Summary
Age affects the immune response to vaccination, with individuals at the extremes of age responding poorly. The initial inflammatory response to antigenic materials shapes the subsequent adaptive response and so understanding is required about the effect of age on the profile of acute inflammatory mediators. In this study we measured the local and systemic inflammatory response after influenza vaccination or infection in neonatal, young adult and aged mice. Mice were immunized intramuscularly with inactivated influenza vaccine with and without the adjuvant MF59 and then challenged with H1N1 influenza. Age was the major factor affecting the inflammatory profile after vaccination: neonatal mice had more interleukin‐1α (IL‐1α), C‐reactive protein (CRP) and granulocyte–macrophage colony‐stimulating factor (GMCSF), young adults more tumour necrosis factor‐α (TNF), and elderly mice more interleukin‐1 receptor antagonist (IL‐1RA), IL‐2RA and interferon‐γ‐induced protein 10 (IP10). Notably the addition of MF59 induced IL‐5, granulocyte colony‐stimulating factor (G‐CSF), Keratinocyte Chemotractant (KC) and monocyte chemoattractant protein 1 (MCP1) in all ages of animals and levels of these cytokines correlated with antibody responses. Age also had an impact on the efficacy of vaccination: neonatal and young adult mice were protected against challenge, but aged mice were not. There were striking differences in the localization of the cytokine response depending on the route of exposure: vaccination led to a high serum response whereas intranasal infection led to a low serum response but a high lung response. In conclusion, we demonstrate that age affects the inflammatory response to both influenza vaccination and infection. These age‐induced differences need to be considered when developing vaccination strategies for different age groups.
Keywords: cytokine, lung, mucosa, vaccination, viral
Introduction
Influenza causes the most severe disease at the extremes of age; infants (under 2 years) and elderly patients (≥ 65 years) have higher influenza attack rates, more frequent influenza‐related hospitalizations and greater rates of influenza‐related mortality.1 Globally, influenza infection results in approximately 374 000 hospitalizations in 1‐year‐old children.2 There is no global estimate for influenza infection in the elderly, but estimates from the USA put the rate of influenza hospitalization in elderly patients (≥ 65 years) as nearly twice that of infants.3 Part of the problem is that vaccination is also less effective in these same age groups4 and therefore better vaccine strategies are required to protect these at risk groups.
Simplistically, a better influenza vaccine would induce qualitatively and quantitatively stronger cellular and antibody responses – ideally with broad cross‐virus protection.5 Although it is widely agreed that responses to influenza vaccination at the extremes of age need to be improved, it is not immediately clear how to achieve this.6 An important step in the induction of an immune response is the production of cytokines and chemokines, which leads to the recruitment and activation of other cells. One assumption in vaccine development is that the young‐adult response is the best response and vaccines should try and replicate this in other age groups. The aim of this study was to compare influenza vaccination in neonatal, young adult and aged mice, to determine how age and the use of an adjvuant affect the inflammatory response and vaccine efficacy. However, vaccines need to be safe because they are a public‐health intervention administered to large numbers of people. Simply increasing inflammation to increase the immune response could lead to vaccines with acute reactogenicity.7 There is likely to be a sweet spot between enough inflammation to induce adaptive immune responses and too much inflammation, resulting in reactogenicity. But this ideal level is not well characterized. A secondary aim of this study was to define the upper limits of inflammation to a vaccine, which is known to be safe, to calibrate future vaccines in development. As part of this calibration, influenza infection was used to represent an unsafe immune outcome.
Different ages of mice were immunized with influenza vaccine, with and without the adjuvant MF59 and serum cytokines were assessed by Luminex at various time‐points after immunization. Distinct patterns of response were seen in the different age groups, associated with reduced vaccine efficacy in aged mice.
Materials and methods
Mouse immunization and infection
CB6F1 mice were obtained from Harlan UK Ltd (Plumstead, UK) or from an internal breeding colony and kept in specific‐pathogen‐free conditions in accordance with the United Kingdom's Home Office guidelines and all work was approved by the Animal Welfare and Ethical Review Board at Imperial College London. Studies followed the ARRIVE guidelines. Neonatal (7–8 days), young adult (6–8 weeks) and aged (≥ 16 months) mice were immunized intramuscularly with 4·5 μg purified surface antigens, 1·5 μg from each influenza strain H1N1 A/California/7/2009, A/Victoria/361/2011 and B/Hubei/Wujiagang/158/2009 (GSK Vaccines, Siena, Italy) in 40 μl (neonates) or 100 μl (young adults and aged). Where used, vaccine was mixed 1 : 1 [volume (vol)/vol] with research grade MF59® emulsion (5% squalene, 0·5% Tween 80, 0·5% Span 85, in citrate buffer (vol/vol)). For infections, mice were anaesthetized using isoflurane and infected intranasally with 2 × 102 plaque‐forming units of influenza virus in 20 μl (neonates) or 5 × 105 plaque‐forming units of influenza virus in 100 μl (young adult and aged mice). Group sizes were determined using a power calculation based on data from previous studies.8
Tissue and cell recovery and isolation
Blood was collected from the tail veins of live mice and sera were isolated after clotting by centrifugation for Luminex analysis; because high‐volume, multiple, sequential bleeds were required, different groups of mice were used for different time‐points. Mice were culled using 100 μl intraperitoneal pentobarbitone (20 mg dose; Pentoject, Animalcare Ltd., York, UK) and tissues collected as previously described.9 Blood was collected from femoral veins and sera were isolated after clotting by centrifugation. Lungs were removed and homogenized by passage through 100‐μm cell strainers, then centrifuged at 200 g for 5 min. Supernatants were removed and stored for Luminex analysis and the cell pellet was treated with red blood cell lysis buffer (ACK; 0·15 m ammonium chloride, 1 m potassium hydrogen carbonate, and 0·01 mm EDTA, pH 7·2) before centrifugation at 200 g for 5 min. The remaining cells were resuspended in RPMI‐1640 medium with 10% fetal calf serum, and viable cell numbers were determined by trypan blue exclusion.
Viral RNA quantification
Virus load in vivo was assessed using Trizol extracted RNA from frozen lung tissue disrupted in a TissueLyzer (Qiagen, Manchester, UK) as described previously.10 RNA was converted into cDNA and quantitative RT‐PCR was carried out using bulk viral RNA, for the influenza M gene and mRNA using 0·1 μm forward primer (5′‐AAGACAAGACCAATYCTGTCACCTCT‐3′), 0·1 μm reverse primer (5′‐TCTACGYTGCAGTCCYCGCT‐3′) and 0·2 μm probe (5′‐FAM‐TYACGCTCACCGTGCCCAGTG‐TAMRA‐3′) on a Stratagene Mx3005p (Agilent Technologies, Santa Clara, CA). M‐specific RNA copy number was determined using an influenza M gene standard plasmid.
Semi‐quantitative antigen‐specific ELISA
Antibodies (IgG) specific to influenza H1N1 in the sera were measured using a standardized ELISA.11 Briefly, MaxiSorp 96‐well plates (Nunc, Roskilde, Denmark) were coated with 1 μg/ml surface proteins or a combination of anti‐murine λ and κ light‐chain‐specific antibodies (AbDSerotec, Oxford, UK) and incubated overnight at 4°. Plates were blocked with 1% BSA in PBS. Bound IgG was detected using horseradish peroxidase‐conjugated goat anti‐mouse IgG (AbD Serotec). A dilution series of recombinant murine IgG was used as a standard to quantify specific antibodies. Tetramethylbenzidine with H2SO4 as stop solution was used to detect the response and optical densities read at 450 nm.
Serum cytokine biomarker quantification
Bio‐Plex Pro™ Mouse Cytokine 25‐plex kits (Bio‐Rad, Watford, UK) were used according to the manufacturer's recommendations with modifications as described below. Briefly, pre‐mixed capture antibody conjugated magnetic beads were placed into each well of two flat‐bottomed 96‐well plates and 50 μl of standard or one in four diluted serum sample was added. Plates were incubated for 30 min, with shaking, then beads were washed. Magnetic beads were incubated with a cocktail of detection antibodies, incubated for 30 min then washed. Streptavidin‐phycoerythrin was added to the beads, which were then incubated for 10 min, washed and read on a Bio‐Plex® 100 Luminex machine (Bio‐Rad).
A custom‐made 5‐plex mouse cytokine panel was also used as described previously.12 Briefly, pre‐mixed capture antibody conjugated magnetic beads were placed into each well of two flat‐bottomed 96‐well plates and 25 μl of standard or one in five diluted serum sample was added. The plates were incubated for 2 hr. Beads were washed twice then incubated with 50 μl biotinylated detection antibody cocktail for 2 hr. After two washes, beads were incubated with 50 μl streptavidin‐phycoerythrin (5 μg/ml) for 30 min, washed and read on a Bio‐Plex® 100 Luminex machine. All Luminex data are available in the Supplementary material (Table S1).
Principal component analysis
Area under the curve values for each cytokine within each age/vaccine group were calculated across the time course using prism 7 (GraphPad Software Inc., La Jolla, CA). Principal component analysis (PCA) using standardized area under the curve values (variables were scaled to have unit variance) was performed using the ‘stats’ package in R V 3.3.1.13 PCA was visualized using the R packages ‘ggfortify’14 and ‘scatterplot3d’.15
Statistical analysis
Calculations as described in the figure legends were performed using prism 7 (GraphPad Software Inc.). Heat maps were generated using Matrix2png (http://www.chibi.ubc.ca/matrix2png/).16 Correlations were calculated using Pearson coefficients in prism 7. Multivariate analysis of variance was performed in R V 3.3.1.
Results
Immunization induces an inflammatory response that is dependent on age and adjuvant
We were interested in the systemic inflammatory response to adjuvanted and non‐adjuvanted vaccines, to define the profile of a safe response and whether this changes with age. Neonatal, young adult and aged mice were immunized with a single dose of the 2012/13 season inactivated influenza vaccine with or without MF59 as an adjuvant, 4·5 μg influenza antigens – 1·5 μg each of H1N1, influenza B, H3N2 (equivalent to 1/10th the human dose). Blood was taken at multiple time‐points after immunization and serum cytokine levels were measured. The age of the mice affected the response to immunization (Fig. 1, Table 1): neonatal mice had significantly elevated levels of interleukin‐1α (IL‐1α), C‐reactive protein (CRP) and granulocyte–macrophage colony‐stimulating factor (GM‐CSF), young adult mice had significantly elevated levels of tumour necrosis factor‐α (TNF‐α), and elderly mice had significantly elevated levels of IL‐10, RANTES, interleukin‐1 receptor A (IL‐1RA), IL‐2RA and interferon‐γ‐induced protein 10 (IP10); notably IL‐1RA, IL‐2RA and IP10 were all elevated before immunization in elderly mice.
Figure 1.
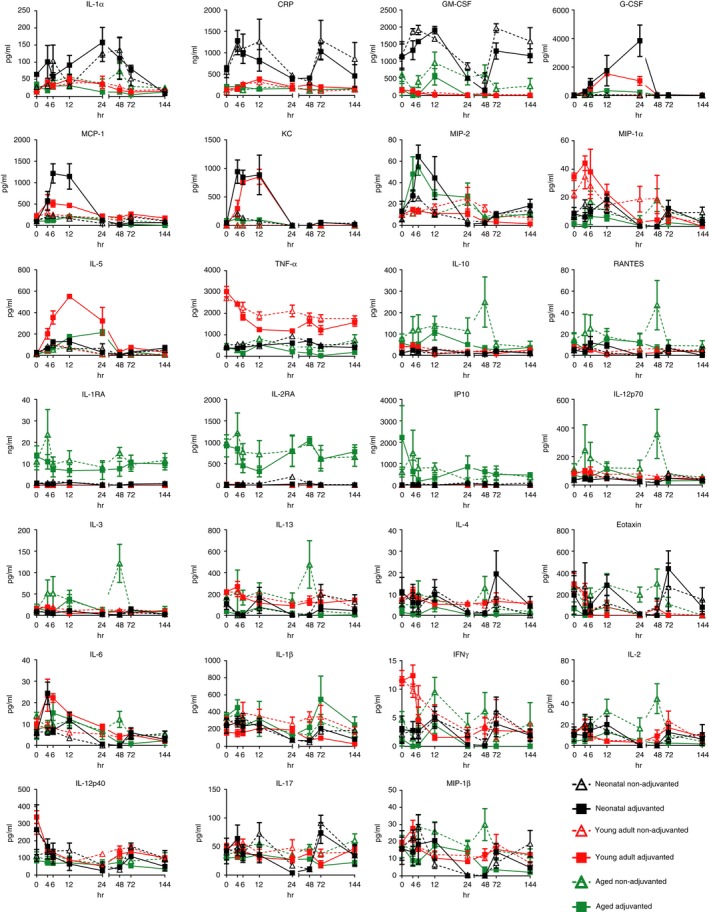
Age affects the cytokine response in the blood to vaccination. Neonatal (7 days old: black symbols), young adult (6–8 weeks old: red symbols) and elderly (16 months: green symbols) mice were immunized intramuscularly with 4·5 μg influenza antigen (1·5 µg each of H1N1, H3N2 and influenza B) with (closed symbols) or without (open symbols) MF59 adjuvant. Serum cytokine levels were measured over 6 days. Data points represent n = 5 animals ± SEM.
Table 1.
Inflammatory response to primary influenza vaccination
| Analyte | Neonatal Non‐Adj | Neonatal Adj | Young Adult Non‐Adj | Young Adult Adj | Aged Non‐Adj | Aged Adj | Significance |
|---|---|---|---|---|---|---|---|
| IL‐1α | 9615 | 11 192 | 3554 | 2955 | 5586 | 1752 | NN vs YA or Aged (**) |
| IL‐1β | 22 512 | 20 404 | 29 742 | 48 642 | 42 260 | 14 140 | NS |
| IL‐2 | 987·2 | 1080 | 1601 | 1499 | 2392 | 433·3 | NS |
| IL‐3 | 669·1 | 959·3 | 1746 | 1156 | 4672 | 1571 | Aged Non Adj vs NN or YA (***) |
| IL‐4 | 456·7 | 1288 | 927·7 | 873·9 | 681·2 | 121 | NS |
| IL‐5 | 5542 | 6558 | 2447 | 18 180 | 2412 | 7589 | YA Adj vs NN or Aged (***) |
| IL‐6 | 502·3 | 613·8 | 707·7 | 870·3 | 983·1 | 462·2 | NS |
| IL‐10 | 2036 | 2096 | 3095 | 2396 | 14 194 | 5670 | Aged Non Adj vs NN or YA (***) |
| IL‐12p40 | 15 924 | 10 655 | 18 896 | 16 507 | 13 062 | 8048 | NS |
| IL‐12p70 | 7091 | 5118 | 9123 | 6853 | 19 057 | 5760 | Aged Non Adj vs NN or YA (***) |
| IL‐13 | 15 298 | 6414 | 21 181 | 18 292 | 20 082 | 1471 | NS |
| IL‐17 | 7196 | 5839 | 5911 | 4711 | 6694 | 3349 | NS |
| Eotaxin | 22 585 | 27 272 | 3221 | 2820 | 19 864 | 1193 | NS |
| G‐CSF | 2508 | 93 076 | 5076 | 38 641 | 5457 | 9950 | NN Adj vs YA or Aged (**) |
| GM‐CSF | 208 321 | 146 713 | 6283 | 2953 | 55 905 | 5394 | NN vs YA or Aged (**) |
| IFN‐γ | 484·1 | 336·7 | 611·4 | 419·6 | 541·4 | 36·43 | NS |
| KC | 5510 | 17 515 | 0 | 11 626 | 0 | 1676 | NN Adj vs YA/Aged Non (**), YA Adj vs NN/Aged Non (***) |
| MCP‐1 | 18 200 | 25 631 | 25 098 | 35 904 | 18 694 | 6774 | NS |
| MIP‐1αa | 1053 | 955·8 | 1599 | 1086 | 1349 | 241·3 | NS |
| MIP‐1β | 1334 | 1187 | 1922 | 1929 | 2590 | 832·6 | NS |
| RANTES | 456·4 | 471·6 | 769·5 | 456·3 | 2444 | 904·8 | Aged Non Adj vs NN or YA (***) |
| TNF‐α | 88 561 | 73 307 | 270 170 | 208 346 | 74 330 | 22 630 | YA vs NN or Aged (***) |
| MIP‐2 | 1492 | 2000 | 1622 | 2042 | 1806 | 716·5 | NS |
| IL‐2RA | 5842 | 1355 | 0 | 88 | 107 018 | 105 290 | Aged vs NN or YA (***) |
| CRP (ng/ml) | 129 467 | 99 374 | 20 858 | 30 704 | 21 838 | 21 639 | NN vs YA or Aged (**) |
| IL‐1RA (ng/ml) | 77·17 | 66·15 | 1·01 | 1·643 | 1624 | 1307 | Aged vs NN or YA (***) |
| IP10 (ng/ml) | 8114 | 2249 | 58·81 | 130·4 | 67 140 | 80 811 | Aged vs NN or YA (***) |
Data presented as area under the curve for cytokine data plotted over 144 hr after immunization with MF59 adjuvant (Adj) or without (Non‐Adj). **P<0·01, ***P<0·001 Significance calculated by multiple t‐test.
The addition of the adjuvant MF59 induced a specific signature that was present to some degree in all ages of mice (Fig. 2a) with increases in IL‐5 (significantly greater than non‐adjuvanted animals in young adults), granulocyte colony‐stimulating factor (G‐CSF) (significantly greater in all ages of mice), Keratinocyte chemottractant (KC) (significantly greater in young adults and neonates), monocyte chemoattractant protein 1 (MCP‐1) and macrophage inflammatory protein 2 (MIP‐2) (both significant in neonatal and young adult) when compared with serum levels of age‐matched mice without adjuvant. Although the ‘MF59 signature’ was present in all age groups, further analysis of the data revealed differences in magnitude of the signature across the ages. Neonatal mice showed the greatest increase in G‐CSF and MCP‐1, while showing similar levels of KC to the young adults. Young adults showed the greatest increase in IL‐5. Aged mice had considerably lower levels of all the cytokines, while maintaining the ‘MF59 signature’, with a notable difference in that the increase in MCP‐1 seen in the neonates and young adults was absent.
Figure 2.
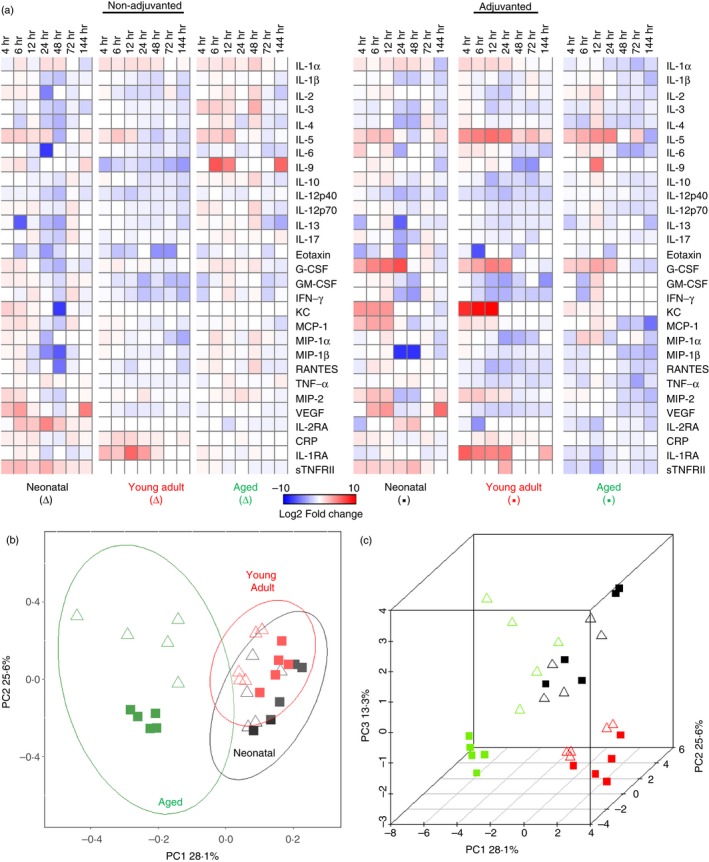
Immunization with MF59 normalizes the response across animals of different ages. Mice were immunized intramuscularly with influenza antigens with or without MF59 adjuvant. Serum cytokine levels were measured over 6 days and heat maps were generated. Heat maps show mean log2‐fold change from the 0 hr measurement of each group of n = 5 mice (a). Two‐dimensional (b) and three‐dimensional (c) principal component analysis was performed to visualize the overall inflammatory response over time using standardized area under the curve values of inflammatory responses plotted in Fig. 1.
To determine whether age or adjuvant was the major contributing factor to variability between groups we used PCA. Reducing the data to two components (Fig. 2b), young adult or neonatal mice grouped closely and the aged mice were significantly different. The addition of MF59 adjuvant led to a greater separation of responses in the elderly mice than in other ages. Applying a third dimension to the analysis separates the responses of young adult from the neonatal mice. Age was the only variable that significantly correlated (P ≥ 0·05) with variance, contributing to 81·9% of the total variance in the first dimension whereas adjuvant only contributed to 0·07%. Further statistical analysis using multivariate analysis of variance revealed that the overall inflammatory response significantly differed between the age groups (P ≥ 0·001). From this we conclude that age has a greater impact on the initial response than adjuvant.
To determine whether the differences seen only occurred during primary exposure to antigen, we compared the effect of age on the inflammatory response to booster immunization (see Supplementary material, Fig. S1). The mice received a second influenza vaccine dose 4 weeks after the first dose; the neonatal mice were now 5 weeks of age. A significant increase in IL‐5, G‐CSF, MIP‐2 and KC was again seen in mice immunized with the MF59‐containing formulation (Fig. 3a), there was less difference in MCP‐1. Significant differences in magnitude across the ages were still observed (P ≤ 0·05, Fig. 3b), but were smaller, age contributed 36·6% of the total variance compared with 81·9% in primary immunization. Aged mice showed the lowest concentrations of IL‐5, G‐CSF and KC, and young adult mice showing either greater (IL‐5, KC) or equivalent (G‐CSF) concentrations compared with the neonates (Fig. 3c). There was a late peak in CCL5, IL‐6, IL‐1β, IL‐17 and MIP‐1β in the adult group receiving adjuvanted vaccine (see Supplementary material, Fig. S1). Therefore age is still a contributory factor to responses to the second exposure to antigen.
Figure 3.
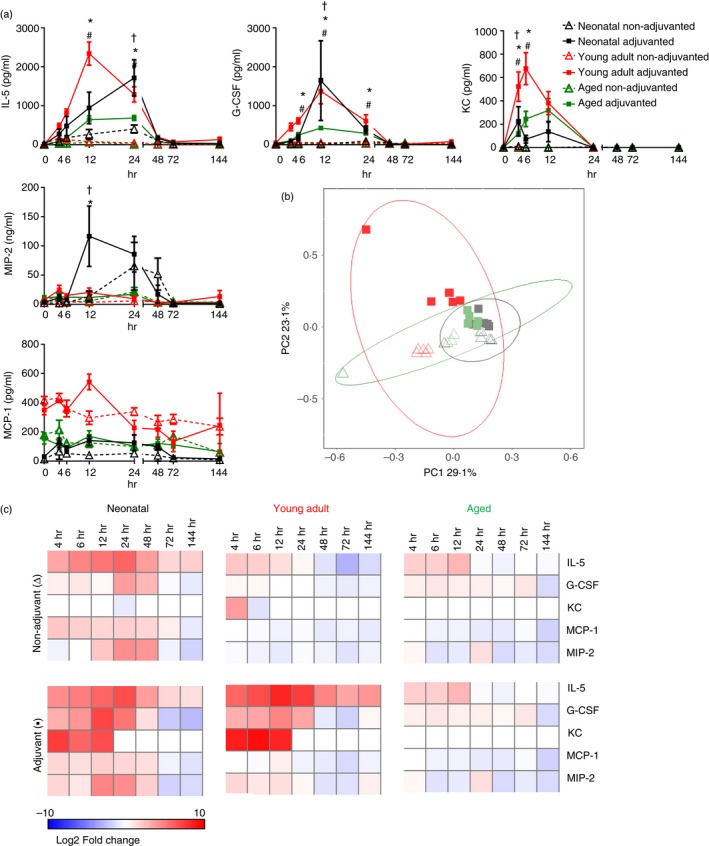
Cytokine response to booster immunization. Mice received two intramuscular immunizations of influenza antigens with (▪) or without (∆) MF59, 4 weeks apart. Serum cytokine levels were measured over 6 days (a). Two‐dimensional principal component analysis was performed to visualize the overall inflammatory response over time using standardized area under the curve values of inflammatory responses (b). Heat maps of log2‐fold change from the 0 hr measurement of each group (c). Points represent mean and SEM of n ≥ 4 mice (a). Symbols represent P < 0·05 between †neonatal, * young adult or # aged animals receiving adjuvant or vaccine alone.
Impact of age on protective efficacy of influenza vaccination
There were clear differences in the inflammatory responses to vaccination in the different aged animals and we wished to see if that translated into differences in antibody response. Levels of serum anti‐H1N1 IgG were measured throughout the vaccination regimen and after challenge. Anti‐H1N1 IgG was detected in the serum of neonates receiving MF59 adjuvanted vaccine from day 6 after immunization and in all neonates by day 28, just before the booster dose (Fig. 4a). There was a slight increase in the group receiving MF59. In the young adults, anti‐H1N1 IgG could be detected in the serum from 6 days after the first vaccination. After the second dose of vaccine, anti‐H1N1 IgG levels continued to rise and levels were significantly greater in MF59‐adjuvanted young adult animals. In the aged mice, anti‐H1N1 IgG could be detected in the MF59‐adjuvanted group 6 days after the first vaccination, but the levels notably increased after the second dose of vaccine. Modest anti‐H1N1 IgG levels were detected in the antigen alone group on day 6 but were much lower throughout the time–course of the experiment (Fig. 4c). Comparing the age groups, the young adults receiving adjuvanted vaccine had the greatest antibody response compared with the other aged animals (Fig. 4d). The incorporation of adjuvant also increased the anti‐H3N2 (Fig. 4e) and anti‐influenza B responses (Fig. 4f).
Figure 4.
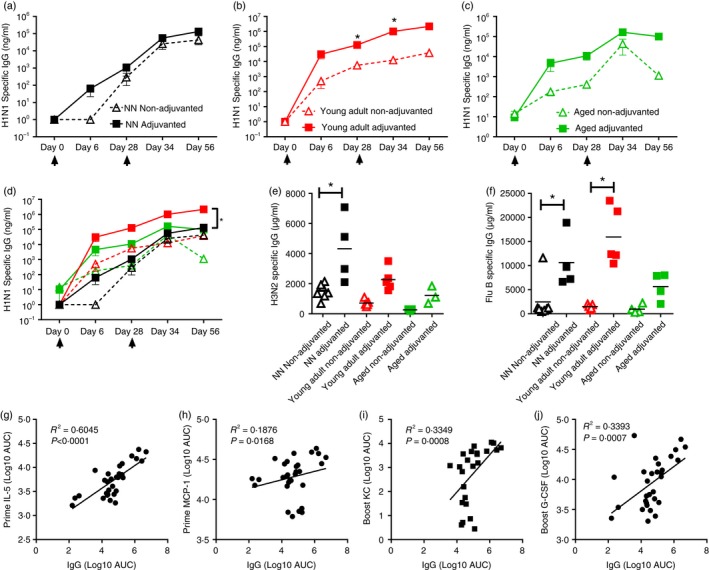
Vaccine‐induced anti‐influenza serum IgG production is dependent on age. Neonatal (a: black symbols), young adult (b: red symbols) and aged (c: green symbols) mice received two intramuscular immunizations of influenza antigens with (closed symbols) or without (open symbols) MF59, 4 weeks apart (day 0 and day 28: indicated by arrows). H1N1‐specific antibody responses measured by ELISA over the time–course of immunization (a–d), H3N2 (e) and Flu B (f) were measured after the second immunization. Correlation between the peak anti‐H1N1 antibody response and IL5 (g), MCP‐1 (h), KC (i) or G‐CSF (j). Lines and points represent mean and SEM of n ≥ 3 mice, * P < 0·05 measured by t‐test (a–c) or one‐way analysis of variance with post test (d–f); correlation by Pearson coefficient.
Since the inclusion of adjuvant increased the antibody response, we wished to determine whether the profile of inflammatory response associated with MF59 correlated with increased antibody. The total IL‐5 response after either primary immunization (Fig. 4g) or secondary immunization (R 2 = 0. 0·2459, P = 0·0053, data not shown) correlated with the peak H1N1 IgG response. MCP‐1 responses to primary immunization (Fig. 4h) but not secondary immunization correlated with antibody response. There was no correlation with MIP‐2 and antibody responses. KC (Fig. 4i) and G‐CSF (Fig. 4j) correlated with antibody responses after secondary immunization. This suggests that the cytokines induced by MF59, IL‐5, KC, G‐CSF and MCP‐1, are important for better antibody responses
On day 56 after the initial vaccine dose, immunized mice were challenged with 5 × 105 plaque‐forming units of H1N1 influenza A/Eng/195/2009 virus and monitored over 6 days, after which they were killed. Responses were compared with age‐matched naive animals; because the mice first immunized as neonates were adults at the time of challenge, responses in this group were compared with naive young adult mice. Unimmunized mice started losing weight on day 1 after infection peaking at 20% weight loss on day 6 after infection (Fig. 5a). In mice immunized with adjuvanted vaccine as neonates or as young adults no significant weight loss was observed. Mice first immunized as neonates without adjuvant lost more weight than MF59 adjuvanted mice at days 3 and 4 (P < 0·001) but these differences resolved by day 5. There was no difference in weight loss between adjuvanted and non‐adjuvanted young adult mice, both groups displayed no signs of disease (Fig. 5b). However, immunization of aged mice did not achieve the same level of protection as seen in neonatal and young adult mice. The vaccine was only partially protective, animals immunized without adjuvant lost 10% body weight by day 6 after infection and did not recover, necessitating humane killing on day 14 after infection. Aged mice immunized with MF59 lost significantly less weight than mice that received antigen alone or naive mice, but still lost weight after infection (Fig. 5c).
Figure 5.
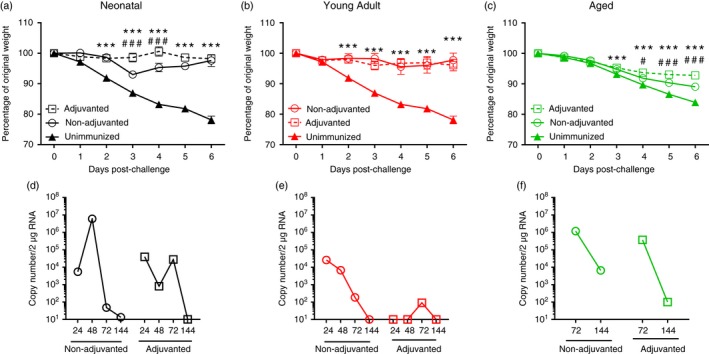
Homologous prime‐boost regimen protects against influenza in neonatal and young adult mice but not aged mice. Mice received two intramuscular immunizations of 1·5 μg HA antigen with or without MF59, 4 weeks apart as neonates (a, d), young adults (b, e) or aged adults (c, f). Four weeks after last vaccination, animals were infected intranasally with 5 × 105 plaque‐forming units A/England/195/2009 H1N1 influenza. Weight change (a–c) and lung viral load (d–f) were measured at multiple time‐points after infection. Lines and points represent mean of n ≥ 4 mice. ***P < 0·001, of immunized mice compared to unimmunized, # P < 0·05, ### P < 0·001 comparing adjuvanted to non‐adjuvanted, measured by two‐way analysis of variance.
Lung viral load was examined by quantitative PCR analysis of a single lung lobe. Mice receiving their priming immunization as neonates cleared virus 144 hr after infection (Fig. 5d), there was no significant difference between the neonatally immunized mice receiving adjuvant or non‐adjuvanted vaccine in the virus load recovered. Mice immunized as young adult mice cleared virus more quickly than mice immunized as neonates (Fig. 5e), and young adult mice immunized with MF59‐adjuvanted vaccine had almost no detectable virus load. However, mice immunized as aged adults, with or without adjuvant, had not cleared the virus by 144 hr after infection (Fig. 5f). Therefore vaccination of aged mice was less protective than for other ages, reflecting the differences seen in the inflammatory response.
Local polarization of cytokine responses to the lung in influenza infection
One of the primary goals of the study was to define a systemic signature of safety after vaccination; the vaccines used in the study are known to be safe and therefore we wanted an ‘unsafe’ event with which to compare them. Influenza infection in mice causes severe disease resulting in pronounced lung inflammation, weight loss and death, so this seemed a good upper comparison to influenza vaccination. Naive mice were infected intranasally with influenza and cytokine levels in the serum and lungs were measured by Luminex (Fig. 6). One of the striking features was the absence of detectable cytokines in the sera after lung infection; the only cytokine elevated above baseline was TNF. However there were significant, age‐specific changes in many of the cytokines detected in the lung. Influenza‐infected neonatal mice had levels of IL‐12p40, MCP‐1, MIP‐1α, MIP‐1β, TNF, eotaxin and vascular endothelial growth factor that rose above base line after infection; nearly all the cytokines measured were elevated in the lungs of young adult mice except IL‐5 and IFN‐γ and the aged mice had increases in IL‐5, IFN‐γ, KC, MCP‐1, MIP‐1α, MIP‐1β, MIP‐2, IL‐1α and G‐CSF at later time‐points (72 and 144 hr). The kinetics of cytokine release was also different among the differently aged animals, with young adults mostly peaking around 48 hr, whereas the aged animals peaked at 144 hr after infection. To see whether differences in disease profile were driven by changes in virus infectivity the viral kinetics was compared in naive neonatal (7‐day‐old), young adult and aged mice. In neonatal and young adult mice virus load was in decline by 144 hr after infection, but not in the aged mice, where it appeared to be increasing.
Figure 6.
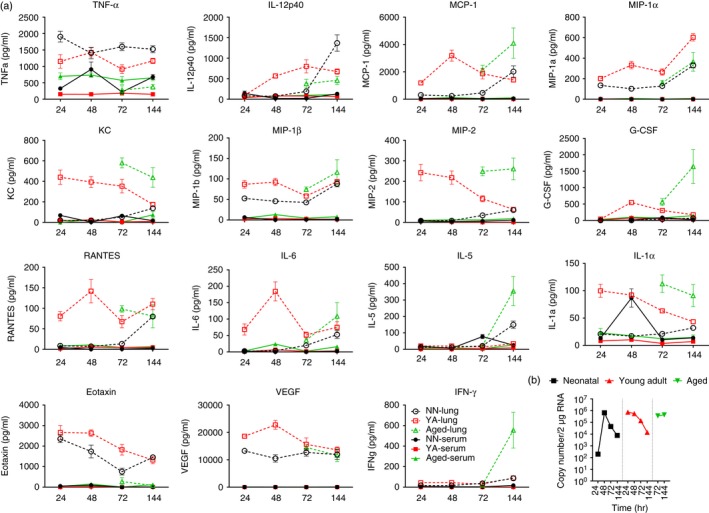
Cytokine expression is polarized into the lungs of influenza‐challenged mice. Mice were infected intranasally with 5 × 105 PFU A/England/195/2009 H1N1 influenza. Serum or lung supernatant cytokine levels were measured over 6 days after influenza challenge (a). Viral load (b). Lines and points represent mean and SEM of n ≥ 4 mice.
We were also interested to see if immunization changed the cytokine profile after infection. We focused on young adult mice and compared the cytokine profile in lungs and sera of immunized and challenged mice to that of unimmunized and challenged mice. Responses were compared with responses in the lungs and sera of naive age‐matched animals (using the same data set presented in Fig. 6). Strikingly, there were significantly elevated levels of IL‐5 in both the lungs and sera of mice immunized with MF59‐adjuvanted vaccine compared with naive animals and mice immunized without adjuvant (Fig. 7). The other cytokines (G‐CSF, KC, MCP‐1, MIP‐2) seen in the sera of MF59‐immunized mice during immunization were not elevated in sera or lungs after infection in immunized animals; though they were significantly greater in the lungs of naive mice compared with immunized mice (P < 0·05).
Figure 7.
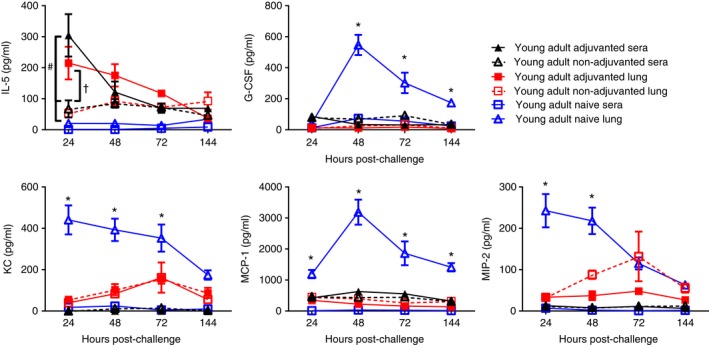
Effect of immunization on cytokine response at challenge. Mice received two intramuscular immunizations of 1·5 μg HA antigen with or without MF59, 4 weeks apart. Four weeks after last vaccination, animals were infected intranasally with 5 × 105 PFU A/England/195/2009 H1N1 influenza. Serum cytokine levels were measured over 6 days after influenza challenge. Lines and points represent mean and SEM of n ≥ 4 mice. * P < 0·05 comparing naive lungs or immunized lungs, # comparing adjuvanted sera with non‐adjuvanted or naive sera, †comparing adjuvanted lung with non‐adjuvanted lung.
Discussion
The aim of this study was to investigate how age affects inflammatory responses to vaccination. There were clear, age‐specific changes, with neonatal and young adult mice responding more strongly to vaccination than aged mice, though there were some cytokines that were constitutively high in aged animals. Age, particularly old age, had an impact on the degree of vaccine‐induced protection against influenza infection. The age of the animal also affected the cytokine response in the lungs during infection with a striking difference between immunization and infection: lung infection led to high local levels of cytokines but very little systemic cytokine response.
This is the first study to perform a complete profile of the inflammatory response to vaccination in three different ages of animals. The cytokine response in the sera was defined by the age of the animals. Infancy has been described as a period of relative immunodeficiency,17 but it is probably more accurate to describe it as period of differential immunity, which has an important impact on vaccine responses.18 Our data support the idea that the neonatal response is different not deficient, with higher IL‐1α, CRP, GM‐CSF, G‐CSF and MCP‐1 than other ages of mice. We19 and others20, 21 have previously investigated adjuvants specifically for use in early life and have shown that MF59 is protective even after a single dose.22
At the other end of life, aged mice had more IL‐10, RANTES, IL‐1RA, IL‐2RA and IP‐10, though many of these seemed to be elevated before immunization. The phenotype of sustained elevation of cytokines has been observed in elderly humans and has been termed ‘inflamm‐aging’.23 Elevated systemic cytokines in the elderly may contribute to elevated susceptibility to bacterial infection by dampening monocyte function,24 but their effect on vaccine responses has not been explored. One possibility is that a suboptimal cytokine response is induced in elderly animals leading to poor immune responses and lack of protection. A previous study compared the response of stimulated splenocyte supernatants in aged and young mice to influenza vaccine, with and without AS03 adjuvant and the responses were greater in the young adult mice,25 likewise vaccination in elderly patients induced lower cytokine expression in peripheral blood mononuclear cells26, 27 and in another study MF59 increased responses, and cytokine production in the serum of aged mice was lower than young adults.27
As seen previously, there were differences in the inflammatory response to infection in the aged mice.28 There was also a striking difference in the virus load between the different ages of mice, in neonatal mice, virus load reached adult levels after 48 hr despite infection with a lower dose and elderly mice had higher and sustained levels of virus. It may be that the increased virus load overwhelmed the immune response in aged mice. Notably, the cytokine signature in the lungs of aged animals spiked at 144 hr after infection for several cytokines, suggesting a delayed influx of cells.
Age also affected the degree of immune protection provided by vaccination; in particular the aged animals were not completely protected, even when adjuvanted vaccine was used. Another study using CpG as an experimental adjuvant has also shown reduced effect of the adjuvant in aged mice.29 Based on our previous studies, we know that antibody is a critical correlate of protection in mouse models of influenza.19 But we have recently confirmed the role of CD8 T cells in the resolution of influenza infection,10 and there are known age‐dependent effects on T cells,31 so targeting T cells may be more effective in the elderly; for example a peptide vaccine coupled to flagellin that induced T cells was protective30 in aged mice.
The efficacy data in this study contrast with efficacy data in the aged human population, where vaccination is effective and MF59 can boost immunogenicity.32, 33, 34, 35 One limitation in comparing our animal studies to the situation in elderly patients is that the mice received their first exposure to influenza antigens as aged animals, whereas elderly patients will have had repeat exposures to influenza viruses and vaccines. A previous study has demonstrated that with regards to the antibody response, age decreases antibody response, but the history of vaccine exposure is a greater factor in the response than age, with pre‐vaccination titre being the best predictor of vaccine response.36 Going forward, there is a complex balance of antigen experience, immunosenescence and inflamm‐aging that all need to be considered in the development and implementation of influenza vaccine strategies for the elderly.37
The adjuvant MF59 is effective in infancy22, 38 and also the elderly.39 The addition of MF59 caused the immune responses of animals at extremes of ages to resemble those of young adults (presumed to be the optimum responders), inducing a very specific cytokine signature of IL‐5, G‐CSF, KC and MCP‐1 in all ages of animals. It was interesting that the IL‐5 response was still detectable after infection, suggesting the induction of IL‐5‐producing T cells. The MF59‐dependent signal identified is consistent with previously published data, which show increases in IL‐5, KC and G‐CSF.40 The cytokines induced by MF59 are important in its mechanism of action and in the current study, cytokine levels correlated with antibody. In previous studies these cytokines led to a rapid recruitment of neutrophils and monocytes that transport antigen to lymph nodes41 and promote the retention of antigen in lymph nodes once there.42 We have previously observed an increase in T follicular helper cells in neonates following vaccination with MF59.22 Why MF59 induces this specific pattern of cytokines is not entirely understood, separating the adjuvant into individual components dramatically reduced the cytokine induction profile and the efficacy43 and previous studies have shown clear differences between different adjuvant formulations in the cytokine responses that they induce.44
One of the striking features of the study was the difference in cytokine response between infection and immunization. The expectation was that influenza infection, because it causes severe disease in mice, would induce systemic cytokine responses. However, there were very low levels of detectable cytokines in the sera of infected animals. Instead there were high levels in the lungs. This may reflect the direction of cellular influx into the lungs with chemokines acting to recruit cells and cytokines activating the cells once they reach the tissue. The compartmentalization of the response is remarkable, and it suggests that disease after influenza infection is not caused by systemic inflammation, but local inflammation affecting lung function. This may have an important implication for cell‐induced protection after vaccination, if we are trying to induce local cell responses, vaccines delivered to the lungs may be more appropriate.
This study was part of the BioVacSafe (Biomarkers for enhanced vaccines immunoSafety)45 consortium. This project aims to identify biomarkers of vaccine safety that will speed up, improve and reduce the cost of vaccine development and the testing and monitoring of vaccine safety before and after market release. A limitation of our study in the context of developing biomarkers to unsafe vaccines, was that we used safe vaccines and therefore the systemic responses to these vaccines may not be indicative of an unsafe vaccine. The comparison to infection was performed to define an upper limit of inflammation, but as we observed, the inflammatory response to lung infection was polarized into the lungs. Therefore ongoing studies will deliver more inflammatory agents systemically to obtain further insight into biomarkers of safety. One observation of interest is that there is a systemic inflammatory response even to ‘safe’ vaccination, which can vary with age and further research is therefore required to define the threshold of this systemic response between safe and unsafe vaccines.
The aim of the current study was to investigate how the interplay of age and adjuvant on the inflammatory response to vaccination, to improve vaccines at the extremes of life. We observed significant differences between animals of different ages. This improved understanding about the effect of these factors on responses to vaccines will enable smarter tailoring of vaccines for specific age groups, possibly through the use of tailored adjuvants.
Disclosures
The authors have no competing interests to declare.
Authorship
JM performed the experiments and analysed data, HG analysed data, ZZ performed experiments, JT designed study, analysed data and wrote paper.
Supporting information
Figure S1. Age affects the cytokine response in the blood to booster vaccination.
Table S1 Complete Data set of inflammatory responses.
Acknowledgements
This work was funded by support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115308, Biovacsafe, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007‐2013) and EFPIA members’ kind contribution.
References
- 1. Preaud E, Durand L, Macabeo B, Farkas N, Sloesen B, Palache A et al Annual public health and economic benefits of seasonal influenza vaccination: a European estimate. BMC Public Health 2014; 14:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lafond KE, Nair H, Rasooly MH, Valente F, Booy R, Rahman M et al Global role and burden of influenza in pediatric respiratory hospitalizations, 1982–2012: a systematic analysis. PLoS Med 2016; 13:e1001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng P‐Y, Steiner C et al Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis 2012; 54:1427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegrist CA, Aspinall R. B‐cell responses to vaccination at the extremes of age. Nat Rev Immunol 2009; 9:185–94. [DOI] [PubMed] [Google Scholar]
- 5. Monto AS, Ohmit SE. Seasonal influenza vaccines: evolutions and future trends. Expert Rev Vaccines 2009; 8:383–9. [DOI] [PubMed] [Google Scholar]
- 6. Principi N, Esposito S, Marchisio P. Present and future of influenza prevention in pediatrics. Expert Opin Biol Ther 2011; 11:641–53. [DOI] [PubMed] [Google Scholar]
- 7. Amanna IJ. Balancing the efficacy and safety of vaccines in the elderly. Open Longev Sci 2012; 6:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tregoning JS, Yamaguchi Y, Harker J, Wang B, Openshaw PJ. The role of T cells in the enhancement of respiratory syncytial virus infection severity during adult reinfection of neonatally sensitized mice. J Virol 2008; 82:4115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Siggins MK, Gill SK, Langford PR, Li Y, Ladhani SN, Tregoning JS. PHiD‐CV induces anti‐Protein D antibodies but does not augment pulmonary clearance of nontypeable Haemophilus influenzae in mice. Vaccine 2015; 33:4954–61. [DOI] [PubMed] [Google Scholar]
- 10. Lambert L, Kinnear E, McDonald JU, Grodeland G, Bogen B, Stubsrud E et al DNA vaccines encoding antigen targeted to MHC Class II induce influenza‐specific CD8+ T cell responses, enabling faster resolution of influenza disease. Front Immunol 2016; 7:321. doi: 10.3389/fimmu.2016.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russell RF, McDonald JU, Lambert L, Tregoning JS. Use of the microparticle nanoscale silicon dioxide as an adjuvant to boost vaccine immune responses against influenza virus in neonatal mice. J Virol 2016; 90:4735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McDonald JU, Ekeruche‐Makinde J, Ho MM, Tregoning JS, Ashiru O. Development of a custom pentaplex sandwich immunoassay using Protein‐G coupled beads for the Luminex® xMAP® platform. J Immunol Methods 2016; 433:6–16. [DOI] [PubMed] [Google Scholar]
- 13. Team R Development Core team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R foundation for statistical computing, 2011. [Google Scholar]
- 14. Tang YHM, Li W. ggfortify: Unified Interface to Visualize Statistical Result of Popular R Packages. The R Journal 2016.
- 15. Ligges UMM. Scatterplot3d – an R Package for visualizing multivariate data. J Stat Softw 2003; 8:1–20. [Google Scholar]
- 16. Pavlidis P, Noble WS. Matrix2png: a utility for visualizing matrix data. Bioinformatics 2003; 19:295–6. [DOI] [PubMed] [Google Scholar]
- 17. Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 2007; 7:379–90. [DOI] [PubMed] [Google Scholar]
- 18. Kollmann TR, Marchant A. Towards predicting protective vaccine responses in the very young. Trends Immunol 2016; 37:523–34. [DOI] [PubMed] [Google Scholar]
- 19. Russell RF, McDonald JU, Lambert L, Tregoning JS. Use of the microparticle NanoSiO2 as an adjuvant to boost vaccine immune responses in neonatal mice against influenza. J Virol 2016; 90:4735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khalil SM, Tonkin DR, Snead AT, Parks GD, Johnston RE, White LJ. An alphavirus‐based adjuvant enhances serum and mucosal antibodies, T cells, and protective immunity to influenza virus in neonatal mice. J Virol 2014; 88:9182–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sherman MP, Pritzl CJ, Xia C, Miller MM, Zaghouani H, Hahm B. Lactoferrin acts as an adjuvant during influenza vaccination of neonatal mice. Biochem Biophys Res Commun 2015; 467:766–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mastelic GB, Eberhardt CS, Auderset F, Castellino F, Seubert A, Tregoning JS et al MF59 mediates its B cell adjuvanticity by promoting T follicular helper cells and thus germinal center responses in adult and early life. J Immunol 2015; 194:4836–45. [DOI] [PubMed] [Google Scholar]
- 23. Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F et al Inflammaging and anti‐inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 2007; 128:92–105. [DOI] [PubMed] [Google Scholar]
- 24. Puchta A, Naidoo A, Verschoor CP, Loukov D, Thevaranjan N, Mandur TS et al TNF drives monocyte dysfunction with age and results in impaired anti‐pneumococcal immunity. PLoS Pathog 2016; 12:e1005368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yam KK, Gupta J, Allen EK, Burt KR, Beaulieu É, Mallett CP et al Comparison of AS03 and alum on immune responses elicited by A/H3N2 split influenza vaccine in young, mature and aged BALB/c mice. Vaccine 2016; 34:1444–51. [DOI] [PubMed] [Google Scholar]
- 26. Bernstein ED, Gardner EM, Abrutyn E, Gross P, Murasko DM. Cytokine production after influenza vaccination in a healthy elderly population. Vaccine 1998; 16:1722–31. [DOI] [PubMed] [Google Scholar]
- 27. Higgins DA, Carlson JR, Van Nest G. MF59 adjuvant enhances the immunogenicity of influenza vaccine in both young and old mice. Vaccine 1996; 14:478–84. [DOI] [PubMed] [Google Scholar]
- 28. Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res 2009; 10:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramirez A, Co M, Mathew A. CpG improves influenza vaccine efficacy in young adult but not aged mice. PLoS ONE 2016; 11:e0150425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leng J, Stout‐Delgado HW, Kavita U, Jacobs A, Tang J, Du W et al Efficacy of a vaccine that links viral epitopes to flagellin in protecting aged mice from influenza viral infection. Vaccine 2011; 29:8147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aspinall R, Henson SM, Pido‐Lopez J. My T's gone cold. I'm wondering why. Nat Immunol 2003; 4:203–5. [DOI] [PubMed] [Google Scholar]
- 32. Gasparini R, Pozzi T, Montomoli E, Fragapane E, Senatore F, Minutello M et al Increased immunogenicity of the MF59‐adjuvanted influenza vaccine compared to a conventional subunit vaccine in elderly subjects. Eur J Epidemiol 2001; 17:135–40. [DOI] [PubMed] [Google Scholar]
- 33. Banzhoff A, Gasparini R, Laghi‐Pasini F, Staniscia T, Durando P, Montomoli E et al MF59‐adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non‐elderly and elderly adults. PLoS ONE 2009; 4:e4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fragapane E, Gasparini R, Schioppa F, Laghi‐Pasini F, Montomoli E, Banzhoff A. A heterologous MF59‐adjuvanted H5N1 prepandemic influenza booster vaccine induces a robust, cross‐reactive immune response in adults and the elderly. Clin Vaccine Immunol 2010; 17:1817–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scheifele DW, McNeil SA, Ward BJ, Dionne M, Cooper C, Coleman B et al Safety, immunogenicity, and tolerability of three influenza vaccines in older adults: results of a randomized, controlled comparison. Hum Vaccin Immunother 2013; 9:2460–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reber AJ, Kim JH, Biber R, Talbot HK, Coleman LA, Chirkova T et al Preexisting immunity, more than aging, influences influenza vaccine responses. Open Forum Infect Dis 2015; 2:ofv052–ofv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lang P‐O, Mendes A, Socquet J, Assir N, Govind S, Aspinall R. Effectiveness of influenza vaccine in aging and older adults: comprehensive analysis of the evidence. Clin Interv Aging 2012; 7:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakaya HI, Clutterbuck E, Kazmin D, Wang L, Cortese M, Bosinger SE et al Systems biology of immunity to MF59‐adjuvanted versus nonadjuvanted trivalent seasonal influenza vaccines in early childhood. Proc Natl Acad Sci USA 2016; 113:1853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frey SE, Reyes MR, Reynales H, Bermal NN, Nicolay U, Narasimhan V et al Comparison of the safety and immunogenicity of an MF59(R)‐adjuvanted with a non‐adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine 2014; 32:5027–34. [DOI] [PubMed] [Google Scholar]
- 40. Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C et al Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci USA 2008; 105:10501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O'Hagan DT et al Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 2011; 29:1812–23. [DOI] [PubMed] [Google Scholar]
- 42. Cantisani R, Pezzicoli A, Cioncada R, Malzone C, De Gregorio E, D'Oro U et al Vaccine adjuvant MF59 promotes retention of unprocessed antigen in lymph node macrophage compartments and follicular dendritic cells. J Immunol 2015; 194:1717–25. [DOI] [PubMed] [Google Scholar]
- 43. Calabro S, Tritto E, Pezzotti A, Taccone M, Muzzi A, Bertholet S et al The adjuvant effect of MF59 is due to the oil‐in‐water emulsion formulation, none of the individual components induce a comparable adjuvant effect. Vaccine 2013; 31:3363–9. [DOI] [PubMed] [Google Scholar]
- 44. Knudsen NP, Olsen A, Buonsanti C, Follmann F, Zhang Y, Coler RN et al Different human vaccine adjuvants promote distinct antigen‐independent immunological signatures tailored to different pathogens. Sci Rep 2016; 6:19570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lewis DJ, Lythgoe MP. Application of “Systems vaccinology” to evaluate inflammation and reactogenicity of adjuvanted preventative vaccines. J Immunol Res 2015; 2015:909406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Age affects the cytokine response in the blood to booster vaccination.
Table S1 Complete Data set of inflammatory responses.


