Summary
Aging leads to hypothalamic inflammation, but does so more slowly in mice whose lifespan has been extended by mutations that affect GH/IGF‐1 signals. Early‐life exposure to GH by injection, or to nutrient restriction in the first 3 weeks of life, also modulate both lifespan and the pace of hypothalamic inflammation. Three drugs extend lifespan of UM‐HET3 mice in a sex‐specific way: acarbose (ACA), 17‐α‐estradiol (17αE2), and nordihydroguaiaretic acid (NDGA), with more dramatic longevity increases in males in each case. In this study, we examined the effect of these anti‐aging drugs on neuro‐inflammation in hypothalamus and hippocampus. We found that age‐associated hypothalamic inflammation is reduced in males but not in females at 12 months of age by ACA and 17αE2 and at 22 months of age in NDGA‐treated mice. The three drugs blocked indices of hypothalamic reactive gliosis associated with aging, such as Iba‐1‐positive microglia and GFAP‐positive astrocytes, as well as age‐associated overproduction of TNF‐α. This effect was not observed in drug‐treated female mice or in the hippocampus of the drug‐treated animals. On the other hand, caloric restriction (CR; an intervention that extends the lifespan in both sexes) significantly reduced hypothalamic microglia and TNF‐α in both sexes at 12 months of age. Together, these results suggest that the extent of drug‐induced changes in hypothalamic inflammatory processes is sexually dimorphic in a pattern that parallels the effects of these agents on mouse longevity and that mimics the changes seen, in both sexes, of long‐lived nutrient restricted or mutant mice.
Keywords: Acarbose, aging, hypothalamus, inflammation, NDGA, longevity, sexual dimorphism, 17‐α Estradiol
Abbreviations
- 17αE2
17‐α‐estradiol
- ACA
acarbose
- ARH
arcuate nucleus of the hypothalamus
- CNS
central nervous system
- CR
calorie restriction
- NDGA
nordihydroguaiaretic acid
Introduction
Increased inflammatory activity accompanies normal brain aging. Aging retardation can be achieved in mice by inhibiting activation of hypothalamic nuclear factor‐kB (NF‐kB) inflammatory pathways (Zhang et al., 2013). Similarly, limiting nutrient availability in the first 3 weeks of life (the crowded litter (CL) model) leads to reduced hypothalamic gliosis in parallel with reduced activation of tumor necrosis factor (TNF‐α) in the aged CL mice (Sadagurski et al., 2015a). Furthermore, the mutations that interfere with GH production or response, in the long‐lived Snell dwarf, Ames dwarf or growth hormone receptor deficient (GHRKO) mice lead to reduced age‐associated hypothalamic inflammation in old mice (Sadagurski et al., 2015b). Recent studies by the NIA Interventions Testing Program (ITP) demonstrated that lifespan can also be increased in male UM‐HET3 mice exposed to three drugs: acarbose (ACA), nordihydroguaiaretic acid (NDGA), and 17‐α‐estradiol (17αE2; Harrison et al., 2013). The genetically heterogeneous UM‐HET3 mice are the ideal model system to study lifespan, because the tested populations are reproducible and represent great genetic diversity (Miller et al., 1999, 2011). NDGA and 17aE2 do not extend lifespan of female UM‐HET3 mice, and the effect of ACA, although statistically significant in both sexes, is dramatically larger in males than in females (Harrison et al., 2013). Neither the site(s) of action responsible for these longevity effects, nor the basis for the sex specificity, are known.
It is well documented that many aspects of carbohydrate and energy metabolism, including pancreatic function and insulin action on the liver and other target organs, are influenced by the hypothalamus via neuronal signals (Myers, 2006) and that obesity, insulin resistance and metabolic syndrome are associated with hypothalamic inflammation (Morton et al., 2006; Thaler et al., 2012). Glial cells are particularly sensitive to homeostatic imbalances, and increased glial activity is a well‐established sign of an inflammatory response (Arvin et al., 1996). Activation of NF‐kB dependent cytokine production and hypothalamic glial activity increases with age, and augmentation of this effect can shorten mouse lifespan (Zhang et al., 2013), while blunting hypothalamic inflammatory signals can increase lifespan in mice (Zhang et al., 2013). Glial activity and production of mRNA for the inflammatory cytokine TNF‐α are diminished in long‐lived, GH‐deficient Ames and Snell dwarf mice, and in GHRKO mice (Sadagurski et al., 2015b), but not in mice with liver‐specific disruption of GHR, a mutant that does not show increased longevity (List et al., 2014).These observations are all consistent with the idea that lower hypothalamic inflammation may modulate aging processes, and lifespan, in mice.
Increased neuro‐inflammatory reaction is frequently observed not only in hypothalamus, but also in the hippocampus during normal brain aging (Gavilan et al., 2007). Age‐related hippocampal inflammatory processes are potentially related to hippocampal neurodegeneration (Mattson & Magnus, 2006). The aging hippocampus does not appear to suffer a generalized loss of cells, although atrophy of the structure may occur in humans, and these changes are thought to be an important contributor to age‐related cognitive impairments (Miller & O'Callaghan, 2005). Activated microglial cells are observed in the hippocampus of aged animals (Lynch et al., 2010), and high levels of several cytokines such as IL‐1β and TNF‐α, produced by the activated microglia, are significantly increased in hippocampus of aged mice and rats (Ye & Johnson, 1999; Norden & Godbout, 2013).
ACA is an inhibitor of intestinal α‐glucosidase (Balfour & McTavish, 1993). It inhibits digestion of polysaccharides and delays the uptake of sugars from the GI tract. It lowers postprandial glucose excursions and is used clinically for the treatment of type 2 diabetes (DiNicolantonio et al., 2015). In UM‐HET3 mice, ACA treatment beginning at 4 months of age increased median and maximal lifespan in males much more strongly than in females (Harrison et al., 2013). When started at 16 months, ACA significantly increased median longevity in males and 90th percentile lifespan in both sexes (Strong et al., 2016). ACA reduced fasting insulin significantly in young males but not in females, while the reduction in body weight and age‐associated decline in voluntary activity was more dramatic in ACA‐fed females than in males (Harrison et al., 2013).
17αE2 is an optical isomer of 17‐β‐estradiol that has reduced affinity for estrogen receptors (Zhurova et al., 2009). This form of estrogen is reported to be neuroprotective in vitro in cultured cells and in vivo in an ischemia–reperfusion animal model (Perez et al., 2005). It also has been reported to protect against neurodegeneration in cell and animal models of Parkinson's disease (Dykens et al., 2005) and cerebrovascular disease (Liu et al., 2005). An initial report using 17aE2 at 4.8 ppm showed increased median and maximal lifespan in males but no effects on lifespan in female mice (Harrison et al., 2013). A second study using 17αE2 at 14.4 ppm robustly extended both median and maximal lifespan in males, but showed no effect in females, and 17aE2‐treated males lived significantly longer than either control or treated females (Strong et al., 2016).
NDGA is both a lipoxygenase inhibitor and potent antioxidant (Lu et al., 2010). Two ITP studies have shown that NDGA increases median lifespan, but only in male mice (Strong et al., 2008; Harrison et al., 2013). The lack of effect of NDGA on female lifespan cannot be explained by effects on body weight (Miller et al., 2007; Harrison et al., 2013) or by differences in blood NDGA concentrations. NDGA inhibits inflammatory responses that are thought to play a role in many age‐related diseases (Ferrandiz & Alcaraz, 1991).
Changes in gene expression and glial localization in the course of normal brain aging are sexually dimorphic (Berchtold et al., 2008). For example, males have more microglia early in postnatal development, whereas females have more microglia with an activated morphology later in life, as young adults (Schwarz et al., 2012). Furthermore, the numbers and diversity of astrocytes are also sexually differentiated across brain regions (McCarthy & Arnold, 2011). Gene expression of a large number of cytokines in the aging brain is highly dependent upon sex and region (Bilbo et al., 2012). These collective data suggest that regional‐, sex‐, and age‐dependent differences in glia cells and their inflammatory capacity could play a role in the sex‐specific lifespan benefit of ACA, 17αE2, and NDGA.
In this study, we evaluated the effect of ACA, 17αE2, and NDGA on age‐associated inflammatory responses in two brain areas that are sensitive to aging and age‐related neurological changes (hypothalamus and hippocampus), to determine whether these are modified by the anti‐aging drugs and whether the effects of the drugs on inflammation differ in males and females.
Results
Effect of ACA and 17αE2 on CNS inflammation in aged mice
We evaluated two indices of inflammatory responses, that is, glial fibrillary acidic protein (GFAP, a marker of astrocyte activation), and production of TNF‐α by microglia, in the hypothalamus and hippocampus of 12‐month‐old UM‐HET3 male and UM‐HET3 female mice that had been exposed to ACA or 17αE2 from 4 months of age.
We used Iba‐1 as a marker of microglial number and analyzed results by analysis of variance to test the main effects of sex and drug exposure and the [sex × drug] interaction term. ACA had a significant effect (P = 0.01) reducing activated microglia in both hypothalamus and hippocampus, with no significant main effect of sex (P = 0.07 for MBH and P = 0.7 for hippocampus; Figs 1 and 2A,B). The interaction term was significant at P = 0.035 for hypothalamus, showing that the drug had greater effects on microglial numbers in males than in females (Fig. 1B). There was no evidence for sex specificity (P = 0.33) in hippocampus (Fig. 2A,B).
Figure 1.
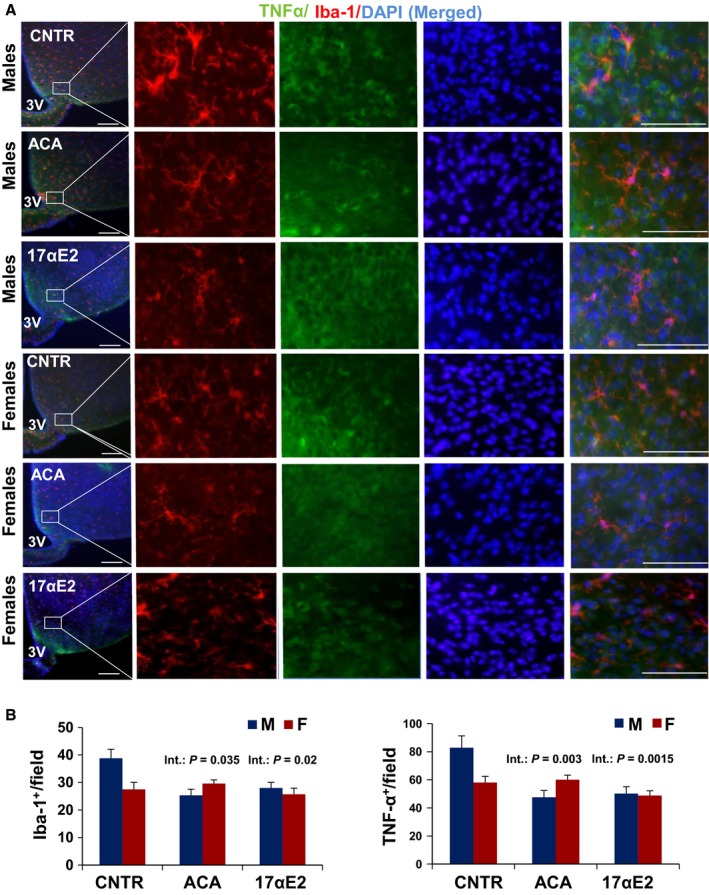
Hypothalamic inflammation in ACA‐ and 17αE2‐treated mice as measured by Iba‐1+ microgial and TNF‐α cells. Brain sections of 12‐month‐old male and female mice were analyzed for hypothalamic microglia and TNF‐α. (A) Representative images showing immunostaining in the MBH of control ACA‐ and 17αE2‐treated mice. Scale bars: 100 μm (far left); 20 μm (right side panels), 3V, third ventricle. (B) Numbers of cells immunoreactive for Iba‐1 or TNF‐α in the hypothalamic mediobasal region (across the confocal microscopic field of serial sections) from indicated male and female mice; error bars show SEM for N = 6 mice of each type. The p‐value shown as ‘Int’ represents the interaction term in a two‐factor ANOVA, testing whether the drug effect differs between male and female mice.
Figure 2.
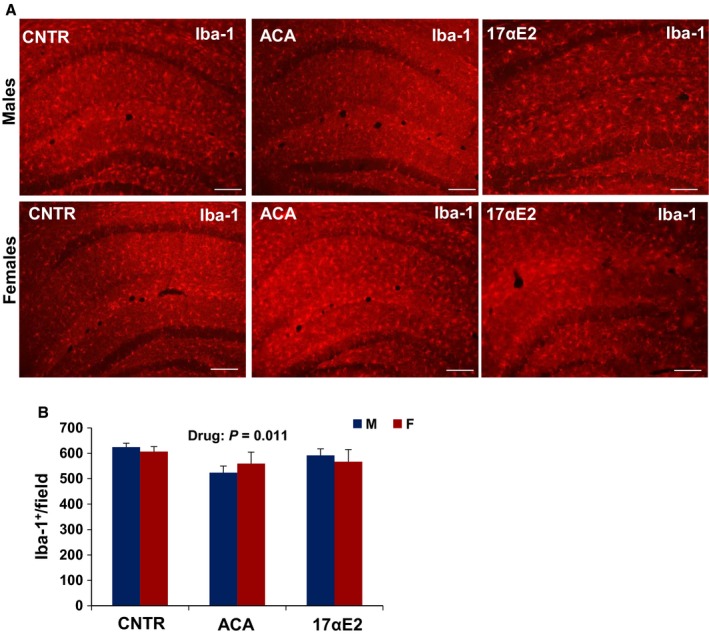
Microglia in the hippocampus of ACA‐ and 17αE2‐treated mice. (A) Representative images of microglia identified by immunofluorescent detection of Iba‐1 protein in coronal sections of CA1, CA3, and dentate gyrus of the hippocampus obtained from 12‐month‐old male and female control, ACA‐, and 17αE2‐treated mice. Scale bars: 100 μm (B) Quantification of Iba‐1 in coronal sections of left CA1, CA3, and dentate gyrus considered together. Bars show mean and SEM for N = 6 mice of each type. Acarbose (ACA) led to a significant decline (P = 0.011) in Iba‐1+ cells, and there was no significant interaction effect for both treatments.
Previous studies by us and others have demonstrated that about 80% of the hypothalamic Iba1+ cells produced tumor necrosis factor‐α (TNF‐α), indicating that they are inflammatory (Zhang et al., 2013; Sadagurski et al., 2015a). Analysis of variance revealed a significant effect of drug (P = 0.008) and a significant interaction of sex and drug on TNF‐α expression in the MBH (P = 0.003), without a main effect of sex (P = 0.29; Fig. 1). Thus, ACA diminishes this aspect of inflammation more dramatically in male than in female mice, with effects demonstrable as early as 12 months of age. We were not able to reliably detect TNF‐α by immunostaining in the hippocampus.
17αE2 treatment had effects similar to those of ACA on hypothalamic microglia (Fig. 1). In male mice evaluated at 12 months of age, 17αE2 diminished hypothalamic microglia (P = 0.02), with significant interaction terms (P = 0.02). In addition, the ANOVA revealed a significant effect of 17αE2 (P = 0.001) and a significant interaction of sex and drug (P = 0.0015) on TNF‐α expression in the MBH of 12‐month‐old 17αE2‐treated animals (Fig. 1). For none of these assays was there a significant main effect of sex per se (Fig. 1). A parallel analysis of the hippocampus showed no effect of 17aE2 (P = 0.83) and no interaction between 17aE2 and sex (P = 0.13; Fig. 2A,B). Astrogliosis with advancing age is correlated with increased expression of (GFAP; Nichols et al., 1995). The number of GFAP‐positive astrocytes in hypothalamus was higher in male control than in female control (P = 0.03) and was diminished by both ACA (P < 0.0001) and 17αE2 (P = 0.001), with significantly more effect in male than in female mice (interaction P = 0.044 for ACA and P = 0.039 for 17αE2; Fig. 3A,B).The number of GFAP‐positive astrocytes in the hippocampus depended on sex at 12 months of age (P = 0.009, with males demonstrating higher GFAP‐positive cell at this age), but there was no significant effect of ACA (P = 0.42) or 17αE2 (P = 0.83) and no significant interaction term in the hippocampus (for ACA, P = 0.48 and for 17αE2, P = 0.13; Fig. 4A,B).
Figure 3.
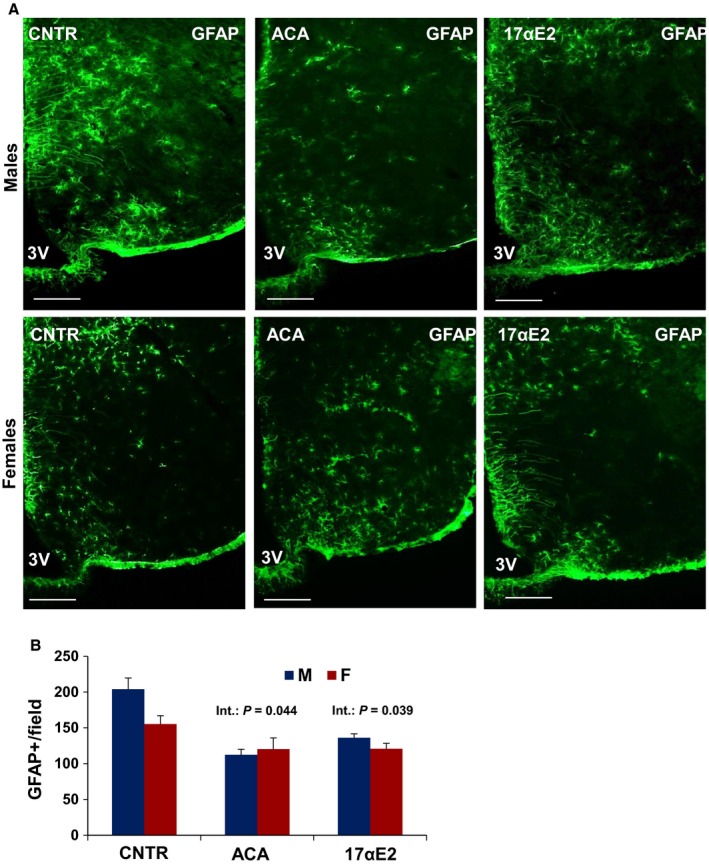
Hypothalamic astrogliosis in ACA‐ and 17αE2‐treated mice. (A) Representative images of astrocytes identified by immunofluorescent detection of GFAP protein in coronal sections of hypothalamus obtained from 12‐month‐old male and female control, ACA‐, and 17αE2‐treated mice. Scale bar: 100 μm. 3V, third ventricle. (B) Quantification of GFAP staining represents number of GFAP‐positive cells per field (error bars indicate SEM; N = 6 mice per group) in the MBH subregion. The significant interaction P‐value (P = 0.044) for ACA and (P = 0.039) for 17αE2 indicates that both drugs have different effects on GFAP + cells in males vs. females.
Figure 4.
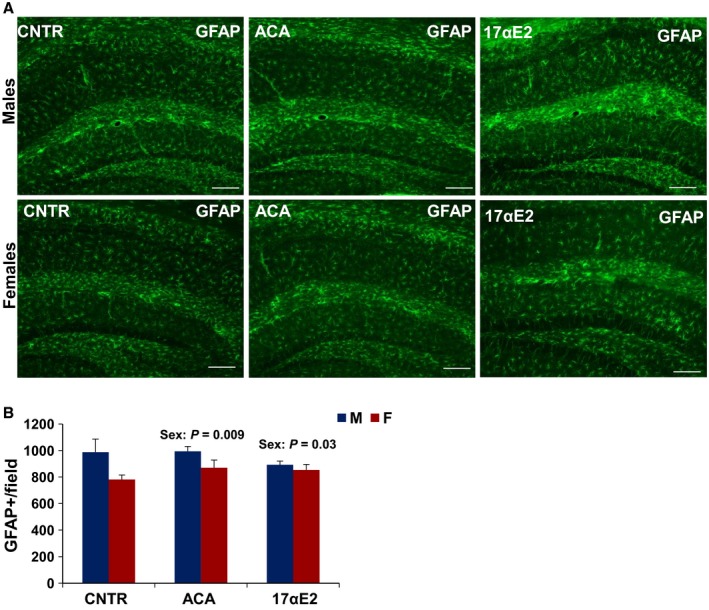
Astrocytes in the hippocampus of ACA‐ and 17αE2‐treated mice. (A) Representative images of astrocytes identified by immunofluorescent detection of GFAP protein in coronal sections of CA1, CA3, and dentate gyrus of the hippocampus obtained from 12‐month‐old male and female control, ACA‐, and 17αE2‐treated mice. Scale bars: 100 μm (B) Quantification of GFAP staining represents number of GFAP‐positive cells in coronal sections of left CA1, CA3, and dentate gyrus considered together. Bars show mean and SEM for N = 6 mice of each type. There was no significant interaction effect for GFAP with either drug. GFAP + cells were significantly higher in male mice regardless of drug treatment.
We detected no significant effects of sex or treatment in the total number of cells detected by DAPI staining (data not shown).
In support of these findings, two‐way ANOVA revealed a significant effect of caloric restriction (an intervention that extends the lifespan in both sexes) on both hypothalamic microglia (P = 0.0001) and TNF‐α (P = 0.0001) without significant interaction of sex and drug for both endpoints at 12 months of age (Fig. S1A,B, Supporting information). The effect of CR on GFAP‐positive astrocytes in the hypothalamus did not reach statistical significance (P = 0.09; Fig. S1C,D, Supporting information). Consistent with the hippocampal results for 12‐month‐old ACA and 17αE2‐treated mice, the number of GFAP‐positive astrocytes in the hippocampus depended on sex (P = 0.002), but there were no significant effects of CR on microglia (P = 0.35) or number of astrocytes (P = 0.07; Fig. S1E–H, Supporting information).
Effect of NDGA on CNS inflammation in old mice
The anti‐inflammatory agent NDGA leads to a male‐specific extension of median lifespan. The effects of NDGA are dose dependent but without an effect on maximal lifespan in either sex (Harrison et al., 2013; Strong et al., 2016). Our previous work has shown continuing age‐related increase in levels of hypothalamic inflammatory markers (TNF‐α, Iba‐1, and GFAP) between 12 and 22 months in UM‐HET3 mice (Sadagurski et al., 2015a). At 22 months of age, we found reduced numbers of GFAP‐immunostained astrocytes and Iba‐1 microglia in the MBH of NDGA‐treated mice (P < 0.01) as compared to control mice (Fig. 5). For astrocytes, the (sex × drug) interaction term, P = 0.052, did not quite reach conventional levels for statistical significance, nor was there evidence for significant sex specificity in the microglial count (P = 0.4; Fig. 5). Similarly, we observed a significant effect of NDGA (P = 0.013) and sex (P = 0.023) on TNF‐α expression in hypothalamic microglia, without a significant interaction between NDGA treatment and sex (Fig. 5A,B). Lastly, we saw no effect of NDGA treatment on astrocytes and microglia activation in the hippocampus of 22‐month‐old mice (Fig. 6).
Figure 5.
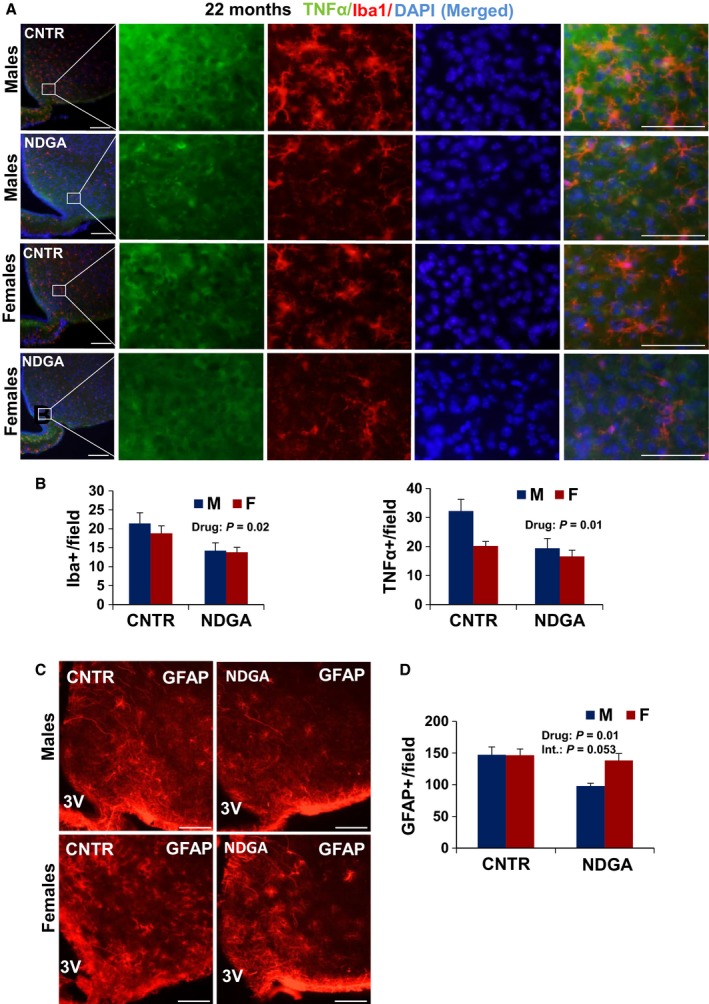
NDGA diminishes hypothalamic inflammation in hypothalamus. Brain sections of 22‐month‐old male and female mice were analyzed for hypothalamic microglia, TNF‐α, and astrocytes. (A) Representative images showing Iba‐1 and TNF‐α immunostaining merged with DAPI (blue) in the hypothalamic mediobasal region (MBH) of control and NDGA‐treated mice. Scale bars: 100 μm (far left); 20 μm (right side panels), 3V, third ventricle. (B) Numbers of cells immunoreactive for Iba‐1 or TNF‐α in the MBH (across the confocal microscopic field of serial sections) from indicated male and female mice. (C) Representative images of astrocytes identified by immunofluorescent detection of GFAP protein in coronal sections of hypothalamus. Scale bar: 100 μm. 3V, third ventricle. (D) Quantification of GFAP staining represents number of GFAP‐positive cells per field in the MBH subregion. Bars show mean and SEM for 4 or 5 mice/group. Two‐factor ANOVA showed a significant effect of drug on microglia numbers and TNF‐α, but no significant interaction (P = 0.14 for TNF‐α cells) between sex and drug treatment. NDGA led to a significant reduction (P = 0.01) in GFAP + cells, and the [sex × drug] interaction term just failed to reach significance at P = 0.053.
Figure 6.
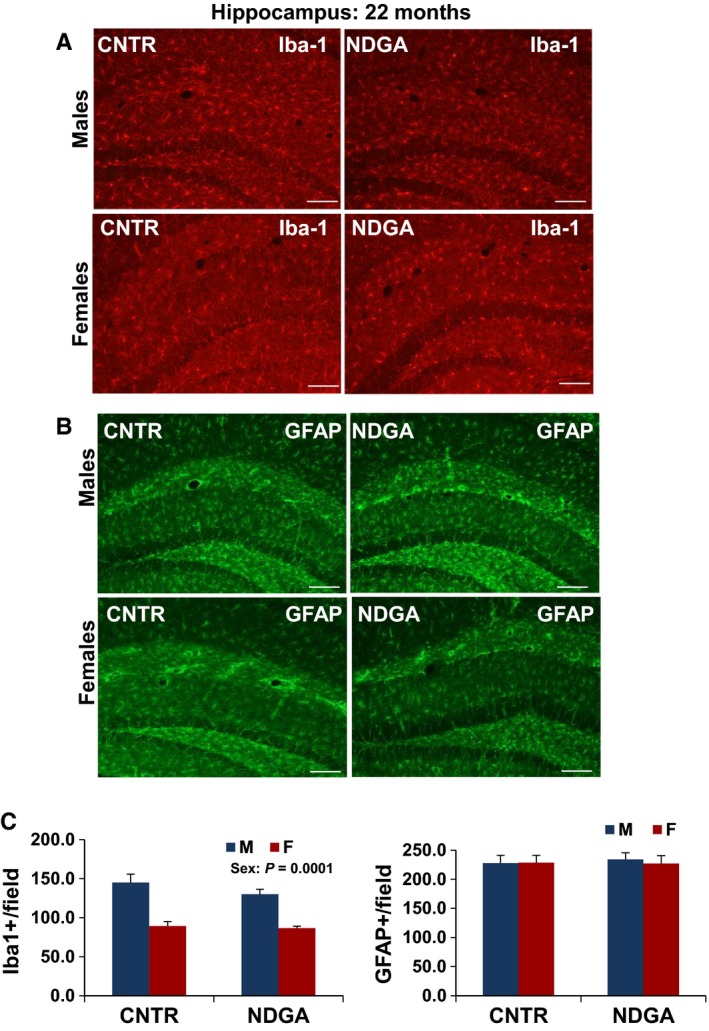
Microglia and astrocytes in the hippocampus of 22‐month‐old NDGA‐treated mice. (A) Representative images of microglia identified by immunofluorescent detection of Iba‐1 protein and (B) astrocytes identified by immunofluorescent detection of GFAP protein in coronal sections of CA1, CA3, and dentate gyrus of the hippocampus obtained from 22‐month‐old male and female NDGA‐treated mice, scale bar: 100 μm. (C) Quantification of Iba‐1 and GFAP staining represents number of Iba‐1 or GFAP‐positive cells in coronal sections of left CA1, CA3, and dentate gyrus. Bars show mean and SEM for N = 6. Iba‐1+ cells were higher in males (P = 0.0001).
Discussion
These data are the first to document the effect of lifespan‐extending drugs on neuro‐inflammatory components, within brain regions that are sensitive to aging. Modulation of lifespan by NDGA, ACA, and 17aE2 is sex specific, with males being more responsive to each of these agents (Strong et al., 2008, 2016; Harrison et al., 2013). We demonstrate here that hypothalamic inflammatory responses in 22‐month‐old NDGA and 12‐month‐old ACA and 17αE2‐treated mice are reduced by these drugs in males but not in females. We saw no consistent effects on inflammation in the hippocampus of the drug‐treated mice, supporting the idea that inhibition of the hypothalamic inflammatory responses may play a particularly important role in mouse lifespan extension (Zhang et al., 2013). Similarly, early‐life caloric restriction (CL between birth and 3 weeks of age; Sadagurski et al., 2015a) and mutations that interfere with GH production or response (Sadagurski et al., 2015b) also lead to reduced hypothalamic inflammation in old mice. Our data now provide evidence that drugs that extend lifespan might be effective in inhibiting hypothalamic inflammatory processes in a sex‐dependent manner.
It seems likely that the sex‐specific effects of these drugs on both inflammation and lifespan may reflect differences in hormonal patterns between the sexes, although our current data do not distinguish between effects of sex hormones in early life (i.e., prior to drug exposure) and possible interactions with endocrine status during the period of drug treatment. Neuron–glial plasticity is heavily influenced by sex steroids and contributes to differential immune responses by sex (Zagni et al., 2016). Sex steroids regulate the transcription of genes, such as cytokines relevant to immune response and immune signaling (Barna et al., 1996). In our study, we did not detect any effects of sex or drug treatment on total cell numbers (DAPI staining), but we cannot exclude the possibility that there may be have effects on neuronal cell numbers. Future studies of the effects of ACA, 17αE2, and NDGA in ovariectomized females and castrated males, or in mice lacking the classical estrogen receptor, or in testosterone‐treated females, would help to clarify the basis for the effects of these drugs on male mice. Information from such studies might suggest ideas about how the health benefits of these three drugs might be extended to female mice and about approaches to development of drugs that postpone aging both in men and women.
In several of the models we have evaluated, drug treatment diminishes inflammation in male mice to the level seen in untreated female mice, but has little effect on the female animals. This observation suggests the possibility of a threshold effect, the idea that inflammation in females may already be at such a low level that drug treatment cannot lead to further diminution. There is substantial evidence for sex‐specific differences in both microglia and astrocytes in the mouse brain (McCarthy & Arnold, 2011; Pfau et al., 2016). Males have more microglia in the CA1, CA3, and dentate gyrus (DG) of the hippocampus and the amygdala early in postnatal development, whereas females have more microglia with an activated morphology later in development, that is, as juveniles and as young 3‐month‐old adults (Schwarz et al., 2012). Our data show that male mice have higher levels of hypothalamic glia than females, consistent with limited data in monkeys (Roberts et al. 2012), changes that may reflect early developmental effects rather than effects of aging. Sex differences in glial number and morphology depend on brain region and may well be modulated differently by drug and endocrine status at later ages as well. We found no significant effect of the anti‐aging drugs on microglia numbers in the hippocampus at 12 or 22 months of age, although females had less microglia than males at the older age. Consistent with this observation, we found no significant effect of the drugs on astrocyte cell counts in hippocampus. A more detailed analysis of separate subregions of the hippocampus would be laborious and would require sections cut at orientations optimized for this purpose, but might provide additional useful detail about regional heterogeneity. Drugs that extend lifespan equally in males and females, or which render females more responsive to the lifespan effects mediated by ACA and 17αE2, will help provide additional insight into this sexual dimorphism.
In contrast to our data, a recent study by Hascup et al. (2016), examining mRNA expression levels, found significant elevation in certain NF‐kB signaling molecules and glutamatergic markers in the hypothalamus and different hippocampus areas in old GHR−/− mice as compared to controls. The methods used by Hascup provided relatively little information about the specific cell type(s) expressing NF‐kB signaling molecules. In contrast, the conclusions of Zhang (Zhang et al., 2013) and from our current and previous studies (Sadagurski et al., 2015b) are based on immunostaining evaluation of inflammatory markers. Such assessment provides an anatomical location of cells within the area of interest suggesting the overall function to which those cells contribute. As differential physiological properties have been reported across hypothalamic areas as well as across septal and temporal hippocampus, a combined approach of comprehensive molecular mapping of inflammatory genes based on in situ hybridization (ISH) data in long‐lived mice can provide additional insight into discrete cellular populations.
Glial cells are particularly sensitive to homeostatic imbalances. Nutritional excess is a key activator of metabolic inflammation in the hypothalamus, suggesting that interventions that can counteract hypothalamic neuro‐inflammation may protect against metabolic disorders (Thaler & Schwartz, 2010). An inflammatory state in the hypothalamus disrupts its ability to sense metabolic abnormalities, affecting energy balance and glucose metabolism, leading to obesity and insulin resistance (Thaler et al., 2012). It was recently demonstrated that there is a sexually dimorphic response to chronic high‐fat diet (HFD) exposure (Morselli et al., 2014). Consistent with some of our findings in aged mice, the work on HFD demonstrated that males, but not females, have hypothalamic inflammation despite comparable weight gain following HFD consumption (Morselli et al., 2014), further emphasizing the physiological relevance of hypothalamic inflammation.
The sphingolipid system has emerged as a key player in modulation of microglia phenotype in pathIogenic forms of neuroinflammation (Assi et al., 2013). Sphingolipids accumulate in the liver and brain during aging, and long‐term food restriction prevents aging‐associated sphingolipid turnover dysregulation (Babenko & Shakhova, 2014). Elevated levels of sphingolipids have previously been associated with reduced insulin signaling and increased inflammation within the CNS (Summers, 2006). Sphingolipids were significantly increased in the hypothalamus of male animals fed with a HFD when compared to the females (Morselli et al., 2014). The recent study by the Gladyshev group showed that brains of old mice had higher concentrations of glycerophospholipids and sphinogmyelins (Ma et al., 2015), key constituents of cell membranes which play a role in neuroinflammation (Yadav & Tiwari, 2014). Future metabolomics analysis from the hypothalamus per se, from normal and drug‐treated mice, would help to elucidate the underlying biochemical mechanisms of sex‐specific drug effects on hypothalamic inflammation in aging.
In previously published work (Harrison et al., 2013), fasting insulin levels in males were higher than in female controls, and ACA reduced insulin levels in males to the level of control females. The male‐specific decline in fasting insulin level in ACA‐treated mice hints that the altered pattern of glucose spikes produced by ACA may lead to improved insulin sensitivity in males, with less effect in females, and that this protection against insulin resistance might in turn contribute to the sexual dimorphism in longevity effect (Harrison et al., 2013) and in the age‐associated hypothalamic inflammation demonstrated in this study. It has been reported that reduction in postprandial glucose peak levels by ACA in patients with type 2 diabetes reduces postprandial activation of the NF‐κB pathway in blood cells (Rudofsky et al., 2004). NDGA lowers blood glucose, ameliorates hypertriglyceridemia in male rats (female rats were not tested; Zhang et al., 2015), and interferes with TNF‐induced NF‐κB‐mediated transactivation in 293 cells (van Puijenbroek et al., 1999). The activity of ACA, NDGA, and 17αE2 as anti‐inflammatory agents opens new avenues for mechanistic testing and will prove to be valuable for studying differences between male and female mice for control of aging and late‐life neurological diseases. It would be of interest to learn whether ACA, NGDA, or 17αE2 modulates additional pathways thought to be relevant to aging and lifespan, such as those linked to ATF4, mTOR, autophagy, adipokine production, proteasome function, and others, and do so in a sex‐specific way.
Methods
Animals
Procedures involved in this study were approved by the University of Michigan Committee on the Use and Care of Animals (UCUCA). UM‐HET3 mice were produced as previously described in detail (Miller et al., 2007). For breeding cages, we used Purina 5008 mouse chow. For weanlings prior to 4 months of age, we used Purina 5LG6. Animals were maintained under temperature‐ and light‐controlled conditions (20–23 °C, 12‐h light–dark cycle).
Experimental diets
17αE2 was purchased from Steraloids Inc. (Newport, RI, USA) and mixed at a dose of 14.4 mg kg−1 diet (14.4 ppm). Mice were fed the 17αE2 diet continuously from 4 months of age. NDGA was purchased from Cayman Chemicals (Ann Arbor, MI, USA) and mixed at a concentration of 2500 ppm and fed to mice beginning at 4 months of age. ACA was purchased from Spectrum Chemical Mfg. Corp., Gardena, CA, USA. It was mixed at a concentration of 1000 mg of ACA per kilogram of diet (1000 ppm); mice were fed continuously from 4 months of age. For CR feeding: starting at 4 months of age, CR mice were given 80% of the amount of food consumed by age‐matched ad lib control mice for 2 weeks. After that and continuing until euthanasia at 12 months of age, the CR mice were given 60% of diet consumed by ad lib control mice, as previously described (Miller et al., 2014).
Perfusion and immunolabeling
Mice were anesthetized (IP) with Avertin and transcardially perfused with phosphate‐buffered saline (PBS; pH 7.5) followed by 4% paraformaldehyde (PFA). Brains were postfixed, dehydrated, and then sectioned coronally (30 μm) using a sliding microtome, followed by immunohistochemical or immunofluorescent analysis as previously described (Sadagurski et al., 2015b). Brains from 22‐month‐old control and NDGA‐treated mice were only postfixed with 4% PFA. For immunohistochemistry, free‐floating brain sections were pretreated by sequential incubations in 0.3% H2O2/1% NaOH, 0.3% glycine, 0.03% SDS, followed by blocking in normal donkey serum (NDS). Sections were incubated with rabbit anti‐GFAP (1:1000; Millipore, Temecula, CA, USA), mouse anti‐TNF‐α (1:500; Abcam, Cambridge, MA, USA) or rabbit anti‐Iba‐1 (1:1000; Wako, Richmond, VA, USA) as primary antibodies followed by AlexaFluor‐conjugated secondary antibodies (Invitrogen, Carlsbad, CA, USA) as previously published (Sadagurski et al., 2012; Zhang et al., 2013).
Sections were mounted onto Superfrost Plus slides (Fisher Scientific, Hudson, NH, USA) and coverslips added with ProLong Antifade mounting medium (Invitrogen). Microscopic images were obtained using an Olympus FluoView 500 Laser Scanning Confocal Microscope (Olympus, Center Valley, PA, USA) equipped with a 10×, 20×, and 40× objectives.
Quantification
For quantification of immunoreactive cells, images of matched brain areas were taken from at least three sections containing the MBH of the hypothalamus for each brain sections between bregma −0.82 mm to −2.4 mm (according to the Franklin mouse brain atlas). Serial brain sections across the hypothalamus were made at 30 μm thickness, and every five sections were represented by one section with staining and cell counting. For quantification of the hippocampus sections, left side of CA1, CA3, and dentate gyrus was considered together. All sections were arranged from rostral to caudal to examine the distribution of labeled glial cells. Iba‐1 and GFAP‐positive cells were counted using ImageJ software with DAPI (nuclear stain). The average of the total number of cells/field of view was used for statistical analysis as described previously (Sadagurski et al., 2015b).
Statistical analysis
Data sets were analyzed using two‐way analysis of variance (ANOVA) followed by Tukey's post hoc test. All data were presented as mean ± SEM. P < 0.05 was considered significant. IBM SPSS v.21 was used for statistical analysis.
Funding
This project was supported by NIA grants AG022303 and the Glenn Foundation for Medical Research. MS was supported by a pilot grant from the UM Pepper Center AG‐024824 and a Feasibility Grant from the Michigan Diabetes Research Center (P30DK020572).
Conflict of interest
No conflict of interest, financial or otherwise, is declared by the authors.
Supporting information
Fig. S1 Microglia and astrocytes in the hypothalamus and hippocampus of CR treated mice.
Acknowledgments
The authors thank Amanda Keedle and Sabrina Van Roekel for husbandry assistance and Taylor Landeryou, Deontay Walker, Annabel Lemke, Tyler Robinson, and Jessica Peck for technical assistance.
References
- Arvin B, Neville LF, Barone FC, Feuerstein GZ (1996) The role of inflammation and cytokines in brain injury. Neurosci. Biobehav. Rev. 20, 445–452. [DOI] [PubMed] [Google Scholar]
- Assi E, Cazzato D, De Palma C, Perrotta C, Clementi E, Cervia D (2013) Sphingolipids and brain resident macrophages in neuroinflammation: an emerging aspect of nervous system pathology. Clin. Dev. Immunol. 2013, 309302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babenko NA, Shakhova EG (2014) Long‐term food restriction prevents aging‐associated sphingolipid turnover dysregulation in the brain. Arch. Gerontol. Geriatr. 58, 420–426. [DOI] [PubMed] [Google Scholar]
- Balfour JA, McTavish D (1993) Acarbose. An update of its pharmacology and therapeutic use in diabetes mellitus. Drugs 46, 1025–1054. [DOI] [PubMed] [Google Scholar]
- Barna M, Komatsu T, Bi Z, Reiss CS (1996) Sex differences in susceptibility to viral infection of the central nervous system. J. Neuroimmunol. 67, 31–39. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Cribbs DH, Coleman PD, Rogers J, Head E, Kim R, Beach T, Miller C, Troncoso J, Trojanowski JQ, Zielke HR, Cotman CW (2008) Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl Acad. Sci. USA 105, 15605–15610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Smith SH, Schwarz JM (2012) A lifespan approach to neuroinflammatory and cognitive disorders: a critical role for glia. J. Neuroimmune Pharmacol. 7, 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNicolantonio JJ, Bhutani J, O'Keefe JH (2015) Acarbose: safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart 2, e000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens JA, Moos WH, Howell N (2005) Development of 17alpha‐estradiol as a neuroprotective therapeutic agent: rationale and results from a phase I clinical study. Ann. N. Y. Acad. Sci. 1052, 116–135. [DOI] [PubMed] [Google Scholar]
- Ferrandiz M, Alcaraz M (1991) Anti‐inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents Actions 32, 283–288. [DOI] [PubMed] [Google Scholar]
- Gavilan MP, Revilla E, Pintado C, Castano A, Vizuete ML, Moreno‐Gonzalez I, Baglietto‐Vargas D, Sanchez‐Varo R, Vitorica J, Gutierrez A, Ruano D (2007) Molecular and cellular characterization of the age‐related neuroinflammatory processes occurring in normal rat hippocampus: potential relation with the loss of somatostatin GABAergic neurons. J. Neurochem. 103, 984–996. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, Nelson JF, Pletcher S, Simpkins JW, Smith D, Wilkinson JE, Miller RA (2013) Acarbose, 17‐alpha‐estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 13, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup ER, Wang F, Kopchick JJ, Bartke A (2016) Inflammatory and glutamatergic homeostasis are involved in successful aging. J. Gerontol. A Biol. Sci. Med. Sci. 71, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F, Stout MB, Zhi X, Sun L, White TA, LeBrasseur NK, Pirtskhalava T, Tchkonia T, Jensen EA, Zhang W, Masternak MM, Kirkland JL, Miller RA, Bartke A, Kopchick JJ (2014) Liver‐specific GH receptor gene‐disrupted (LiGHRKO) mice have decreased endocrine IGF‐I, increased local IGF‐I, and altered body size, body composition, and adipokine profiles. Endocrinology 155, 1793–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wen Y, Perez E, Wang X, Day AL, Simpkins JW, Yang SH (2005) 17 β‐Estradiol attenuates blood‐brain barrier disruption induced by cerebral ischemia‐reperfusion injury in female rats. Brain Res. 1060, 55–61. [DOI] [PubMed] [Google Scholar]
- Lu JM, Nurko J, Weakley SM, Jiang J, Kougias P, Lin PH, Yao Q, Chen C (2010) Molecular mechanisms and clinical applications of nordihydroguaiaretic acid (NDGA) and its derivatives: an update. Med. Sci. Monit. 16, RA93–RA100. [PMC free article] [PubMed] [Google Scholar]
- Lynch AM, Murphy KJ, Deighan BF, O'Reilly JA, Gun'ko YK, Cowley TR, Gonzalez‐Reyes RE, Lynch MA (2010) The impact of glial activation in the aging brain. Aging Dis. 1, 262–278. [PMC free article] [PubMed] [Google Scholar]
- Ma S, Yim SH, Lee SG, Kim EB, Lee SR, Chang KT, Buffenstein R, Lewis KN, Park TJ, Miller RA, Clish CB, Gladyshev VN (2015) Organization of the mammalian metabolome according to organ function, lineage specialization, and longevity. Cell Metab. 22, 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Magnus T (2006) Ageing and neuronal vulnerability. Nat. Rev. Neurosci. 7, 278–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP (2011) Reframing sexual differentiation of the brain. Nat. Neurosci. 14, 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP (2005) Aging, stress and the hippocampus. Ageing Res. Rev. 4, 123–140. [DOI] [PubMed] [Google Scholar]
- Miller RA, Austad S, Burke D, Chrisp C, Dysko R, Galecki A, Jackson A, Monnier V (1999) Exotic mice as models for aging research: polemic and prospectus. Neurobiol. Aging 20, 217–231. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Strong R (2007) An Aging Interventions Testing Program: study design and interim report. Aging Cell 6, 565–575. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de CR, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R (2011) Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 66, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L, Strong R (2014) Rapamycin‐mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 13, 468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli E, Fuente‐Martin E, Finan B, Kim M, Frank A, Garcia‐Caceres C, Navas CR, Gordillo R, Neinast M, Kalainayakan SP, Li DL, Gao Y, Yi CX, Hahner L, Palmer BF, Tschop MH, Clegg DJ (2014) Hypothalamic PGC‐1alpha protects against high‐fat diet exposure by regulating ERalpha. Cell Rep. 9, 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW (2006) Central nervous system control of food intake and body weight. Nature 443, 289–295. [DOI] [PubMed] [Google Scholar]
- Myers MG Jr (2006) Role reversal: brain insulin and liver STAT3. Cell Metab. 3, 231–232. [DOI] [PubMed] [Google Scholar]
- Nichols NR, Finch CE, Nelson JF (1995) Food restriction delays the age‐related increase in GFAP mRNA in rat hypothalamus. Neurobiol. Aging 16, 105–110. [DOI] [PubMed] [Google Scholar]
- Norden DM, Godbout JP (2013) Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 39, 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez E, Liu R, Yang SH, Cai ZY, Covey DF, Simpkins JW (2005) Neuroprotective effects of an estratriene analog are estrogen receptor independent in vitro and in vivo. Brain Res. 1038, 216–222. [DOI] [PubMed] [Google Scholar]
- Pfau DR, Hobbs NJ, Breedlove SM, Jordan CL (2016) Sex and laterality differences in medial amygdala neurons and astrocytes of adult mice. J. Comp. Neurol. 524, 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Puijenbroek AA, Wissink S, van der Saag PT, Peppelenbosch MP (1999) Phospholipase A2 inhibitors and leukotriene synthesis inhibitors block TNF‐induced NF‐κB activation. Cytokine 11, 104–110. [DOI] [PubMed] [Google Scholar]
- Roberts DE, Killiany RJ, Rosene DL (2012) Neuron numbers in the hypothalamus of the normal aging rhesus monkey: stability across the adult lifespan and between the sexes. J. Comp. Neurol. 520, 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudofsky G Jr, Reismann P, Schiekofer S, Petrov D, Von Eynatten M, Humpert P, Isermann B, Müller‐Hoff C, Thai T‐P, Lichtenstein S (2004) Reduction of postprandial hyperglycemia in patients with type 2 diabetes reduces NF‐κB activation in PBMCs. Horm. Metab. Res. 36, 630–638. [DOI] [PubMed] [Google Scholar]
- Sadagurski M, Leshan RL, Patterson C, Rozzo A, Kuznetsova A, Skorupski J, Jones JC, DePinho RA, Myers MG Jr, White MF (2012) IRS2 signaling in LepR‐b neurons suppresses FoxO1 to control energy balance independently of leptin action. Cell Metab. 15, 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagurski M, Landeryou T, Cady G, Bartke A, Bernal‐Mizrachi E, Miller RA (2015a) Transient early food restriction leads to hypothalamic changes in the long‐lived crowded litter female mice. Physiol. Rep. 3, e12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagurski M, Landeryou T, Cady G, Bartke A, Kopchick J, List EO, Berryman DE, Miller RA (2015b) Growth hormone modulates hypothalamic inflammation in long‐lived pituitary dwarf mice. Aging Cell 14, 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD (2012) Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 120, 948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Harrison DE (2008) Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell 7, 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, de Magalhaes JP, Martinez PA, McCord JM, Miller BF, Muller M, Nelson JF, Ndukum J, Rainger GE, Richardson A, Sabatini DM, Salmon AB, Simpkins JW, Steegenga WT, Nadon NL, Harrison DE (2016) Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha‐glucosidase inhibitor or a Nrf2‐inducer. Aging Cell 15, 872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers SA (2006) Ceramides in insulin resistance and lipotoxicity. Prog. Lipid Res. 45, 42–72. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Schwartz MW (2010) Minireview: inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology 151, 4109–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW (2012) Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RS, Tiwari NK (2014) Lipid integration in neurodegeneration: an overview of Alzheimer's disease. Mol. Neurobiol. 50, 168–176. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW (1999) Increased interleukin‐6 expression by microglia from brain of aged mice. J. Neuroimmunol. 93, 139–148. [DOI] [PubMed] [Google Scholar]
- Zagni E, Simoni L, Colombo D (2016) Sex and gender differences in central nervous system‐related disorders. Neurosci. J. 2016, 2827090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D (2013) Hypothalamic programming of systemic ageing involving IKK‐beta, NF‐kappaB and GnRH. Nature 497, 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li Y, Hu J, Shen WJ, Singh M, Hou X, Bittner A, Bittner S, Cortez Y, Tabassum J, Kraemer FB, Azhar S (2015) Effect of creosote bush‐derived NDGA on expression of genes involved in lipid metabolism in liver of high‐fructose fed rats: relevance to NDGA amelioration of hypertriglyceridemia and hepatic steatosis. PLoS ONE 10, e0138203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhurova EA, Zhurov VV, Chopra D, Stash AI, Pinkerton AA (2009) 17Alpha‐estradiol x 1/2 H2O: super‐structural ordering, electronic properties, chemical bonding, and biological activity in comparison with other estrogens. J. Am. Chem. Soc. 131, 17260–17269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Microglia and astrocytes in the hypothalamus and hippocampus of CR treated mice.


