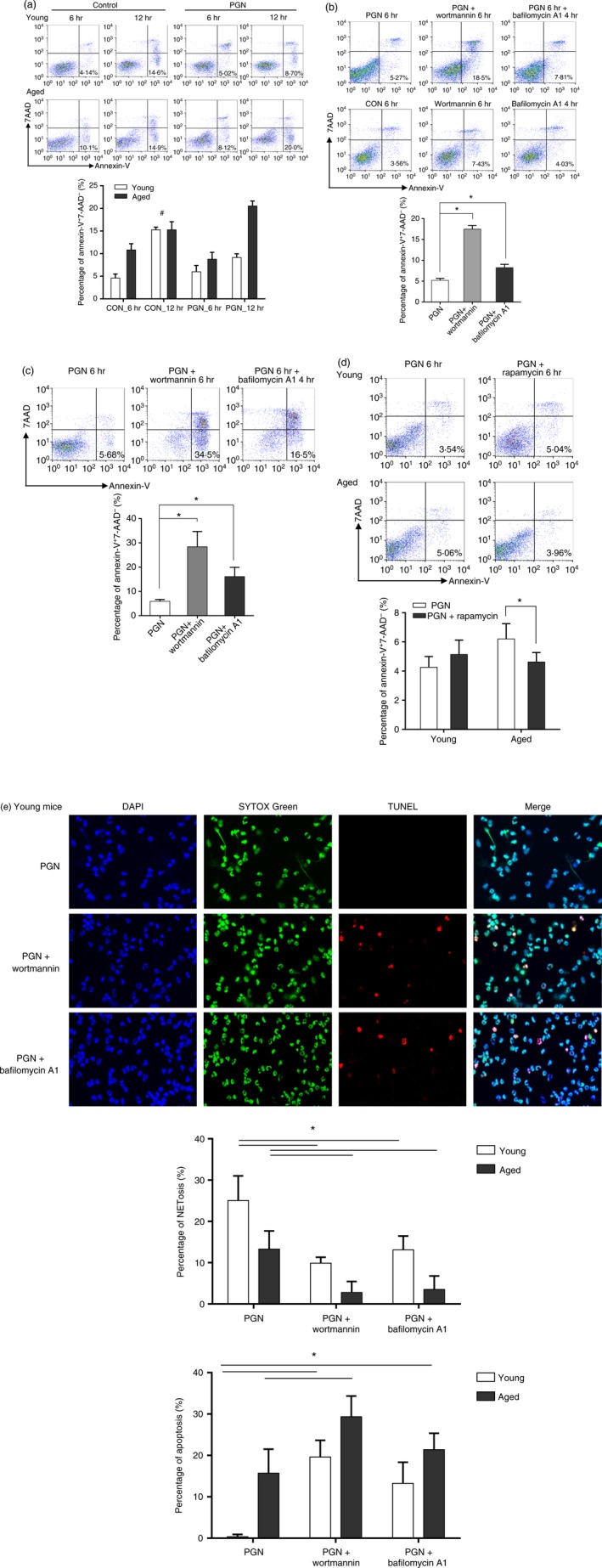
Polymorphonuclear leucocytes (PMNs) from aged mice are prone to undergoing apoptosis rather than NETosis. (a) Neutrophil apoptosis measured by flow cytometry after peptidoglycan (PGN) challenge or left untreated. Statistically significant between young and aged mice except for the group marked with #. (b) Neutrophil apoptosis in young mice measured by flow cytometry with inhibitors of autophagy. *P < 0·05. (c) Neutrophil apoptosis in aged mice measured by flow cytometry with inhibitors of autophagy. *P < 0·05. (d) Neutrophil apoptosis measured by flow cytometry with an enhancer of autophagy. *P < 0·1. (e) Neutrophil NETosis and apoptosis measured by confocal microscopy. *P < 0·05. Fluorescent dyes used in confocal microscopy: blue, Hoechst 33342; Green, SYTOX Green; Red, TMR Red. Data are represented as the mean ± SD of at least triplicate samples. [Colour figure can be viewed at wileyonlinelibrary.com]
Autophagy and apoptosis are usually cross‐regulated, mostly in a reciprocal suppression manner.23 Studies have shown that autophagy can reduce the propensity of cells to undergo apoptosis.24, 25 Based on these, the decrease in NETosis while apoptosis increases, as we see here in aged mice, may be regulated by autophagic activity. Inhibitors (wortmannin and bafilomycin A1) and an enhancer (rapamycin) of autophagy were used to treat PMNs in combination with PGN stimulation. The addition of wortmannin or bafilomycin A1 to PGN increased the percentage of apoptotic cells in young (Fig. 8b) and aged (Fig. 8c) mice. The use of either inhibitor alone had no significant effect on apoptosis. On the other hand, use of rapamycin reduced the percentage of apoptosis in aged mice, but not in young mice (Fig. 8d). Consistent with the above results, the formation of NETs was reduced, whereas apoptosis was increased, when wortmannin or bafilomycin A1 was applied under confocal microscopy (Fig. 8e).
Discussion
In the present study, we showed that a variety of TLR2 ligands were able to induce a ROS‐dependent formation of NETs. Low autophagic activity led to decreased NETosis and increased compensatory apoptosis after stimulation with TLR2 ligands in PMNs from aged mice. Atg5 might be the key molecule contributing to the defected autophagic activity in aged PMNs, as summarized in Fig. 9.
Figure 9.
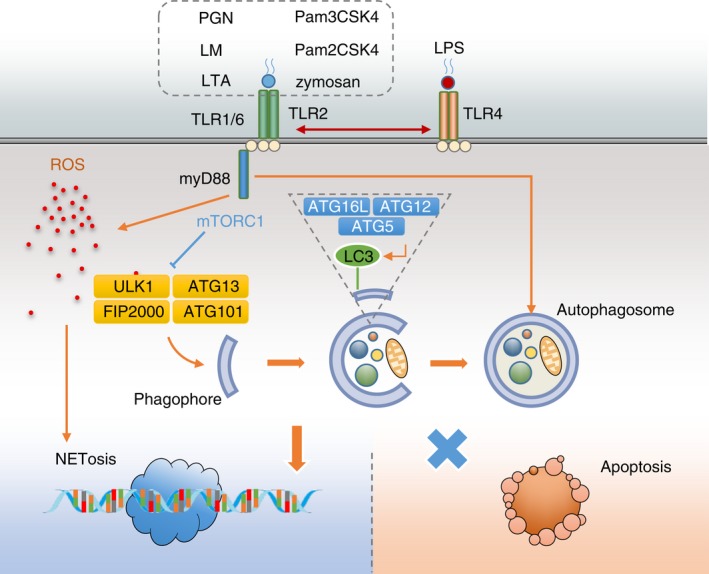
Schematic diagram of the present study. A variety of Toll‐like receptor 2 (TLR2) ligands are able to induce reactive oxygen species (ROS) ‐dependent neutrophil extracellular trap (NET) formation. Autophagy regulates the trend of neutrophils to undergo NETosis or apoptosis. In aged mice, the defect in Atg5 contributes to a low autophagic activity, which further leads to decreased NETosis and increased compensatory apoptosis after TLR2 stimulation. [Colour figure can be viewed at wileyonlinelibrary.com]
As an important function of neutrophils, NETs play a critical role in the innate immune system. It is of great significance to elucidate whether the ability to produce NETs is impaired in aged mice, and to explore the underlying mechanisms.
We started our research from TLR2 ligand‐induced NETosis. TLRs are similar to each other in many ways. It is quite understandable that TLR2 ligands, often as bacterial components, are capable of inducing NETosis. TLR2 recognizes diverse cell‐wall components, including those from Gram‐positive bacteria (peptidoglycan matrix, lipoteichoic acid and lipoproteins), mycobacteria (lipoarabinomannan) and yeast (zymosan).26 This also implies that TLR2 ligand may be one reason why NETosis can be induced by a broad spectrum of pathogenic microorganisms. In a previous report, Yipp et al. found that stimulation with bacteria but not Pam3CSK4 could induce NETs in vivo, whereas TLR2 KO animals were incapable of releasing histones or nuclear DNA.11 However, results from other research supported the notion that NETosis could be induced by the TLR2 agonists Pam3CSK4 and FSL‐1.12 We believe the reasons why Yipp et al. obtained a negative result may include: (i) Pam3CSK4 is not as potent as live bacteria in inducing NETs; (ii) it is harder to observe NET formation in vivo than in vitro; and (iii) the time course was relatively short (about 1 hr). The present study confirmed the important role of TLR2 in NET formation.
It takes several hours for ligands to induce NETs in vitro. The long time course it needed seems to restrict the significance of NETs as a weapon to prevent pathogens from spreading. However, research implies that bacteria are much more effective than simply pathogen‐associated molecules. A small amount of bacteria can induce NET formation in vivo in a relatively short time period (minutes).27
Many pathogens express ligands for multiple pattern recognition receptors, i.e. lipoproteins for TLR2 and LPS for TLR4; and mixed infection is not rare in the clinic. Therefore, chances are that multiple TLRs might be activated at the same time. We demonstrated a synergistic effect between PGN and LPS in inducing NETosis. To release NETs in circumstances where only small amounts of pathogens are present may be a natural defence mechanism of the innate immunity.
It was reported that there was a significant age‐related decline in LPS‐ and IL‐8‐induced NET formation in peripheral blood neutrophils isolated from healthy human subjects.18 Tseng et al.28 also reported that challenged with methicillin‐resistant Staphylococcus aureus, neutrophils isolated from aged mice showed defective NET formation. In the current study, we confirmed these results with in vivo and in vitro experiments. Hazeldine et al.18 found that the expression of TLR4 on neutrophils was similar between young and old subjects. And the defect in LPS‐ or IL‐8‐induced NETs was associated with reduced ROS production.18 The surface expression of TLR2 and ROS production were also observed in our experiments. However, we believed that there might be other factors that could influence NET formation.
Emerging evidence showed that NETosis is linked to autophagy. Remijsen et al. found that autophagy was involved in vacuolization during PMA‐induced NETosis.9 Another study showed that pharmacological inhibition of mTOR accelerated NET release.29 We detected a reduced autophagic activity in neutrophils from aged mice. Actually, decreased autophagic activity can be observed in most cell types and tissues as the organism becomes aged. Decreased autophagy in phagocytes may not only impair their ability to kill pathogens, but also cause dysregulated activation of the inflammasome.30 The mechanisms causing autophagic malfunction in aged individuals are still poorly understood.31 Several studies reported that the expression of ATG genes was down‐regulated in aged tissues.32, 33 Lipinski et al.32 demonstrated reduced expression of Atg5, Atg7 and Beclin 1 in normal aged brain. Atg5 takes part in the ubiquitin‐like conjugation system Atg12–Atg5–Atg16 complex, which plays an essential role in phagophore expansion and autophagosome formation. Both of the other two candidate proteins (ULK1 and Atg13) are involved in the initiation of autophagy, and are inhibited by mTORC1 under normal conditions (Fig. 9). Age‐related increase in mTOR activity has been reported extensively.34 In our study, PGN‐induced mTOR expression in PMNs from aged mice was higher than that from young counterparts. However, the phosphorylation of its downstream targets, such as 4EBP1, S6K and eIF4E, was not confirmed. Therefore, the role of mTOR in aging‐related NET formation is still not clear. Moreover, the screen of differentially expressed genes was based on microarray data, which is prone to be false negative due to the small sample size and technical errors. Hence, it is quite possible that other molecules, or other Atg genes, may participate in the defect of autophagy in the elderly.
Apoptosis is the main death form of neutrophils. There is evidence for increased apoptosis in neutrophils associated with aging.35, 36 Additionally, delayed apoptosis is a well‐known phenomenon in TLR‐activated neutrophils.37, 38 In this study, we found that PMNs from the aged were prone to apoptosis. Besides, unlike PMNs from young mice, delayed apoptosis was not obvious in aged counterparts. These results reflect reduced resistance to stress in aging subjects.
There is a complex relationship between autophagy and apoptosis. Usually autophagy is the instinct of cells to cope with external or internal stress, and apoptosis is the outcome of excessive stress, which cannot be resolved. Multiple mechanisms result in the inhibition of apoptosis by autophagy. Mitophagy, the selective degradation process to remove damaged mitochondria, stops the cell from running into intrinsic apoptosis.39 Autophagy also prevents cells from programmed death by selectively degrading the accumulated pro‐apoptotic proteins in the cytosol.40 On the other hand, there is also evidence that autophagy facilitates the activation of apoptosis. Autophagosomes can serve as a platform for caspase 8 activation.41 Autophagy also induces apoptosis by degrading its endogenous inhibitors. Conversely, apoptosis can also inhibit autophagy by digesting essential autophagy proteins.42, 43 Autophagy inhibitors were used to determine the relationship between autophagy and apoptosis in this study. Wortmannin is a phosphoinositide 3‐kinase inhibitor, which had been demonstrated to interfere with the formation of autophagosome. Bafilomycin A1 is a specific vacuolar‐type H (+)‐ATPase inhibitor, which was reported to prevent the maturation of autophagic vacuoles by inhibiting autophagosome–lysosome fusion.44 Our results showed that the inhibition of autophagy increased apoptosis in PMNs, suggesting an inhibitory effect of autophagy. However, a role of apoptosis on autophagy in turn cannot be completely ruled out. Wortmannin had a greater effect on inducing apoptosis than bafilomycin A1, indicating that autophagosome formation may be more important than the complete process of autophagy in mediating the shift from undergoing NETosis to apoptois. Moreover, wortmannin inhibits phosphoinositide 3‐kinase, which has an impact on a wide range of intracellular signalling pathways. This may also explain why wortmannin has a stronger effect than bafilomycin A1.
In the present study, we used an assay to quantify NETs with fluorescent dyes, which is currently one of the most commonly used methods. However, molecular probes, such as SYTOX Green, cannot distinguish the source of bounded DNA. Therefore, fluorescence intensity can also be detected when there are other causes of DNA release, such as other forms of cell death. Hence, all quantitative assays in this study were performed when NETs were believed to be the predominant origin of extruded DNA. Other protocols, like counting the percentage of NETotic cells, were used when wortmannin, bafilomycin A1, or rapamycin was added, to avoid any possible interference.
In conclusion, we demonstrated that TLR2 ligands were able to induce a ROS‐dependent NET formation. The ability to release NETs was impaired in aged mice. An Atg5‐related defect in autophagy contributed to the decreased NETosis and an increased compensatory apoptosis after TLR2 ligand stimulation. Our results suggest an important role of Atg5 and autophagy in maintaining PMN function in response to infection and in regulating neutrophil death. Targeting autophagy‐promoted NETs may present a therapeutic strategy to improve infection defence in the aged population.
Author contributions
FX, LC and CZ planned and performed experiments including cell isolation and treatment, confocal microscopy, Western blotting and flow cytometry; they also performed the animal experiments; YL, TRB, MAW, XS and JF planned the project and conceived the experiments; FX, EKF, XS and JF analysed the data and wrote the manuscript.
Disclosure
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the National Institutes of Health Grant R01‐HL‐079669 (JF and MAW), National Institutes of Health Grant R56‐HL‐123882 (JF), National Institutes of Health Grant R01‐HL‐076179‐09 (JF), VA Merit Award 1I01BX002729 (JF), National Natural Science Foundation of China 81470262 (JF), Key Program of Medical Science Development of PLA BWS12J027 (XS), National Natural Science Foundation of China 81372103 (XS) and National Natural Science Foundation of China 81671944 (XS).
Contributor Information
Xueyin Shi, Email: shixueyin1128@163.com.
Jie Fan, Email: jif7@pitt.edu.
References
- 1. Economic Do, Nations SAU . World population ageing: 1950–2050. UN; 2002. [Google Scholar]
- 2. Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O'Donnell M, Sullivan R, et al The burden of disease in older people and implications for health policy and practice. Lancet (London, England) 2015; 385(9967):549–562. [DOI] [PubMed] [Google Scholar]
- 3. Kline KA, Bowdish DME. Infection in an aging population. Curr Opin Microbiol 2016; 29:63–67. [DOI] [PubMed] [Google Scholar]
- 4. Fortin CF, McDonald PP, Lesur O, Fulop T Jr. Aging and neutrophils: there is still much to do. Rejuvenation Res 2008; 11(5):873–882. [DOI] [PubMed] [Google Scholar]
- 5. Simell B, Vuorela A, Ekstrom N, Palmu A, Reunanen A, Meri S, et al Aging reduces the functionality of anti‐pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine 2011; 29(10):1929–1934. [DOI] [PubMed] [Google Scholar]
- 6. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al Neutrophil extracellular traps kill bacteria. Science (New York, NY) 2004; 303(5663):1532–1535. [DOI] [PubMed] [Google Scholar]
- 7. Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ 2011; 18(4):581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007; 176(2):231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Remijsen Q, Vanden Berghe T, Wirawan E, Asselbergh B, Parthoens E, De Rycke R, et al Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res 2011; 21(2):290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, et al The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll‐like receptors. Proc Natl Acad Sci U S A 2000; 97(25):13766–13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, et al Infection‐induced NETosis is a dynamic process involving neutrophil multitasking in vivo . Nat Med 2012; 18(9):1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marcos V, Nussbaum C, Vitkov L, Hector A, Wiedenbauer EM, Roos D, et al Delayed but functional neutrophil extracellular trap formation in neonates. Blood 2009; 114(23):4908–4911; author reply 4911‐4902. [DOI] [PubMed] [Google Scholar]
- 13. Toscano MG, Ganea D, Gamero AM. Cecal ligation puncture procedure. J Vis Exp 2011; 7(51): pii: 2860. doi: 10.3791/2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vong L, Sherman PM, Glogauer M. Quantification and visualization of neutrophil extracellular traps (NETs) from murine bone marrow‐derived neutrophils. Methods Mol Biol 2013; 1031:41–50. doi: 10.1007/978‐1‐62703‐481‐4_5. [DOI] [PubMed] [Google Scholar]
- 15. Jung YO, Cho ML, Lee SY, Oh HJ, Park JS, Park MK, et al Synergism of toll‐like receptor 2 (TLR2), TLR4, and TLR6 ligation on the production of tumor necrosis factor (TNF)‐α in a spontaneous arthritis animal model of interleukin (IL)‐1 receptor antagonist‐deficient mice. Immunol Lett 2009; 123(2):138–143. [DOI] [PubMed] [Google Scholar]
- 16. Stoiber W, Obermayer A, Steinbacher P, Krautgartner WD. The role of reactive oxygen species (ROS) in the formation of extracellular traps (ETs) in humans. Biomolecules 2015; 5(2):702–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shaw AC, Goldstein DR, Montgomery RR. Age‐dependent dysregulation of innate immunity. Nat Rev Immunol 2013; 13(12):875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hazeldine J, Harris P, Chapple IL, Grant M, Greenwood H, Livesey A, et al Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell 2014; 13(4):690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 2009; 43:67–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vieira da Silva Pellegrina D, Severino P, Vieira Barbeiro H, Maziero Andreghetto F, Tadeu Velasco I, Possolo de Souza H, et al Septic shock in advanced age: transcriptome analysis reveals altered molecular signatures in neutrophil granulocytes. PLoS One 2015; 10(6):e0128341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iba T, Hashiguchi N, Nagaoka I, Tabe Y, Murai M. Neutrophil cell death in response to infection and its relation to coagulation. J Intensive Care 2013; 1(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luo HR, Loison F. Constitutive neutrophil apoptosis: mechanisms and regulation. Am J Hematol 2008; 83(4):288–295. [DOI] [PubMed] [Google Scholar]
- 23. Marino G, Niso‐Santano M, Baehrecke EH, Kroemer G. Self‐consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol 2014; 15(2):81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bennett HL, Fleming JT, O'Prey J, Ryan KM, Leung HY. Androgens modulate autophagy and cell death via regulation of the endoplasmic reticulum chaperone glucose‐regulated protein 78/BiP in prostate cancer cells. Cell Death Dis 2010; 1:e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deng L, Feng J, Broaddus RR. The novel estrogen‐induced gene EIG121 regulates autophagy and promotes cell survival under stress. Cell Death Dis 2010; 1:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oliveira‐Nascimento L, Massari P, Wetzler LM. The role of TLR2 in infection and immunity. Front Immunol 2012; 3:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yipp BG, Petri B, Salina D, Jenne CN, Scott BNV, Zbytnuik LD, et al Dynamic NETosis is carried out by live neutrophils in human and mouse bacterial abscesses and during severe gram‐positive infection. Nat Med 2012; 18(9):1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tseng CW, Kyme PA, Arruda A, Ramanujan VK, Tawackoli W, Liu GY. Innate immune dysfunctions in aged mice facilitate the systemic dissemination of methicillin‐resistant S. aureus . PLoS ONE 2012; 7(7):e41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Itakura A, McCarty OJ. Pivotal role for the mTOR pathway in the formation of neutrophil extracellular traps via regulation of autophagy. Am J Physiol Cell Physiol 2013; 305(3):C348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet 2008; 24(12):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubinsztein David C, Mariño G, Kroemer G. Autophagy and Aging. Cell 2011; 146(5):682–695. [DOI] [PubMed] [Google Scholar]
- 32. Lipinski MM, Zheng B, Lu T, Yan Z, Py BF, Ng A, et al Genome‐wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer's disease. Proc Natl Acad Sci USA 2010; 107(32):14164–14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carames B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging‐related loss is linked with cell death and osteoarthritis. Arthritis Rheum 2010; 62(3):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age‐related disease. Nature 2013; 493(7432):338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jr FT, Fouquet C, Allaire P, Perrin N, Lacombe G, Stankova J, et al Changes in apoptosis of human polymorphonuclear granulocytes with aging. Mech Ageing Dev 1997; 96(1–3):15. [DOI] [PubMed] [Google Scholar]
- 36. Gupta S, Agrawal A, Agrawal S, Su H, Gollapudi S. A paradox of immunodeficiency and inflammation in human aging: lessons learned from apoptosis. Immun Ageing 2006; 3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Francois S, El Benna J, Dang PM, Pedruzzi E, Gougerot‐Pocidalo MA, Elbim C. Inhibition of neutrophil apoptosis by TLR agonists in whole blood: involvement of the phosphoinositide 3‐kinase/Akt and NF‐κB signaling pathways, leading to increased levels of Mcl‐1, A1, and phosphorylated Bad. J Immunol 2005; 174(6):3633–3642. [DOI] [PubMed] [Google Scholar]
- 38. Power CP, Wang JH, Manning B, Kell MR, Aherne NJ, Wu QD, et al Bacterial lipoprotein delays apoptosis in human neutrophils through inhibition of caspase‐3 activity: regulatory roles for CD14 and TLR‐2. J Immunol 2004; 173(8):5229–5237. [DOI] [PubMed] [Google Scholar]
- 39. Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL‐2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 2014; 15(1):49–63. [DOI] [PubMed] [Google Scholar]
- 40. Amir M, Zhao E, Fontana L, Rosenberg H, Tanaka K, Gao G, et al Inhibition of hepatocyte autophagy increases tumor necrosis factor‐dependent liver injury by promoting caspase‐8 activation. Cell Death Differ 2013; 20(7):878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Young MM, Takahashi Y, Khan O, Park S, Hori T, Yun J, et al Autophagosomal membrane serves as platform for intracellular death‐inducing signaling complex (iDISC)‐mediated caspase‐8 activation and apoptosis. J Biol Chem 2012; 287(15):12455–12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oral O, Oz‐Arslan D, Itah Z, Naghavi A, Deveci R, Karacali S, et al Cleavage of Atg3 protein by caspase‐8 regulates autophagy during receptor‐activated cell death. Apoptosis 2012; 17(8):810–820. [DOI] [PubMed] [Google Scholar]
- 43. Luo S, Rubinsztein DC. Apoptosis blocks Beclin 1‐dependent autophagosome synthesis: an effect rescued by Bcl‐xL. Cell Death Differ 2010; 17(2):268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang YP, Hu LF, Zheng HF, Mao CJ, Hu WD, Xiong KP, et al Application and interpretation of current autophagy inhibitors and activators. Acta Pharmacol Sin 2013; 34(5):625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]


