Summary
Regulatory T (Treg) cells play a key role in dampening excessive immune activation. However, antiretroviral therapy (ART) ‐naive HIV‐1 infection maintains the immune system in a sustained state of activation that could alter both Treg cell surface markers and functions. As Treg cell surface markers are directly linked to their functions the overall objective of this study was to determine how ART‐naive HIV infection affects the phenotypic properties of Treg cells. Our data showed that in ART‐naive HIV‐1 infection, Treg cells are dominated by effector (CD45RA + CD27− CCR7− CD62L−) and effector memory (CD45RA − CD27− CCR7− CD62L−) cells. In contrast Treg cells from HIV‐negative individuals were mainly naive (CD45RA + CD27+ CCR7+ CD62L+) and central memory (CD45RA − CD27+ CCR7+ CD62L+) cells. Whereas effector and effector memory Treg cells showed enhanced expression of CD39 (P < 0·05), CD73 (P < 0·001), HLA‐DR and CD38 (P < 0·001); naive and central memory Treg cells showed a significant reduction in the expression of these markers. Overall Treg cell frequencies within total CD4+ T cells correlated positively with plasmatic HIV‐1 viral load. As increased viral load is associated with augmented CD4+ T‐cell destruction; this could suggest a resistance of peripheral Treg cells to HIV‐1 destruction. Hence the modulation of Treg cell phenotype and frequencies could be considered in designing immunotherapeutic strategies targeting immune system restoration during HIV‐1 infection.
Keywords: antiretroviral therapy‐naive HIV infection, phenotype, regulatory T cells, sustained activation
Abbreviations
- ART
antiretroviral therapy
- FoxP3
forkhead box P3
- MFI
mean fluorescence intensity
- PBMCs
peripheral blood mononuclear cells
- Treg
CD4+ CD25+ FoxP3+ regulatory T cells
Introduction
Chronic infection with HIV‐1 maintains the immune system in a sustained state of activation ultimately culminating in CD4+ T‐cell depletion and immune suppression.1 A direct effect of this sustained activation is the multiplication of HIV‐1 infection targets, which as a consequence augments viral replication and exacerbates helper CD4+ T‐cell destruction. Sustained destruction of helper CD4+ T cells during antiretroviral therapy (ART) ‐naive HIV infection can impair essential functions of forkhead box P3 (FoxP3) ‐expressing CD4+ regulatory T (Treg) cells, including the maintenance of peripheral tolerance and immune homeostasis.2, 3 Such Treg cell dysfunctions have been linked to several diseases including allergy, autoimmunity, cancers and early graft rejection.4
Regulatory T cells are expected in steady states to dampen excessive immune activation, thereby limiting tissue damage and preventing immunopathologies.3 However, in the context of a challenging persistent infection such as HIV, Treg cell function in vivo is probably limited as a result of either direct infection of Treg cells by HIV5 or poor interaction of Treg cells with other immune cells like dendritic cells in the destroyed tissue micro‐environment.6 Nevertheless, previous studies have demonstrated the beneficial effect of Treg cells in reducing HIV‐1‐associated immune activation and inflammation.7, 8, 9 Treg cells have also been shown to suppress both HIV‐specific T‐cell proliferation and cytokine production. This on the one hand can result in a reduction of the available target cells for HIV replication, thereby limiting disease progression. On the other hand, the suppression of critical virus‐specific immune responses could be deleterious to the individual, especially with respect to unchecked viral expansion and inflammation.10, 11, 12
The phenotype of Treg cells in vivo is vital to their function, so we employ multiparametric flow cytometry to assess the phenotype of Treg cells freshly purified by magnetic sorting from peripheral blood mononuclear cells (PBMCs) obtained from ART‐naive HIV‐infected participants from the CIRCB AFRODEC cohort. Our hypothesis being that Treg cell phenotype in the context of ART‐naive HIV infection when associated with viral load and helper CD4 T‐cell count could be used in predicting the role of Treg cells in the challenging environment created by HIV infection.
The need to purify Treg cells during this study arises because they represent a small fraction of CD4+ T cells (5–10%) in steady state, which are further depleted during ART‐naive HIV‐1 infection, making it difficult to obtain an adequate amount for in vitro studies with bulk PBMCs. As Treg cells constitutively express CD25, the interleukin‐2 receptor α chain component,13 FoxP3, the forkhead box P3 transcription factor protein,14 and low levels of CD127, the interleukin‐7 receptor α‐chain,15 we decided to use these markers to track Treg cells during multiparametric FACS analysis.
The purity of the magnetically sorted Treg cells ranged between 90 and 95% for both PBMCs from ART‐naive HIV‐infected and PBMCs from sero‐negative participants. As CD127 has been shown to be inversely correlated with FoxP3,16 we assessed whether the combination of CD127lo CD25+ can provide a good alternative to FoxP3 for tracking Treg cells of ART‐naive HIV‐infected individuals. We demonstrated that FoxP3+ expression of both ART‐naive HIV‐positive and ‐negative people correlated positively with the numbers of CD127lo CD25+ thereby implying that these markers could be used for tracking their Treg cells.
Our data showed that in ART‐naive HIV‐1‐infected people Treg cells consist of a heterogeneous population of CD4+ CD25+ CD127lo cells dominated by effector (CD45RA+ CD27− CCR7− CD62L−) and effector memory (CD45RA− CD27− CCR7− CD62L−) phenotypes in contrast to Treg cells from HIV‐negative people, which displayed a predominantly naive (CD45RA+ CD27+ CCR7+ CD62L+) and central memory (CD45RA− CD27+ CCR7+ CD62L+) phenotype. Both effector and effector memory Treg cells showed significant increases in CD39 (P < 0·05), CD73 (P < 0·001), HLA‐DR and CD38 (P < 0·001) expression, which have been associated with immunomodulatory suppressive functions of Treg cells.17, 18, 19 Overall, the total number of Treg cells correlated positively with the helper CD4+ T‐cell count and inversely with plasmatic HIV viral load. However, when Treg cell frequencies were considered they correlated inversely with CD4+ T‐cell count and positively with plasmatic HIV viral load. These results allowed us to categorize ART‐naive HIV‐1‐infected individuals into two categories. First, those with low Treg cell frequencies (helper CD4 T‐cell count > 350 cells/mm3) with comparatively less plasmatic viral load, and second, people displaying high Treg cell frequencies (helper CD4+ T‐cell count < 350 cells/mm3) with severe immune suppression and elevated viral load. This implies that the success of immunotherapeutic strategies targeting Treg cells in the context of ART‐naive HIV infection could be assessed by considering Treg phenotypes and frequencies. These results are highly relevant because they provide a framework for defining the Treg cell phenotypes and frequencies that are desirable during targeted immotherapeutic strategies aimed at restoring Treg cell function in HIV infection.
Materials and methods
Study populations
Thirty‐one ART‐naive HIV‐1‐infected participants (22 female and 9 male) were recruited from the CIRCB AFRODEC cohort. Seventeen HIV‐negative participants (12 female and 5 male) were also recruited as controls. In addition to people who did not provide consent, participants who had been diagnosed with hepatitis B virus, hepatitis C virus, Dengue virus, Mycobacterium tuberculosis, or malaria were excluded from the study. Absolute numbers of helper CD4+ T cells for both HIV+ and HIV− participants were determined in fresh whole blood by BD multitest CD3/CD8/CD45/CD4 and TruCount tubes (BD Biosciences, San Jose, USA) according to the manufacturer's instructions. Plasmatic HIV‐1 viral load was determined on the m2000rt machine using the Abbott Real‐Time HIV‐1 Assay protocol.
Cell preparation
Isolation of PBMCs
The PBMCs were isolated from whole blood within 2–4 hrs of sampling by density gradient centrifugation using Ficoll‐Paque Plus (GE Healthcare Bio‐Sciences, Uppsala, Sweden). Briefly, venous blood was diluted with an equal volume of 1 × PBS without Ca2+ and Mg2+ (Mediatech, Corning, NY), then carefully overlaid on Ficoll‐Paque Plus and centrifuged at 300 g at 21° for 20 min. The mononuclear‐cell‐rich interface was harvested, washed twice in 1 × PBS without Ca2+ and Mg2+ and counted on a bright‐line hemocytometer (improved Neubauer, 0·100 mm deep; Hausser Scientific, Horsham, USA). The cells were finally re‐suspended at a final concentration of 1 × 107 cells/ml either in Magnetic Activated Cell Sorting buffer (MACS BSA stock solution 1 : 20 autoMACS rinsing solution; Miltenyi Biotec, Bergish Gladbach, Germany) or in FACS buffer (1 × PBS with Ca2+ and Mg2+ + 2% heat inactivated fetal bovine serum; Mediatech) for Treg cell purification or staining, respectively.
Purification of Treg cells
The Treg cells were isolated from PBMCs using the CD4+ CD25+ CD127dim/− Treg cell isolation kit II supplied by Miltenyi Biotec using the manufacturer's protocol (Miltenyi Biotech). Firstly, CD4+ T cells were negatively isolated from PBMCs with CD4+ CD25+ CD127dim/− T‐cell biotin–antibody cocktail II and anti‐biotin microbeads. Isolated cells were then washed and depleted of CD4– and CD127high cells using Miltenyi LD columns. Next, Treg cells (CD4+ CD25+ CD127dim/− Treg cells) were purified from CD4+ T cells by positive selection using Miltenyi CD25 microbeads II. The purity of Treg cells was assessed by flow cytometry using a BD Fortessa X‐20 (BD Biosciences).
Partial purification of Treg cells
CD4+ CD25+ Treg cells were isolated from PBMCs using the BD IMag human regulatory T lymphocyte separation kit (BD Biosciences) according to the manufacturer's instructions. Briefly, non‐CD4+ were stained following incubation of PBMCs with Treg separation cocktail for 15 min at room temperature. After washing away excess antibody, two steps of separation were performed. First, CD4+ T cells were negatively selected following incubation with streptavidin particles for 30 min at room temperature. They were then transferred to a BD Falcon tube, placed within the magnetic field of the BD IMagnet (Cat. No. 552311) and depleted of labelled cells. This negative selection was repeated twice to increase the yield of the enriched fraction. Second, the CD25+ Treg cells were isolated from the twice‐enriched fraction by positive selection using the BD IMagnet after incubation with Anti‐APC particles for 30 min at room temperature. To increase its purity, positive selection was repeated three times.
Antibodies
The monoclonal antibodies used for surface staining in this study included Brilliant Violet (BV)‐421‐labelled anti‐CD127 (clone 9HIL‐7R‐M21), BV‐650 labelled anti‐CD39 (clone TU66), BV605‐conjugated anti‐CD62L (clone DREG‐56) and Fixable Viability stain 510, all purchased from BD Biosciences. Alexa‐Fluor 700 labelled anti‐HLA‐DR (clone LN3), peridinin‐chlorophyll protein‐eFluor 710 conjugated anti‐CCR7 (clone 3D12) and FITC‐labelled anti‐CD27 (clone LG.7F9) were obtained from eBiosciences (San Diego, CA, USA). Phycoerythrin (PE) ‐conjugated anti‐CD25, PE‐cyanine 7 (PE‐Cy7) ‐labeled anti‐CD73 (clone AD2), PE‐Cy5‐labelled anti‐CD45RA (clone HI100) and allophycocyanin (APC) ‐labelled anti‐CD38 were provided by BD Pharmingen (San Diego, CA, USA). APC Vio770‐conjugated anti‐CD3 (clone BW 264/56) and PE‐Texas Red‐conjugated anti‐CD4 were obtained from Miltenyi Biotec and Beckman Coulter (Brea, Fullerton, CA, USA) respectively.
PE‐Cy7‐labelled anti‐FoxP3 (clone PCH101) was supplied by eBioscience and used for intracellular staining.
Optimal concentration was determined for each antibody by testing serial dilutions starting with the concentration recommended by the manufacturers.
Phenotypic characterization of Treg cells
Bulk PBMCs or sorted Treg cells were first incubated for 20 min at 4° in the dark, with 1% FcR blocking solution (Miltenyi Biotec) diluted in FACS buffer and then stained on the surface with a cocktail of fluorochrome‐labelled antibodies to CD3, CD4, CD25, CD127, HLA‐DR, CD38, CD45RA, CD27, CD39, CD73, CCR7, CD62L, CCR7, CD27 and Live/Dead. After incubation for 20 min at 4° in the dark, the cells were washed with FACS buffer, and then fixed and permeabilized with a FoxP3 fixation/permeabilization buffer (eBioscience) at 4° for 45 min in the dark. Cells were stained intracellularly with anti‐FoxP3 for 30 min at 4° protected from the light. Two rounds of cell washes with FoxP3 permeabilization buffer (eBioscience) were employed before and after intracellular labelling. Next, the cells were re‐suspended in FACS buffer and acquired on BD Fortessa X‐20 cytometer using bd facs diva software (BD Biosciences, San Jose, CA, USA). One thousand events were collected within a gate set on CD4+ CD127lo cells.
Statistical analysis
All flow cytometric data were analysed using flowjo software (Tree Star, Ashland , OR, USA) version 9·8·5 and statistical analyses were performed using graphpad prism version 5·0 (Graphpad, San Diego, CA, USA). The data were shown as median (25–75th percentile). Comparisons of medians among two groups were performed using the Mann–Whitney U‐test. A Kruskal–Wallis test with Dunn's multiple comparisons post‐test was used to test for differences between more than two groups. Correlations were made with Spearman test and P‐values < 0·05 were considered to be statistically significant.
Ethical considerations
This study received ethical approval from the Cameroon National Ethics Committee for Human Health Research. (Reference number No. 2015/03/569/CE/CNERSH/SP). All participants provided written informed consent. Data were processed using specific identifiers for privacy and confidentiality purposes. Clinical data generated during the course of this study were provided free of charge to all participants.
Results
Study population
A total of 48 participants (31 HIV+ and 17 HIV−) were included in this study. Most (71%) were women, aged 37 (32·50–42·50) and 34 (25–38·50) years for HIV+ and HIV− participants, respectively. HIV+ men were older than their sex‐matched HIV− controls [45 (37·50–46) versus 31 (28·50–36·50) years old; P < 0·05].20 As a consequence of ART‐naive HIV‐1 infection, median CD4 counts were lower in HIV+ participants than healthy controls: 436 cells/mm3 (278·5–587·5) versus 1050 cells/mm3 (745–1497) in men (P < 0·001) and 393 cells/mm3 (298·5–609) versus 899 cells/mm3 (675–1032) in women (P < 0·0001). Whereas the majority of HIV+ participants (55%) showed no significant immune suppression (CD4 > 500 cells/mm3), 29% had mild immunosuppression (350–499 cells/mm3) and 16% had advanced immunosuppression (200–349 cells/mm3). All these participants were in WHO clinical stage 1. Women showed a 0·68 Log lower plasmatic viral load than men (Table 1). The female domination of our participants is a reflection of the global prevalence of HIV‐1 infection within our sub region (UNAIDS, 2015)
Table 1.
Study population
| Variable | HIV‐negative participants (n = 17) | HIV‐1‐infected participants (n = 31) | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Participants (%) | 5 (30) | 12 (70) | 9 (29) | 22 (71) |
| Median age (IQR) | 31 (28·50–36·50)* | 34 (25–38·50) | 45 (37·50–46) | 37 (32·50–42·50) |
| Median CD4 count (cells/mm3) | 1050 (745–1497) | 899 (675–1032) | 436 (278·5–587,5)** | 393 (298·5–609)*** |
| CD4 > 500 cells/mm3 (n/%) | 4 (13) | 13 (42) | ||
| 350–499 cells/mm3 (n/%) | 4 (13) | 5 (16) | ||
| 200–349 cells/mm3 (n/%) | 1(3) | 4 (13) | ||
| Median viral load (Log10 copies/ml) | NA | NA | 4·66 (3·31–5·63) | 3·98 (2·56–5·04) |
*P < 0·05; **P < 0·001; ***P < 0·0001; NA, not applicable; IQR, interquartile range; n = number of participants.
A total of 31 HIV‐positive participants were recruited for this study with 71% being female and the rest (29%) male. The median ages were 37 (32·50–42·50) years for women and 45 (37·50–46) years for men. Seventeen HIV‐negative participants were recruited as controls. Relative to the controls, there was a significant reduction (P < 0·001 for men and P < 0·0001 for women) of helper CD4 counts as a consequence of antiretroviral naive HIV‐1 infection. Whereas a most participants (55%) showed no significant immune suppression (CD4 > 500 cells/mm3), 29% had mild immunosuppression (350–499 cells/mm3) and 16% had advanced immunosuppression (200–349 cells/mm3). Comparatively, female participants showed a 0·68 Log lower plasmatic viral load than male participants. All participants were in WHO clinical stage 1.
Tracking FoxP3 expressing cells using a combination of CD25+ CD127lo markers in magnetically sorted Treg cells from participant's PBMCs
Regulatory T cells were defined as CD4+ CD127lo CD25+ FoxP3+ cells.21 They were analysed using the gating strategy shown in Fig. 1a. To determine their total number in ART‐naive HIV‐infected participants, we did a FoxP3 staining either in bulk PBMCs or in magnetically sorted Treg cells. As shown in Fig. 1(b,c) and in the Supplementary material (Figure S1), previous purification of Treg cells resulted in higher numbers of CD4+ CD127lo CD25+ cells [852 cells/mm3 (690–908)] relative to partially purified samples [364 cells/mm3 (259–672); P < 0·001] and analysis with bulk PBMCs [320 cells/mm3 (173–514); P < 0·0001]. This was also reflected in total FoxP3 expressing cells as a fully purified group showed the highest numbers of FoxP3 expressing Treg cells [591 cells/mm3 (415–636)] followed by partially purified samples [214 cells/mm3 (126–401); P < 0·001] and lastly by bulk PBMCs [138 cells/mm3 (77–223); P < 0·0001]. A similar trend was also observed in HIV‐negative controls (compare Fig. 1b,c). Next, we assessed the relationship between the surface expression of a combination of CD25 and CD127 and the transcription factor, FoxP3 which is the signature marker of Treg cells.14 To achieve this, we plotted the number of CD4+ T cells expressing a combination of CD25+ and CD127lo against the number of cells positive for FoxP3. Using Spearman correlation analysis, we could demonstrate a positive correlation between CD25+ CD127lo and FoxP3 expression in all the categories listed above. This correlation was determined for PBMCs (r = 0·94; P < 0·0001; Fig. 1d), partially purified Treg cells (r = 0·86; P < 0·0001; Fig. 1e) and fully purified Treg cells (r = 0·71; P < 0·0002; Fig. 1f).
Figure 1.
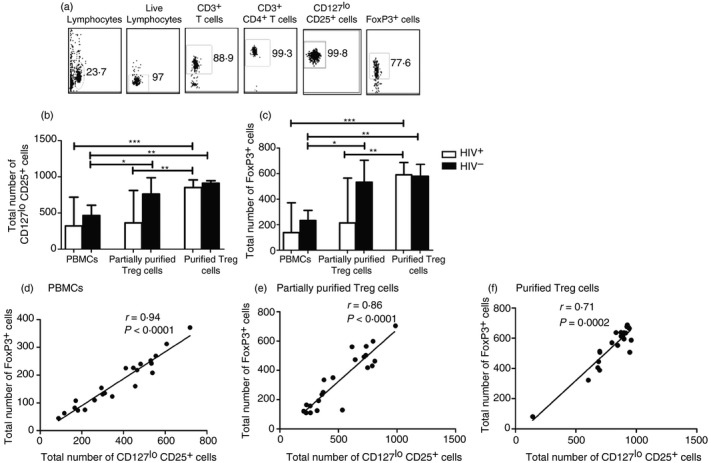
Tracking FoxP3‐expressing cells using a combination of CD25+ CD127lo markers in either magnetically sorted regulatory T (Treg) cells or bulk peripheral blood mononuclear cells (PBMC). (a) Representative dot plots of the gating strategy for magnetically purified Treg cells. Following identification of lymphocytes based on forward and side scatter, CD3+ CD4+ T cells were selected from live lymphocytes and CD127lo T cells were gated from this population; 1000 events were collected from the CD127lo gate to detect cells that were positive for CD25 and FoxP3 expression. (b, c) Magnetic sorting of Treg cells resulted in a significant increase in the desired population in both HIV + (n = 16) and HIV – (n = 6) participants. (d–f) There was a positive correlation between CD127lo CD25+ and FoxP3+ irrespective of whether staining was done with bulk PBMCs (r = 0·94; P < 0·0001; d), partially purified Treg cells (r = 0·86; P < 0·0001; e) or fully purified Treg cells (r = 0·71; P = 0·0002; f) Horizontal bars represent the median. Values were compared using Kruskal–Wallis test followed by Dunn's multiple comparison test.(*P <0·05; **P <0·001; ***P <0·0001) Each dot represents a single individual and correlation was assessed using Spearman test.
Due to this strong correlation we therefore selected CD4+ CD25+ CD127lo expression for subsequent tracking of Treg cell phenotypes.
Relationship between helper CD4+ T‐cell count and Treg cells
CD4+ T cells are the primary target of HIV‐1 and the depletion of helper CD4+ T cells is a hallmark of HIV‐1 disease progression. As Treg cells are a subpopulation of CD4+ T cells, we assessed whether Treg cell numbers are affected by HIV infection by examining the relationship between helper CD4+ T‐cell count and either total Treg cell numbers or Treg cell frequencies. As shown in Fig. 2(a), Treg cell numbers declined proportionately with a reduction in helper CD4+ T‐cell counts. A significant reduction was observed in ART‐naive HIV‐1‐infected participants with helper CD4+ T‐cell counts < 350 cells/mm3 (72 cells/mm3; 66–147; P < 0·05). In contrast, Treg cell frequencies increased as helper CD4+ T‐cell counts decreased (Fig. 2b). The highest Treg cell frequencies were observed in samples from ART‐naive HIV‐1‐infected participants with helper CD4+ T‐cell counts < 350 cells/mm3 (5%; 4·44–6·89) comparative to samples with helper CD4+ T‐cell counts between 350 and 499 cells/mm3 (1·84%; 1·17–3·33; P < 0·05) and to those with helper CD4+ T‐cell counts ≥500 cells/mm3 (1·48%; 1·23–2·3; P < 0,001). In addition, helper CD4+ T‐cell counts correlated positively with total Treg cell numbers (r = 0·60, P = 0·0005; Fig. 2c) and negatively with Treg cell frequencies (r = −0·58, P = 0·0009; Fig. 2d) using the Spearman test.
Figure 2.

Relationship between helper CD4+ T‐cell count and regulatory T (Treg) cells. HIV‐infected participants were grouped into three categories according to their helper CD4+ T‐cell counts [CD4 < 350 cells/mm3 (n = 5); 350–499 cells/mm3 (n = 9); >500 cells/mm3 (n = 17)]. When compared with uninfected participants (n = 17), the median number of Treg cells diminished proportionately with the helper CD4+ T‐cell count (a). The lowest values were observed in HIV‐infected participants with CD4 < 350 cells/mm3 (P < 0·05). However, this group showed significant increase in Treg cell percentages within total CD4+ T cells compared with other groups (b) with helper CD4+ T‐cell count between 350 and 499 cells/mm3 (P < 0·05) or > 500 cells/mm3 (P < 0·001). (c, d) Helper CD4+ T‐cell count of HIV‐infected participants correlated positively with the total number of Treg cells and negatively with the percentages of Treg cells. Bars represent the median values, which were compared using Kruskal–Wallis test followed by Dunn's multiple comparison test. (*P <0·05; **P <0·001) Each dot represents a single individual and the correlation was calculated using Spearman test.
HIV infection modulates Treg cell frequencies and numbers
We further evaluated the relationship between plasmatic HIV‐1 viral loads and the total number of Treg cells on the one hand and the proportions of Treg cells on the other. Our results indicated that plasmatic HIV‐1 viral loads correlated negatively with the total number of Treg cells (r = −0·51; P = 0·011; Fig. 3a,c) and positively with Treg cell frequencies (r = 0·55; P = 0·007; Fig. 3b,d).
Figure 3.
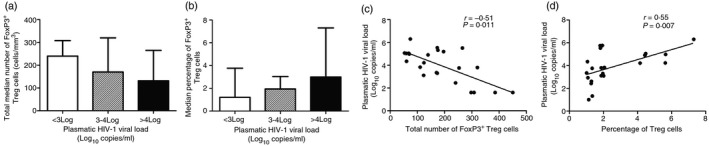
HIV infection modulates regulatory T (Treg) cell frequencies and numbers. HIV‐infected participants were divided into three groups according to their plasmatic HIV viral loads [VL< 3 Log copies/ml (n = 8); 3–4 (n = 8); > 4 (n = 12)]. The total number of Treg cells decreased when the plasmatic HIV viral load increased (a), whereas an inverse tendency was observed when Treg cell frequencies were considered (b) although the differences were not significant using Kruskal–Wallis test. However, plasmatic HIV viral load correlated negatively with total Treg cell numbers (r = −0·51; P = 0·011; c) and positively with Treg cell frequencies (r = 0·55; P = 0·007; d) using Spearman test.
Our data indicate that the Treg cell frequencies within total CD4+ T cells could be relevant in predicting the degree of CD4+ T‐cell depletion in ART‐naive HIV‐infected people.
Identification of Treg cell subsets in ART‐naive HIV infection using CD45RA, CD27, CD62L and CCR7 cell surface markers
Using multi‐parametric FACS analysis, Treg cells from peripheral blood of study participants displayed a heterogeneous expression profile relative to CD45RA, CD27, CD62L and CCR7 cell surface markers (Fig. 4b,c). However, we focused on CD45RA+ CD27+ CCR7+ CD62L+, CD45RA+ CD27− CCR7− CD62L−, CD45RA− CD27+ CCR7+ CD62L+ and CD45RA− CD27− CCR7− CD62L−, phenotypes representing naive, effector, central memory and effector memory subsets respectively (Fig. 4a). As shown in Fig. 4(b,c), whereas uninfected people displayed predominantly naive (P < 0·001) and central memory (P < 0·05) phenotypes, HIV‐infected participants showed significantly elevated numbers of effector (P < 0·001) and effector memory (P < 0·05) Treg cell subsets.
Figure 4.
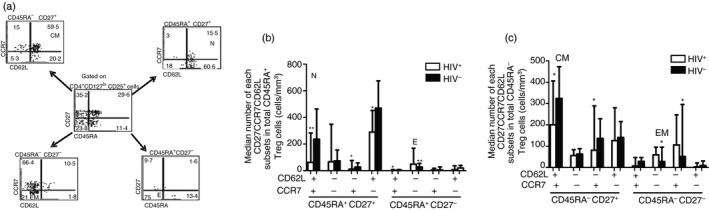
Identification of regulatory T (Treg) cell subsets in antiretroviral‐naive HIV infection using CD45RA, CD27, CD62L and CCR7 cell surface markers. (a) Representative dot plots of the gating strategy of different Treg cell subsets. Purified Treg cells obtained from peripheral blood mononuclear cells (PBMCs) of HIV + (n = 31) and HIV – (n = 17) participants were stained using monoclonal antibodies specific for CD3, CD4, CD25, CD127, CD45RA, CD27, CD62L and CCR7 and then analysed by multiparametric flow cytometry. Dot plots showing co‐expression of both CD27 and CD45RA on CD4+ CD25+ CD127lo Treg cells are represented in the middle panel. The data on CCR7 and CD62L are shown in each CD27 CD45RA subset. Distribution of Treg cell subsets analysed on CD45RA + or CD45RA – Treg cells are shown in (b) and (c), respectively. Whereas seronegative people displayed predominantly naive (P < 0·05) and central memory (P < 0·001) phenotypes, antiretroviral therapy‐naive HIV‐infected participants showed significantly elevated numbers of effector (P < 0·001) and effector memory (P < 0·05) Treg cell subsets. Bars represent the median and Mann–Whitney U‐test was used to compare each subset between HIV‐infected and uninfected controls. *P<0·05; **P<0·001.
ART‐naive HIV‐infection reduces CD39 and CD73 expression in naive and central memory Treg cells
CD39, a member of the ectonucleoside triphosphate diphosphohydrolases, is strongly associated with suppressive immunomodulatory functions by hydrolysing the pro‐inflammatory ATP to AMP, which in turn is converted to adenosine by the ecto‐5′‐nucleotidase CD73.18 Here we showed a significant increase in the number of effector and effector memory Treg cells from ART‐naive HIV‐infected participants expressing CD39 relative to seronegative controls [24 (11–26·75) versus 13 (8–17) cells/mm3 and 11 (5·5–31·50) versus 3 (0·25–8·75) cells/mm3; P < 0·05, respectively]. A similar trend was observed in CD73 expression [18 (15·5–33·5) versus 11 (7–18) cells/mm3 and 24 (17·25–31) versus 8 (6–16·50) cells/mm3; P < 0·001, respectively, for effector and effector memory Treg cells]. In contrast, naive and central memory Treg cells from uninfected people displayed increased expression of both CD39 and CD73 (P < 0·0001; Fig. 5f). The same tendency was observed when the expression was analysed in mean fluorescence intensity (MFI). The MFI of both CD39 and CD73 are shown in the Supplementary material (Figure S2). As Treg cells in seronegative participants are dominated by naive and central memory phenotypes, the reduction in the expression of these markers in ART‐naive HIV‐infected people could be relevant in Treg cell function impairment.
Figure 5.
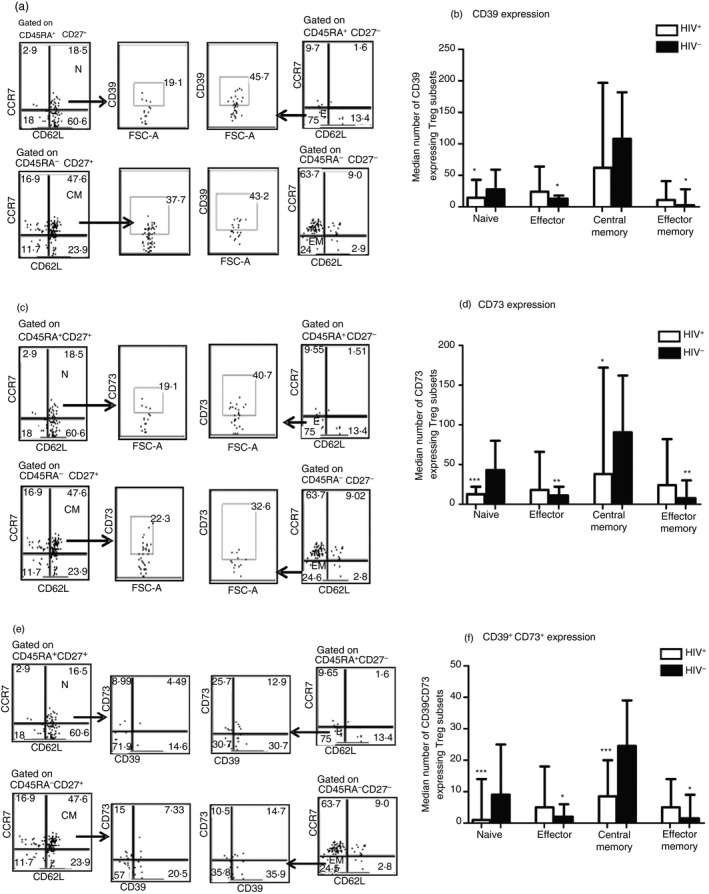
Antiretroviral therapy‐naive HIV‐infection reduces CD39 and CD73 expression in naive and central memory regulatory T (Treg) cells. (a, c, e) Representative dot plots illustrating the gating strategy of CD39 and CD73 expression. The median numbers of each Treg subset expressing CD39, CD73 or both markers are shown in (b), (d) and (f), respectively. The differences between HIV + and HIV – participants were calculated using Mann–Whitney U‐test. Effector and effector memory Treg cells from antiretroviral therapy‐naive HIV‐infected participants showed significant increase in CD39 (P < 0·05) and CD73 (P < 0·001) expression. In contrast, these markers were highly expressed instead on naive (P < 0·05 and P < 0·0001 respectively for CD39 and CD73) and central memory (P < 0·05 for CD73) Treg cells from uninfected individuals. A similar trend was observed for the combined expression of both CD39 and CD73. *P <0·05, **P <0·001, ****P <0·0001.
Expression of HLA‐DR and CD38 on Treg cell subsets
The activation status of Treg cells from ART‐naive HIV‐infected participants was assessed following HLA‐DR and CD38 expression analysis in different Treg cell subsets (Fig. 6). In HIV‐infected participants, HLA‐DR expression was significantly higher in effector [28 (19·75–34·50) cells/mm3] and effector memory [46 (38·50–62·75) cells/mm3] Treg cells relative to seronegative controls [16 (12·75–19·25) cells/mm3] and [16 (11–18) cells/mm3] respectively (P < 0·001). A similar trend was observed in CD38 expression [37 (34–67·5) versus 20 (15·75–27) cells/mm3 and 48 (46–58) versus 21 (18–31) cells/mm3; P < 0·001 respectively for effector and effector memory Treg cells]. When seronegative participants are considered, naive Treg cells were more activated (P < 0·05, Mann–Whitney test). The same tendency was observed when the expression was analysed in MFI as shown in the Supplementary material (Figure S3).
Figure 6.

Expression of HLA‐DR and CD38 on regulatory T (Treg) cell subsets. In (a) the gating strategy for HLA‐DR Treg cell surface expression is shown. The median values of HLA‐DR and CD38 are represented as bars with range (b and c). The differences in HLA‐DR/CD38 expression for each Treg subset was assessed between HIV + and HIV – participants using Mann–Whitney U‐test (*P <0·05; **P <0·001). While naive Treg cells from uninfected people displayed a significant increase in the expression of HLA‐DR/CD38 (P < 0·05), as shown in (b) and (c), these markers were highly expressed by effector and effector memory Treg cells from antiretroviral therapy‐naive HIV‐infected participants (P < 0·001).
Discussion
The present study was designed to determine phenotypic features of Treg cells in the context of ART‐naive HIV infection for possible application in optimizing Treg cell targeted immunotherapeutic strategies. The choice of ART‐naive HIV infection becomes expedient because it represents the most appropriate situation where the interaction between HIV and the immune system can be explored without external confounding factors. The CIRCB AFRODEC cohort of ART‐naive HIV‐infected people has been in existence since 2011, permitting us to go back in time and correlate virological and immunological parameters with purified Treg cell phenotypes. To date CD4, CD127, CD25 and FoxP3 represent the most used markers for Treg cell identification.15, 21 In previous studies the combination of CD25+ CD127lo/− expression on CD3+ CD4+ T cells has been used in place of CD3+ CD4+ CD25+ FoxP3+ as an alternative phenotype to track and characterize Treg cells.16, 22 Therefore, we used this approach to track FoxP3 expression in purified Treg cells from both ART‐naive HIV+ and HIV– people. There was a positive correlation between CD127lo CD25+ and FoxP3+ irrespective of whether staining was done with HIV+ or HIV– purified samples. Hence a positive correlation between CD127lo CD25+ and FoxP3+ was established either through the staining of bulk PBMCs (r = 0·94; P < 0·0001; Fig. 1d), partially purified (r = 0·86; P < 0·0001; Fig. 1e) or fully purified Treg cells (r = 0·71; P = 0·0002; Fig. 1f).16, 23 This allowed us to use CD4+ CD25+ CD127lo as an alternative to CD3+ CD4+ CD25+ FoxP3+ for tracking and phenotypic characterization of Treg cells of ART‐naive HIV‐infected people from the CIRCB AFRODEC cohort.
To understand the nature of Treg cells in freshly purified PBMCs we assessed the relationship between both total Treg cell numbers and Treg cell frequencies (i.e. Treg cell proportion in total CD4+ T cells) with respect to helper CD4+ T‐cell count and HIV plasmatic viral load as markers of disease progression. As reported in previous studies10, 15, 21, 24, 25 there was a proportionate reduction of Treg cell numbers vis‐à‐vis helper CD4+ T‐cell counts (r = 0·60, P = 0·0005). In contrast to plasmatic HIV load, which increased inversely with Treg cell numbers, here significantly (P < 0·05) lower Treg cell numbers were observed in HIV‐infected participants with helper CD4+ T‐cell counts < 350 cells/mm3 [72 cells/mm3 (66–147)] compared with uninfected controls [216 cells/mm3 (179–296)]. This is probably due to exacerbated destruction of Treg cells alongside helper CD4+ T cells during ART‐naive HIV infection. Treg cells as a subset of CD4+ T cells have previously been demonstrated to express chemokine receptors CCR5 and CXCR4, which are required for HIV‐1 entry into host cells and are therefore potential targets for HIV‐1 infection and destruction.5, 26, 27, 28
Another probable scenario may be the recruitment of HIV‐specific Treg cells to sites of HIV infection and replication, such as the mucosa and lymph nodes.3, 17 However, when considered in terms of Treg cell frequencies within total CD4+ T cells there was a negative correlation between helper CD4+ T‐cell counts and Treg cell frequencies (r = −0·58, P = 0·0009) on the one hand and a positive correlation between plasmatic HIV‐1 viral loads and Treg cell frequencies (r = 0·55; P = 0·007; Fig. 3d) on the other hand in samples with advanced immuno‐depression (CD4 < 350 cells/mm3). This would imply that as HIV infection disease progresses the proportion of Treg cells within the total CD4+ T‐cell sub‐population seemed to increase in the periphery. These elevated peripheral Treg cell frequencies can result from the reported high rate of conversion of conventional CD4+ T cells into Treg cells during HIV‐1 infection. Moreover, such Treg cells in the context of HIV‐1 infection have also been shown to be significantly more proliferative than other memory CD4+ T‐cell subsets.17, 29 This coupled with the suggested low susceptibility of peripheral Treg cells to R5 viruses28 could account for the apparent resistance of Treg cells to destruction by the HIV‐1 in comparison with other CD4+ T‐cell subsets. In contrast to our data Gaardbo et al., reported similar Treg cell numbers and frequencies in HIV‐infected participants and healthy controls.30 The lack of difference between their study groups was probably due to similarity in their helper CD4+ T‐cell counts, which were all within the normal range (> 500 cells/mm3). Hence, whereas their study population was mainly people with no detectable immunosuppression by CDC categorization, the differences in Treg cell frequencies observed by our group were mainly in people with advanced immunosuppression. Increased in Treg cell frequencies have generally been reported in advanced immunosuppression which in essence could be an indication of Treg cell‐mediated suppression against CD4+ T cells.19 This is in contrast to a reduction in Treg cell numbers, which probably suggest a decrease in their suppression capacity on other immune cells. This assertion is supported by the fact that efficacious ART is generally accompanied by a progressive decrease of Treg cell frequencies to normal levels17, 31, 32, 33, 34 and a proportionate increase of Treg cell counts with CD4+ T‐cell counts.17, 31, 32, 33, 35 This implies that immunotherapeutic approaches targeting Treg cells during HIV‐1 infection should aim at reducing Treg cell frequencies in people with advanced immunosuppression, which in effect can diminish their immunosuppressive effect on CD4+ T cells.
When phenotypic properties of magnetically purified Treg cells were assessed using CD45RA, CD27, CD62L and CCR7 surface markers, we found a heterogeneous population of Treg cell subsets including naive (CD45RA+ CD27+ CCR7+ CD62L+), effector (CD45RA+ CD27– CCR7– CD62L–), central memory (CD45RA– CD27+ CCR7+ CD62L+) and effector memory (CD45RA– CD27– CCR7– CD62L–) phenotypes. Whereas uninfected people displayed predominantly naive (P < 0·001) and central memory (P < 0·05) phenotypes, HIV‐infected participants showed significantly elevated levels of effector (P < 0·001) and effector memory (P < 0·05) Treg cell subsets. To discriminate Treg cell subsets other groups have used CD45RA and CD27 expression levels.24, 36 One major limitation of this strategy is that without taking into consideration the chemokine receptor CCR7 and the l‐selectin receptor CD62L which are critical for T‐cell homing into lymphoid tissues; it is impossible to clearly delineate the various Treg cell subsets. By including CCR7 and CD62L for example we were able to extend the surface markers used for delineating the four groups listed above to four as against just CD45RA and CD27 previously reported by the groups mentioned above. By so doing we could also delineate certain populations (up to 16 Treg cell subsets, see Fig. 4b,c), which could be relevant in appreciating Treg cell phenotypes.
Following an in‐depth analysis of the levels of CD39, CD73, HLA‐DR and CD38 on the aforementioned four Treg cell subsets we found that effector and effector memory Treg cells in ART‐naive HIV‐infected participants expressed increased levels of CD39, CD73, HLA‐DR and CD38. These markers have been associated with disease progression or immune activation17, 37 and have also been shown to be involved in the suppression of HIV‐specific responses and/or in Treg cell survival in the inflammatory environment created by HIV infection.15, 19 ART‐naive HIV infection drives persistent immune activation, which is directly linked with CD4+ T‐cell depletion and disease progression.38, 39 In this context the role of Treg cells in limiting HIV‐1‐mediated persistent immune activation is conflicting because by limiting immune activation they also invariably would inhibit HIV‐1‐specific immune responses. Nevertheless, low immune activation has been associated with slower disease progression40 but the general consensus remains that sustained immune activation accelerates disease progression irrespective of viral load.39 On the contrary Gaardbo et al., recently suggested increased activation of Treg cells to be relevant in preserving CD4+ T cells in long‐term non‐progressors and elite controllers. This is probably true in circumstances where there is no significant immunosuppression. However, the differences observed between Treg cell activation in elite controllers, long‐term non‐progressors and viral controllers may be because viral control is a transitory state. Viral controllers might ultimately become either long‐term non‐progressors or progressors following prolonged interaction with the immune system.
The functional characterization of the Treg cells is in progress.
In summary our work lays the foundation for an in‐depth analysis of Treg cells in ART‐naive HIV infection and could provide a framework for designing immunotherapeutic strategies to improve HIV infection prognosis by targeted modulation of Treg cell phenotypes and functions. Specifically, Treg cells are known to inhibit HIV‐specific immune responses, so when immunotherapeutic strategies are aimed at decreasing Treg cell frequencies, this could result in a reduction in their immunosuppressive functions.
Author Contributions
NWG conceived and designed the experiments; ANG performed the experiments; technical assistance was provided by NNL, NNN, SNC, LA, TTF, TJ, SM and NEC; ANG and NWN analysed the data; and ANG, NWN, NEC and EF wrote the paper.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 Tracking FoxP3‐expressing cells using a combination of CD25+ CD127lo markers in either magnetically sorted regulatory Tcells or bulk peripheral blood mononuclear cells.
Figure S2 Antiretroviral therapy‐naive HIV infection increases CD39 and CD73 expression in effector and effector memory regulatory T cells.
Figure S3 Antiretroviral therapy‐naive HIV infection increases activation of effector and effector memory regulatory T cells.
Acknowledgements
We thank all participants who consented to participate in this study. We would like to thank the personnel of unites techniques of CIRCB for their help in collecting the blood samples. This project was funded by CIRCB, EDCTP, TWAS, Canada grand challenge and African Korean collaborative grant.
References
- 1. Paiardini M, Muller‐Trutwin M. HIV‐associated chronic immune activation. Immunol Rev 2013; 254:78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor α‐chains (CD25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J Immunol 1995; 155:1151–64. [PubMed] [Google Scholar]
- 3. Keynan Y, Card CM, McLaren PJ, Dawood MR, Kasper K, Fowke KR. The role of regulatory T cells in chronic and acute viral infections. Clin Infect Dis 2008; 46:1046–52. [DOI] [PubMed] [Google Scholar]
- 4. Sakaguchi S, Sakaguchi N. Regulatory T cells in immunologic self‐tolerance and autoimmune disease. Int Rev Immunol 2005; 24:211–26. [DOI] [PubMed] [Google Scholar]
- 5. Pion M, Jaramillo‐Ruiz D, Martinez A, Munoz‐Fernandez MA, Correa‐Rocha R. HIV infection of human regulatory T cells downregulates Foxp3 expression by increasing DNMT3b levels and DNA methylation in the FOXP3 gene. AIDS 2013; 27:2019–29. [DOI] [PubMed] [Google Scholar]
- 6. O'Brien M, Manches O, Bhardwaj N. Plasmacytoid dendritic cells in HIV infection. Adv Exp Med Biol 2013; 762:71–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiss L, Donkova‐Petrini V, Caccavelli L, Balbo M, Carbonneil C, Levy Y. Human immunodeficiency virus‐driven expansion of CD4+ CD25+ regulatory T cells, which suppress HIV‐specific CD4 T‐cell responses in HIV‐infected patients. Blood 2004; 104:3249–56. [DOI] [PubMed] [Google Scholar]
- 8. Velavan TP, Ojurongbe O. Regulatory T cells and parasites. J Biomed Biotechnol 2011; 2011:520940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Angin M, King M, Altfeld M, Walker BD, Wucherpfennig KW, Addo MM. Identification of HIV‐1‐specific regulatory T‐cells using HLA class II tetramers. AIDS 2012; 26:2112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kinter AL, Hennessey M, Bell A, Kern S, Lin Y, Daucher M et al CD25+CD4+ regulatory T cells from the peripheral blood of asymptomatic HIV‐infected individuals regulate CD4+ and CD8+ HIV‐specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med 2004; 200:331–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suchard MS, Mayne E, Green VA, Shalekoff S, Donninger SL, Stevens WS et al FOXP3 expression is upregulated in CD4T cells in progressive HIV‐1 infection and is a marker of disease severity. PLoS One 2010; 5:e11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Macatangay BJ, Szajnik ME, Whiteside TL, Riddler SA, Rinaldo CR. Regulatory T cell suppression of Gag‐specific CD8 T cell polyfunctional response after therapeutic vaccination of HIV‐1‐infected patients on ART. PLoS One 2010; 5:e9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood 2012; 119:4430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol 2003; 4:330–6. [DOI] [PubMed] [Google Scholar]
- 15. Presicce P, Orsborn K, King E, Pratt J, Fichtenbaum CJ, Chougnet CA. Frequency of circulating regulatory T cells increases during chronic HIV infection and is largely controlled by highly active antiretroviral therapy. PLoS One 2011; 6:e28118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu W, Putnam AL, Xu‐Yu Z, Szot GL, Lee MR, Zhu S et al CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 2006; 203:1701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schulze Zur Wiesch J, Thomssen A, Hartjen P, Toth I, Lehmann C, Meyer‐Olson D et al Comprehensive analysis of frequency and phenotype of T regulatory cells in HIV infection: CD39 expression of FoxP3+ T regulatory cells correlates with progressive disease. J Virol 2011; 85:1287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A et al Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007; 204:1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simonetta F, Bourgeois C. CD4+ FOXP3+ regulatory T‐cell subsets in human immunodeficiency virus infection. Front Immunol 2013; 4:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gaardbo JC, Nielsen SD, Vedel SJ, Ersboll AK, Harritshoj L, Ryder LP et al Regulatory T cells in human immunodeficiency virus‐infected patients are elevated and independent of immunological and virological status, as well as initiation of highly active anti‐retroviral therapy. Clin Exp Immunol 2008; 154:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horta A, Nobrega C, Amorim‐Machado P, Coutinho‐Teixeira V, Barreira‐Silva P, Boavida S et al Poor immune reconstitution in HIV‐infected patients associates with high percentage of regulatory CD4+ T cells. PLoS One 2013; 8:e57336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seddiki N, Santner‐Nanan B, Martinson J, Zaunders J, Sasson S, Landay A et al Expression of interleukin (IL)‐2 and IL‐7 receptors discriminates between human regulatory and activated T cells. J Exp Med 2006; 203:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miyara M, Sakaguchi S. Human FoxP3+ CD4+ regulatory T cells: their knowns and unknowns. Immunol Cell Biol 2011; 89:346–51. [DOI] [PubMed] [Google Scholar]
- 24. Rallón NI, López M, Soriano V, García‐Samaniego J, Romero M, Labarga P et al Level, phenotype and activation status of CD4+ FoxP3+ regulatory T cells in patients chronically infected with human immunodeficiency virus and/or hepatitis C virus. Clin Exp Immunol 2008; 155:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li L, Liu Y, Bao Z, Chen L, Wang Z, Li T et al Analysis of CD4+ CD25+ Foxp3+ regulatory T cells in HIV‐exposed seronegative persons and HIV‐infected persons with different disease progressions. Viral Immunol 2011; 24:57–60. [DOI] [PubMed] [Google Scholar]
- 26. Antons AK, Wang R, Oswald‐Richter K, Tseng M, Arendt CW, Kalams SA et al Naive precursors of human regulatory T cells require FoxP3 for suppression and are susceptible to HIV infection. J Immunol 2008; 180:764–73. [DOI] [PubMed] [Google Scholar]
- 27. Cao W, Jamieson BD, Hultin LE, Hultin PM, Detels R. Regulatory T cell expansion and immune activation during untreated HIV type 1 infection are associated with disease progression. AIDS Res Hum Retroviruses 2009; 25:183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moreno‐Fernandez ME, Zapata W, Blackard JT, Franchini G, Chougnet CA. Human regulatory T cells are targets for human immunodeficiency virus (HIV) infection, and their susceptibility differs depending on the HIV type 1 strain. J Virol 2009; 83:12925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chachage M, Pollakis G, Kuffour EO, Haase K, Bauer A, Nadai Y et al CD25+ FoxP3+ memory CD4 T cells are frequent targets of HIV infection in vivo . J Virol 2016; 90:8954–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gaardbo JC, Ronit A, Hartling HJ, Gjerdrum LM, Springborg K, Ralfkiaer E et al Immunoregulatory T cells may be involved in preserving CD4 T cell counts in HIV‐infected long‐term nonprogressors and controllers. J Acquir Immune Defic Syndr 2014; 65:10–8. [DOI] [PubMed] [Google Scholar]
- 31. Bi X, Suzuki Y, Gatanaga H, Oka S. High frequency and proliferation of CD4+ FOXP3+ Treg in HIV‐1‐infected patients with low CD4 counts. Eur J Immunol 2009; 39:301–9. [DOI] [PubMed] [Google Scholar]
- 32. Jiao Y, Fu J, Xing S, Fu B, Zhang Z, Shi M et al The decrease of regulatory T cells correlates with excessive activation and apoptosis of CD8+ T cells in HIV‐1‐infected typical progressors, but not in long‐term non‐progressors. Immunology 2009; 128:e366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Montes M, Sanchez C, Lewis DE, Graviss EA, Seas C, Gotuzzo E et al Normalization of FoxP3+ regulatory T cells in response to effective antiretroviral therapy. J Infect Dis 2011; 203:496–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhuang Y, Wei X, Li Y, Zhao K, Zhang J, Kang W et al HCV coinfection does not alter the frequency of regulatory T cells or CD8+ T cell immune activation in chronically infected HIV+ Chinese subjects. AIDS Res Hum Retroviruses 2012; 28:1044–51. [DOI] [PubMed] [Google Scholar]
- 35. Angin M, Kwon DS, Streeck H, Wen F, King M, Rezai A et al Preserved function of regulatory T cells in chronic HIV‐1 infection despite decreased numbers in blood and tissue. J Infect Dis 2012; 205:1495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen T, Zheng J, Liang H, Xu C, Chen X, Zhang T et al Characteristics and PD‐1 expression of peripheral CD4+ CD127lo CD25hiFoxP3+ Treg cells in chronic HCV infected‐patients. Virol J 2011; 8:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xiao J, Qian KL, Cao QH, Qiu CL, Qiu C, Xue YL et al HLA‐DR expression on regulatory T cells is closely associated with the global immune activation in HIV‐1 infected subjects naive to antiretroviral therapy. Chin Med J (Engl) 2011; 124:2340–6. [PubMed] [Google Scholar]
- 38. Sousa AE, Carneiro J, Meier‐Schellersheim M, Grossman Z, Victorino RM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV‐1 and HIV‐2 but only indirectly to the viral load. J Immunol 2002; 169:3400–6. [DOI] [PubMed] [Google Scholar]
- 39. Chevalier MF, Weiss L. The split personality of regulatory T cells in HIV infection. Blood 2013; 121:29–37. [DOI] [PubMed] [Google Scholar]
- 40. Shaw JM, Hunt PW, Critchfield JW, McConnell DH, Garcia JC, Pollard RB et al Increased frequency of regulatory T cells accompanies increased immune activation in rectal mucosae of HIV‐positive noncontrollers. J Virol 2011; 85:11422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Tracking FoxP3‐expressing cells using a combination of CD25+ CD127lo markers in either magnetically sorted regulatory Tcells or bulk peripheral blood mononuclear cells.
Figure S2 Antiretroviral therapy‐naive HIV infection increases CD39 and CD73 expression in effector and effector memory regulatory T cells.
Figure S3 Antiretroviral therapy‐naive HIV infection increases activation of effector and effector memory regulatory T cells.


