Summary
Several host factors have been implicated in resistance to HIV infection in individuals who remain HIV‐seronegative despite exposure. In a cohort of HIV‐serodiscordant heterosexual couples, we investigated interactions between systemic inflammation and T‐cell activation in resistance to HIV infection. Males and females in stable long‐term relationships with either HIV‐infected or uninfected partners were recruited, blood T‐cell activation (CD38, HLA‐DR, CCR5 and Ki67) and plasma cytokine concentrations were evaluated. The HIV‐negative exposed individuals had significantly lower frequencies of CCR5+ CD4+ and CD8+ T cells than unexposed individuals. Mean fluorescence intensity of CCR5 expression on CD4+ T cells was significantly lower in HIV‐negative exposed than unexposed individuals. Protective CCR5 haplotypes (HHA/HHF*2, HHF*2/HHF*2, HHC/HHF*2, HHA/HHA, HHA/HHC and HHA/HHD) tended to be over‐represented in exposed compared with unexposed individuals (38% versus 28%, P = 0·58) whereas deleterious genotypes (HHC/HHD, HHC/HHE, HHD/HHE, HHD/HHD and HHE/HHE) were under‐represented (26% versus 44%; P = 0·16). Plasma concentrations of interleukin‐2 (P = 0·02), interferon‐γ (P = 0·05) and granulocyte–macrophage colony‐stimulating factor (P = 0·006) were lower in exposed compared with unexposed individuals. Activation marker expression and systemic cytokine concentrations were not influenced by gender. We conclude that the dominant signature of resistance to HIV infection in this cohort of exposed but uninfected individuals was lower T‐cell CCR5 expression and plasma cytokine concentrations.
Keywords: CCR5, HIV, immune activation, resistance
Abbreviation
- ACD
acid citrate dextrose
- AIDS
acquired immunodeficiency syndrome
- CCR5
C‐C chemokine receptor type 5
- CD
cluster of differentiation
- GM‐CSF
granulocyte‐macrophage colony‐stimulating factor
- HIV
human immunodeficiency virus
- IFN‐γ
interferon gamma
- IL‐2
interleukin‐2
- ORF
open reading frame
- PBMC
peripheral blood mononuclear cell
- PCR
polymerase chain reaction
- RANTES
Regulated on Activation, Normal T Cell Expressed and Secreted
- SNP
single nucleotide polymorphism
- TNF‐α
tumor necrosis factor‐alpha
- Treg
regulatory T cell
Introduction
Important insights into mechanisms underlying resistance to HIV infection have come from studies in exposed–uninfected individuals. Although HIV transmission is inefficient, substantial efforts have focused on identifying co‐factors enhancing infection and defining potential correlates of protection, relevant to the design of HIV prevention strategies. As such, key populations including commercial sex workers,1 HIV‐serodiscordant couples,2, 3, 4 injectable drug users,5 infants born to HIV‐infected mothers,6 occupational exposure in healthcare workers7 and men having sex with men8 have provided some important clues.
Although the mechanisms of protection from HIV infection in HIV‐exposed individuals have been investigated, they are likely to be multifactorial and remain largely undefined. Several host factors have been associated with protection against HIV infection in these individuals; such as: CCR5 co‐receptor susceptibility,9 certain innate and adaptive immune responses;10, 11 and cytokines (RANTES, secretory leucocyte protease inhibitor 1, macrophage inflammatory protein‐1α and macrophage inflammatory protein‐1β).12, 13 In addition, higher frequencies of HIV‐specific interferon‐γ (IFN‐γ) and interleukin‐2 (IL‐2) ‐secreting T cells were found in HIV‐exposed–uninfected individuals than unexposed individuals.14, 15
Lower levels of immune activation and immunological quiescence are strongly associated with resistance to HIV infection in exposed–uninfected individuals.16, 17, 18, 19 In addition, specific CCR5 genotypes have been associated with resistance/protection from HIV infection. This includes the CCR5Δ32 genotype, characterized by a 32‐bp deletion in the CCR5 open reading frame (ORF), directly impacting on both susceptibility to HIV‐1 infection and rate of disease progression.20, 21 CCR5 receptor density, linked to CCR5 genotype, exerts a direct influence on susceptibility to HIV infection.20, 22 In addition to CCR5Δ32 genotype, multiple other CCR5 ORF mutations, single nucleotide polymorphisms (SNPs) and/or haplotypes in the CCR5 regulatory/promoter region have been shown to be capable of influencing CCR5 receptor density; hence HIV susceptibility and rate of disease progression.23, 24, 25, 26
Card et al.27 suggested that lower levels of immune activation in individuals resistant to HIV infection were associated with higher frequencies of regulatory T (Treg) cells. Although lower levels of immune activation are associated with resistance to HIV infection, the mechanism for the long‐term maintenance of the low levels of immune activation has yet to be identified. In this study, we investigated the interaction between systemic inflammation (measured by soluble cytokine concentrations), immune activation of T cells and the influence of CCR5 genotype in resistance to HIV infection in HIV‐exposed but uninfected individuals in HIV serodiscordant relationships.
Materials and methods
Study participants
Two hundred and fifteen HIV‐negative black South African men and women who were in stable long‐term heterosexual relationships were recruited from the Empilisweni Clinic in Gugulethu, Cape Town.28, 29 HIV‐positive participants had to be naive to therapy to be eligible for enrolment. Of the couples enrolled, 48% (103/215) of couples included partners who were both HIV‐negative (unexposed) and 52% (112/215) included HIV serodiscordant partners where one was HIV‐positive (HIV‐exposed). The study was approved by the Faculty of Health Sciences Human Research Ethics Committee of the University of Cape Town and informed written consent was obtained from all individuals before enrolment.
Specimen collection and processing
Blood (16 ml) was collected by venepuncture into sterile ACD anti‐coagulated vacutainer tubes (BD Biosciences, Plymouth, UK) and processed within 4 hr of collection. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll‐Hypaque (Sigma‐Aldrich, St Louis, MO) density gradient centrifugation in Leucosep® tubes, and cryopreserved in liquid nitrogen. Plasma was split into aliquots and preserved at −80° for cytokine measurement.
Measurement of cytokine concentrations in plasma
The concentrations of IL‐1β, IL‐6, IL‐12p70, tumour necrosis factor‐α (TNF‐α), IL‐10, IL‐2, IFN‐γ, IL‐7 and granulocyte–macrophage colony‐stimulating factor (GM‐CSF) were measured in blood plasma using High Sensitivity Human Cytokine LINCOplex kits (sensitivity range: 0·01–0·48 pg/ml; LINCO Research, St Charles, MO). Interplate variation for cytokines was measured by the inclusion of duplicates for 76 samples, distributed across the seven plates assayed. Spearman rank correlation coefficients were used to assess degree of variation, with the least deviation seen for IL‐1β (R 2 = 0·96, P < 0·0001) and the most for IL‐12p70 (R 2 = 0·38, P = 0·0007; see Supplementary material, Table S1). Data were collected using a Bio‐Plex™ Suspension Array Reader (Bio‐Rad Laboratories Inc®, Minneapolis, MN) and a 5 PL regression formula was used to calculate cytokine concentrations from the standard curves. Data were analysed using BIO‐plex manager software (version 4; Bio‐Rad Laboratories Inc.). Cytokine concentrations that were below the detection limit of the assay were reported as the mid‐point between the lowest concentration measured for each cytokine and zero.
Staining for markers of T‐cell activation by flow cytometry
Thawed PBMCs from a convenient subset of 38/103 HIV‐unexposed and 30/112 HIV‐exposed–uninfected individuals were stained and 1 million PBMCs were used per staining reaction per participant.30 PBMCs were incubated with LIVE/DEAD® Fixable Violet Dead Cell Stain for 20 min at room temperature, then washed with 1% fetal calf serum (FCS) ‐supplemented PBS. The pelleted cells were resuspended in a dead volume and stained for 30 min at room temperature with phenotypic marker peridinin chlorophyll protein‐Cy5.5 labelled anti‐CD4 (Clone SK7; BD Pharmingen, San Jose, CA), QDot605‐labelled anti‐CD8 (Clone 3B5; Invitrogen, Carlsbad, CA), allophycocyanin‐labelled anti‐CD195/CCR5 (Clone 2D7; BD Biosciences, San Jose, CA), phycoerythrin‐Cy7‐labelled anti‐CD38 (Clone HB7; BD Biosciences), phycoerythrin‐labelled anti‐HLA‐DR (Clone L243; BD Biosciences), and finally Pacific Blue‐labelled anti‐CD14 (Clone M5E2; BD Pharmingen) and anti‐CD19 (Clone SJ25‐C1; Invitrogen). Anti‐CD14 and anti‐CD19 antibodies were included as dump markers to exclude monocytes and B cells from analysis, respectively. Cells were then washed twice with 1% FCS‐supplemented PBS, fixed and permeabilized with BD CytoPerm/CytoFix (BD Biosciences) for 20 min at room temperature. Cells were washed with Perm/Wash buffer (BD Biosciences) and stained intracellularly with allophycocyanin‐H7‐labelled anti‐CD3 (Clone SK7; BD Biosciences) and FITC‐labelled anti‐Ki67 (BD Biosciences; Clone B56). Cells were washed and fixed with BD Cell Fix (BD Biosciences). Cell fluorescence was assessed using a BD LSR Fortessa flow cytometer (BD Immunocytometry Systems, San Jose, CA). Fluorescence minus one was used to distinguish continuous populations. Compensation and analysis of data were performed using flowjo software (Tree Star, Ashland, OR). For the gating strategy: cell doublets/aggregates were removed by gating on singlets. Live CD3+ T‐cell populations were differentiated into CD4+ and CD8+ T‐cell subsets. Overall expression frequencies of activation markers as well as those of the permutations of expression that contributed to that frequency were then evaluated for CD4+ and CD8+, respectively.
CCR5 genotyping and haplotype assignment
Individuals were genotyped as described previously.31 Genomic DNA was extracted from PBMC samples; a continuous region encompassing the CCR5 ORF and the promoter 1 region was PCR amplified in overlapping sections using Expand High Fidelity PCR System (Roche, Mannheim, Germany) and sequenced using BigDye Terminator version 3·1 chemistry (Applied Biosystems, Foster City, CA). Sequenced fragments were electrophoresed using the automated 3100 Genetic Analyzer (Applied Biosystems) and HAPLOTYPER software was used to infer haplotypes.32
CCR5 ‐4223 C/T SNP genotyping
A real‐time SYBR green CT (cycle threshold)‐shift assay was designed to genotype the CCR5 ‐4223 C/T SNP reported to disrupt the CpG ‐41 site.19 Primer sequences were as follows (square brackets denote lock nucleic acid modified nucleotides), C allele‐specific reverse primer: 5ˈ‐CCATTTCCTCATCTGTTAAATGAC[G]‐3ˈ; T allele‐specific reverse primer: 5ˈ‐CCATTTCCTCATCTGTTAAATGAC[A]‐3ˈ; common forward primer: 5ˈ‐GTGGAGTAACGCACACTGCAA‐3ˈ. Hence, two PCR were conducted per sample, one with each allele‐specific primer. PCR were run in an Applied Biosystems 7500 Real‐Time PCR system. To analyse the PCR data, the difference in CT of the two reactions was calculated, in a heterozygous individual, both PCR should amplify similarly with minimal CT difference (fewer than two cycles in this assay) and in homozygous wild‐type (C allele) individuals a CT difference of eight or more cycles was consistently attained. Homozygosity for the mutant allele (T) was not observed.
Statistical analysis
Comparison of unpaired responses was performed using the Mann–Whitney U‐test. Statistical inferences on binary outcomes were performed using the Fisher's exact test. All tests were two‐tailed and P‐values of ≤ 0·05 were considered significant. Adjustment for multiple comparisons was performed using a false discovery rate step‐down approach. Statistical analyses were performed using graphpad prism version 5·0 for Windows (GraphPad Software, San Diego, CA) and stata™ (version 11, StataCorp, College Station, TX).
Result
Two hundred and fifteen HIV‐negative men and women and their long‐term heterosexual partners were included to investigate the role of sexual partner HIV status on systemic immune activation, CCR5 haplotype and expression, and inflammation in South African individuals, as potential correlates of HIV risk or protection (Table 1). Of these, 48% (103/215) were HIV‐negative unexposed (stable partners also HIV‐negative) and 52% (112/215) were HIV‐negative but exposed to HIV (partners were HIV‐positive). All couples were black South African isiXhosa speaking individuals. HIV‐negative exposed individuals were more likely to be men (73% versus 50%; P = 0·001) in this cohort and were slightly older than HIV‐unexposed individuals (40 versus 36; P = 0·01; Table 1). The age difference is expected because significantly more men were enrolled in the exposed group, and overall men were older than women (40 versus 36 years; P = 0·02). In addition, HIV‐negative exposed individuals reported having had a longer sexual history than their HIV‐negative unexposed counterparts (21 versus 19 years; P = 0·01; Table 1) and, although age at sexual debut did not differ significantly between the groups, exposed individuals did tend to have initiated sexual activity at a younger age (17 versus 18 years; P = 0·20; Table 1). HIV‐unexposed individuals reported higher frequencies of sex acts in the last month and lower frequencies of condom use than HIV‐exposed individuals. The significantly higher condom usage among discordant couples was not surprising as couple‐based counselling would have informed these participants about risk‐reducing behaviours. CD4 percentages, proportion of individuals cohabiting, or having either ulceration or discharge were similar in HIV‐negative exposed and unexposed individuals (Table 1). All the men included in this study had undergone traditional isiXhosa circumcision.
Table 1.
Clinical and socio‐behavioural characteristics of participants
| Characteristics | Unexposed | Exposed | P‐value |
|---|---|---|---|
| Male [% (n/N)] | 49 (50/103) | 65 (73/112) | 0·001 |
| Age [year; median (IQR)] | 36 (29–43) | 40 (33–48) | 0·01 |
| Living together with partner [% (n/N)] | 72 (74/103) | 56 (63/112) | 0·2 |
| Age at first sex [median (IQR)] | 18 (16–18) | 17 (15–18) | 0·2 |
| Sexual exposure [median years of sex (IQR)] | 19 (12–26) | 21 (17–30) | 0·01 |
| Blood CD4% [median (IQR)] | 71 (59–86) | 74 (61–81) | 0·9 |
| Sex acts in the last month [median (IQR)] | 4 (3–8) | 3 (2–5) | 0·004 |
| Condom usage [% (n/N)] | 29 (27/92a) | 75 (77/102a) | < 0·0001 |
| Genital ulceration in the last 6 months [% (n/N)] | 2 (2/93a) | 5 (5/1111) | 0·5 |
| Vaginal discharge in the last 6 months [% (n/N)] | 6 (3/511) | 10 (3/30a) | 0·5 |
Number of responses for each characteristic varied based on availability of data in participants’ folders and samples in the repository.
IQR, Interquartile range.
Partner HIV status and systemic cytokines
The concentrations of TNF‐α, IL‐β, IL‐6, IL‐10, IL‐7, GM‐CSF, IL‐12p70, IL‐2 and IFN‐γ were measured in plasma from HIV‐negative exposed and unexposed individuals (Table 2). Concentrations of adaptive cytokines IL‐2 (P = 0·02) and IFN‐γ (P = 0·05), and haematopoietic GM‐CSF (P = 0·006) were significantly lower in exposed compared with unexposed individuals. Differences in GM‐CSF remained significant after adjustment for multiple comparisons. No significant differences were observed in inflammatory cytokines (IL‐β, IL‐6, IL‐12p70, TNF‐α) between the groups. Although exposed individuals were more likely to be men, gender did not significantly influence concentrations of systemic cytokines (Table 2).
Table 2.
Impact of partner HIV status and gender on plasma cytokine concentrations
| Function | Cytokine | Median cytokine conc (IQR; pg/ml) | P‐value | Median cytokine conc (IQR; pg/ml) | P‐value | ||
|---|---|---|---|---|---|---|---|
| Unexposed (n = 103) | Exposed (n = 112) | Male (n = 123) | Female (n = 92) | ||||
| Inflammatory | IL‐β | 0·45 (0·07–1·43) | 0·35 (0·04–1·25) | 0·5 | 0·31 (0·028–1·18) | 0·50 (0·10–1·50) | 0·82 |
| IL‐6 | 4·66 (2·36–7·87) | 3·90 (1·75–7·50) | 0·2 | 3·77 (1·81–8·01) | 4·66 (2·75–7·04) | 0·90 | |
| IL‐12p70 | 0·005 (0·005–0·83) | 0·005 (0·005–0·58) | 0·8 | 0·005 (0·005–0·50) | 0·005 (0·005–1·15) | 0·44 | |
| TNF‐α | 6·00 (3·94–8·14) | 5·85 (4·02–7·78) | 0·7 | 6·20 (4·08–8·06) | 5·32 (3·95–7·57) | 0·07 | |
| Regulatory | IL‐10 | 10·43 (5·60–22·26) | 9·08 (5·88–17·95) | 0·7 | 9·68 (5·87–18·25) | 10·01 (5·75–22·41) | 0·55 |
| Adaptive | IL‐2 | 0·28 (0·005–1·03) | 0·008 (0·005–0·69) | 0·02 | 0·03 (0·005–0·67) | 0·32 (0·005–1·31) | 0·97 |
| IFN‐γ | 0·94 (0·14–2·91) | 0·64 (0·02–1·93) | 0·05 | 0·64 (0·02–2·19) | 0·83 (0·16–2·59) | 0·21 | |
| Haematopoietic | IL‐7 | 1·86 (0·68–3·49) | 1·51 (0·69–3·46) | 0·7 | 1·49 (0·68–3·13) | 2·00 (0·71–3·80) | 0·54 |
| GM‐CSF | 0·64 (0·20–1·46) | 0·32 (0·12–0·82) | 0·006 | 0·35 (0·098–0·82) | 0·70 (0·21–1·47) | 0·14 | |
Mann–Whitney U‐tests were applied to compare cytokine concentrations between groups.
More than half (57%) of the HIV‐positive partners in this study had plasma HIV loads > 1500 cps/ml (data not shown), and so would be considered ‘infectious’ to their HIV‐negative partners, according to the estimates published by Quinn et al.33 Several plasma cytokines were positively associated with male partners’ plasma HIV loads [IFN‐γ (r = 0·44, P = 0·018), IL‐2 (r = 0·43, P = 0·021), IL‐6 (r = 0·44, P = 0·018), IL‐7 (r = 0·56, P = 0·002), IL‐12p70 (r = 0·39, P = 0·041) and GM‐CSF (r = 0·41, P = 0·29)].
Partner HIV status and T‐cell activation
CCR5, CD38, HLA‐DR and Ki67 expression by CD4+ and CD8+ T cells were compared individually or in biologically relevant combinations [HLA‐DR+ CD38+ (representing highly activated T cells), Ki67+ CCR5+ (representing proliferating T cells, which may be susceptible to HIV infection), CD38+ CCR5+ (representing susceptible, activated T cells) and CD38+ Ki67+ (representing activated, proliferating T‐cells)] in exposed versus unexposed individuals to investigate the relationship between partner status and activation of T cells in HIV‐negative individuals (Fig. 1a–d). HIV‐exposed individuals had significantly lower frequencies of CD4+ T cells expressing the HIV co‐receptor CCR5, alone or in combination with Ki67 or CD38, than unexposed individuals (P = 0·007 for CCR5 alone, P = 0·001 for CCR5+ Ki67+ and P = 0·005 for CCR5+ CD38+; Fig. 1a,c). All of these comparisons remained significantly different after adjusting for the potential contributions of confounding variables age, gender and condom use. These data suggest that lower frequencies of activated and proliferating CCR5+ CD4+ T cells may be potentially induced by HIV exposure in those protected from HIV infection by their HIV‐positive partners.
Figure 1.
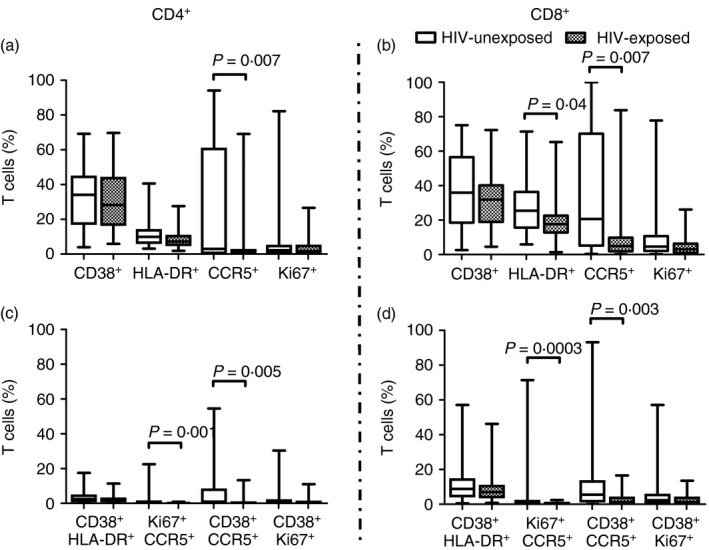
Impact of partner HIV status on specific T‐cell activation (expression of CD38, HLA‐DR, or Ki67) and CCR5 expression in HIV‐unexposed (open) versus HIV exposed–uninfected (shaded) individuals. Frequency of specific activation marker expression (Ki67, HLA‐DR, CD38) and CCR5 expression on CD4+ (a and c) and CD8+ (b and d) T cells derived from the blood of HIV‐exposed (n = 30) and unexposed (n = 38) was assayed. The % of T cells in each group of individuals is depicted by box‐and‐whisker plots indicating the median (middle line), 25th (bottom line) and 75th centiles (top line), and the range (whiskers) of the frequencies of T cells expressing the respective activation markers. Assessments of differences between exposed and unexposed participants were carried out using the Mann–Whitney U‐test.
Similarly, HIV‐negative exposed individuals had significantly lower frequencies of CCR5, alone or in combination with Ki67 and CD38, on the surface of their CD8+ T cells than their unexposed counterparts (P = 0·007 for CCR5 alone, P = 0·0003 for CCR5+ Ki67+, P = 0·003 for CCR5+ CD38+; Fig. 1b,d). These differences remained significantly different for CCR5 alone and CCR5+ CD38+ after adjusting for age, gender and condom usage. HIV‐negative exposed individuals also had lower frequencies of CD8+ T cells expressing HLA‐DR than HIV‐negative unexposed individuals (P = 0·04; Fig. 1b). Furthermore, CCR5 mean fluorescence intensity was lower in HIV‐exposed than unexposed individuals, significantly so for CD4+ T cells (P = 0·04 for CD4+ and P = 0·07 for CD8+ T‐cell subsets, Fig. 2). This suggests that HIV‐exposed individuals had significantly fewer CD4+ CCR5+ T cells, which also expressed significantly lower amounts of CCR5 than unexposed individuals. Activation marker expression was similar in men and women (Fig. 3). No association was found between T‐cell activation, proliferation or CCR5 expression by HIV‐negative women's cells and partner's plasma HIV load (data not shown). Together with the cytokine data, this suggests that the protection conferred in HIV‐negative partners could be the result of both HIV exposure and a natural resistance in the form of a lowered CCR5 expression.
Figure 2.
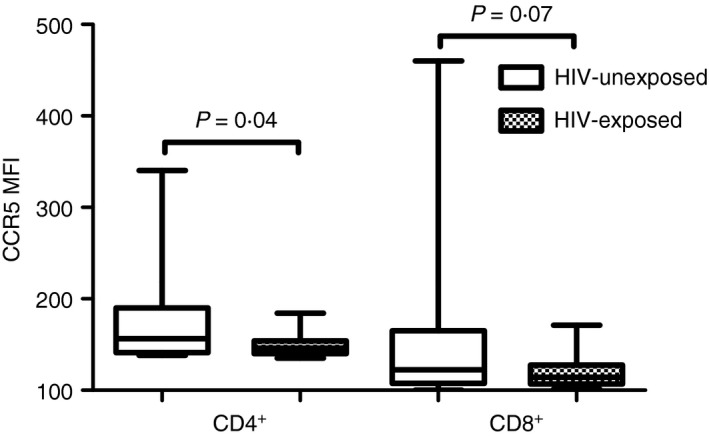
Impact of partner HIV status on density of CCR5 expression by T cells (measured by mean fluorescence intensity). The cumulative MFI in each group of individuals is depicted by box‐and‐whisker plots indicating the median (middle line), 25th (bottom line) and 75th centiles (top line), and the range (whiskers). Assessments of differences between exposed and unexposed participants were carried out using the Mann–Whitney U‐test.
Figure 3.
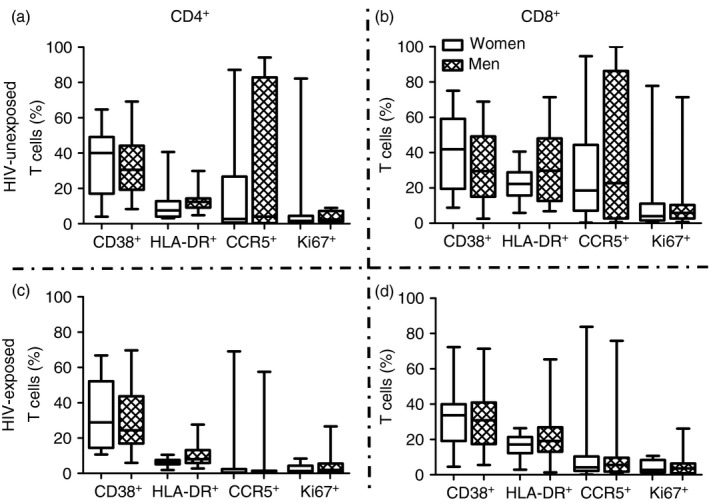
Impact of gender on specific T‐cell activation (expression of CD38, HLA‐DR, or Ki67) and CCR5 expression. Frequency of specific activation marker expression (Ki67, HLA‐DR, CD38) and CCR5 expression on CD4+ (a and c) and CD8+ (b and d) T cells derived from the blood of men and women was assayed. The % of T cells in each group of individuals is depicted by box‐and‐whisker plots indicating the median (middle line), 25th (bottom line) and 75th centiles (top line), and the range (whiskers) of the frequencies of T cells expressing the respective activation markers. Assessments of differences between men and women was carried out using the Mann–Whitney U‐test.
CCR5 polymorphisms and CCR5 expression
As particular CCR5 polymorphisms have been associated with susceptibility to HIV infection, we evaluated the haplotype and genotype distribution of CCR5 in this cohort. None of the participants had the Δ32 CCR5 mutation associated with deficient expression of CCR5 and HIV resistance. The most common haplotypes (Fig. 4a) in exposed individuals were HHA (24%), HHC (21%) and HHE (21%), although the most common in unexposed individuals were HHE (28%), HHA (22%) and HHD (19%). Based on CCR5 haplotyping, individuals were categorized into one of three categories defined as haplotype pairs exhibiting: (i) protective genotypes (HHA/HHF*2, HHF*2HHF*2, HHC/HHF*2, HHA/HHA, HHA/HHC and HHA/HHD), (ii) deleterious genotypes (HHC/HHD, HHC/HHE, HHD/HHE, HHD/HHD and HHE/HHE), and (iii) neutral genotypes (with no described phenotype in the context of HIV‐1 infection, and including any combination not considered protective or deleterious). Protective genotypes were defined as haplotype pairs associated with decreased HIV‐1 susceptibility and/or slower disease progression; deleterious genotypes were defined as haplotype pairs associated with faster disease progression to AIDS and death and/or increased susceptibility to HIV‐1 infection; and haplotype pairs for which there were no designated disease altering effects were reported as neutral.34 HIV‐exposed participants had a greater proportion of individuals with protective genotypes than unexposed individuals (38% versus 28%, respectively; P = 0·58, Fig. 4b) compared with those with deleterious genotypes (26% versus 44%; P = 0·18, Fig. 4b) and a larger proportion of genotypes designated as neutral (36% versus 28%; P = 0·59, Fig. 4b).
Figure 4.
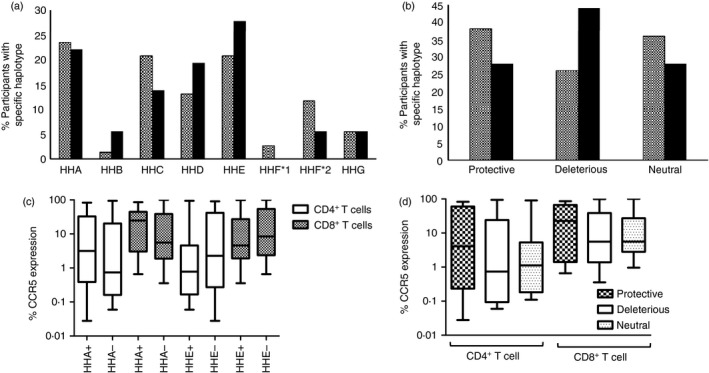
Distribution of CCR5 haplotypes and potential phenotypes across HIV‐exposed and unexposed individuals and their association with CCR5 expression level. (a) Distribution of CCR5 haplotypes previously described.24 (b) Distribution of phenotypes based on pre‐established sets of genotypes24, 33 conferring protection (Protective), increased risks (Deleterious) or no effect (Neutral) in the context of HIV‐1 infection and AIDS disease. (c) Influence of HHA and HHE haplotypes on CCR5 expression within CD4+ and CD8+ T‐cell subsets. Individuals with or without an HHA allele were designated as HHA+ and HHA− respectively. Individuals with or without an HHE allele were designated as HHE+ and HHE−, respectively. Matching CCR5 expression data and CCR5 haplotypes was available for 33 participants (n = 14 for HHA+, 19 for HHA−, 16 for HHE+ and 17 for HHE−). (d) Influence of protective, deleterious, or neutral CCR5 genotypes on CCR5 expression by CD4+ and CD8+ T‐cell subsets.
Haplotype HHA has been associated with slower disease progression in African American individuals,24 whereas haplotype HHE has been linked to an increased risk of HIV‐1 acquisition and a faster disease progression in populations that were ethnically divergent.26, 35, 36 Therefore, the potential impact of the presence or absence of the HHA and HHE haplotypes on CCR5 expression on T cells was investigated. Haplotypes HHA and HHE did not significantly impact CCR5 expression on CD4+ and CD8+ T cells, although HHA+ individuals tended to have higher frequencies of CCR5+ T cells compared with HHA– individuals whereas HHE+ individuals tended to have lower CCR5+ T‐cell frequencies (Fig. 4c). As it is an individual's genotype (including the sum effects of the combination of CCR5 haplotypes) that is likely to determine the overall CCR5 expression, CCR5 expression levels of individuals were compared across defined phenotypes (protective, deleterious and neutral). No significant differences were found when comparing CCR5 expression levels on T cells across the three groups (Fig. 4d), although individuals with protective CCR5 phenotypes tended to have higher frequencies of CCR5+ T cells than those with deleterious CCR5 phenotypes.
CCR5 ‐4223 C/T SNP genotyping
It was previously shown that the CCR5 −4223C/T SNP, which disrupts the CpG −41 site and alters the core consensus motif of the CREB1‐binding site of CCR5, is uniquely present in persons from southern Africa, and occurs on the background of the ancestral protective HHA haplotype.19 This SNP showed a trend to over‐representation in individuals with reduced risk of acquiring HIV‐1 and in those with more effective control of disease progression.19 We looked at the presence of this particular genotype across our groups of exposed and unexposed individuals. Exposed–uninfected individuals had a higher representation of this SNP than unexposed controls, although this was not significant (11% versus 5%, respectively, P = 0·70, data not shown). Furthermore, the presence of this SNP did not impact on CCR5 expression (data not shown).
Discussion
HIV‐exposed–uninfected individuals from South Africa provide a unique opportunity to determine correlates of protection associated with natural resistance to HIV infection in a region with some of the highest HIV prevalence and incidence rates globally. HIV serodiscordant couples represent an important opportunity to study correlates of protection, as exposure to HIV is more predictable than those who engage in high‐risk behaviour with partners unaware of their status. Here, we found that these HIV‐exposed–uninfected individuals had lower frequencies of T cells expressing CCR5, lower densities of CCR5 per cell, higher prevalence of protective CCR5 genotypes with lower prevalence of deleterious CCR5 genotypes, lower frequencies of activated CD38+ and proliferating Ki67+ T cells, and lower concentrations of certain adaptive (IL‐2 and IFN‐γ) and haematopoietic (GM‐CSF) cytokines than their unexposed South African counterparts, suggesting that those who remained uninfected had a smaller subset of HIV‐susceptible target cells compared with unexposed individuals.
CCR5 expression is central to productive HIV infection of HIV target cells.37 A key difference between non‐progressing primate hosts for simian immunodeficiency virus infection (Sooty Mangabeys and African Green Monkeys) and susceptible primate species (macaques and baboons) was found to be comparatively reduced numbers of CD4+ CCR5+ T cells in blood and lymphoid tissues (bone marrow and lymph nodes).38 Paxton et al.9 linked the decreased ability to infect CD4+ T cells from exposed–uninfected individuals to both attenuated expression of CCR5 on T cells, and concurrent elevated production of β‐chemokines. Wu et al.39 showed that the level of CCR5 expression correlated with the ability to infect host cells with macrophage‐tropic HIV in vitro. Similarly, Blaak et al.40 confirmed that susceptibility of activated PBMCs to infection with HIV was associated with levels of CCR5 expression and β‐chemokine production.
Although resistance to HIV infection has been attributed in some cohorts to homozygosity for the mutant alleles of the CCR5 receptor (CCR5Δ3241), this mutation is present at very low frequencies in African populations.34 None of the individuals included in this study expressed this mutant allele. We previously found marked differences in CCR5 haplotype prevalence and CCR5 expression in distinct South African populations,31, 34, 42 and that specific CCR5 haplotypes were associated with reduced CCR5 expression. CCR5 HHA haplotype has been suggested to predict slower HIV disease progression in HIV‐1‐infected individuals of African ancestry,24 and have the lowest transcriptional activity in vitro.43 However, we observed widely varying frequencies of CCR5 expression in HHA+ and HHA− individuals in this study that largely overlapped although median frequencies of T cells expressing CCR5 tended to be higher in HHA+ than HHA− individuals. Gonzalez et al.24 argued that the combined genotype (haplotype pairing rather than the individual haplotypes of CCR5) is more likely to affect the relationship between CCR5 phenotype and risk for HIV infection or disease progression. In line with this, we found that protective CCR5 genotypes (including HHA/HHF*2, HHF*2HHF*2, HHC/HHF*2, HHA/HHA, HHA/HHC and HHA/HHD34) were more prevalent among HIV‐exposed individuals than unexposed individuals, whereas deleterious CCR5 genotypes (including HHC/HHD, HHC/HHE, HHD/HHE, HHD/HHD and HHE/HHE) were less prevalent. The southern African CCR5 −4223C/T SNP showed a trend to higher representation among high‐risk HIV‐exposed uninfected individuals and in those infected individuals with a better disease outcome during HIV infection.19 Similarly, this SNP was found at higher frequencies in this study in the HIV‐exposed compared with the unexposed individuals; however, this was not significant and did not impact on CCR5 expression. These data collectively highlight the importance of testing these CCR5 genetic variants on larger sample numbers – to establish their contribution to protection from infection that also considers the extent of immune activation as an epigenetic modifier influencing risk of infection.
In addition to differences in CCR5 expression and genotypes, we found that exposed individuals had reduced levels of activated CD8+ T cells, marked by reduced expression of HLA‐DR, compared with unexposed individuals, suggesting that immune‐quiescence may have contributed to protection in this cohort as proposed by others in cohorts from Kenya,27, 44 Central African Republic,17 Ivory Coast,18 and the Netherlands.16 Clerici et al.45 suggested that immune activation in individuals residing in Africa was environmentally driven and not genetically pre‐determined. Comparing HIV‐negative Ugandans and Italians residing in Italy with their counterparts living in Africa, they showed that surface expression of CCR5 was higher in those residing in Africa compared with those in Italy, irrespective of ancestry. They suggested that environmental factors, such as parasites, other chronic infections, or nutrition, might influence immune activation. Cohen et al.46 found that cervical T cells from Kenyan women were more activated than those from US women, with elevated levels of CD4+ CD69+, CD4+ CD69+ CCR5+ and CD8+ CD69+ T‐cell subsets, possibly contributing to susceptibility and the higher HIV incidence in young women from sub‐Saharan Africa. We have previously shown that levels of T‐cell activation in blood broadly correlate with activation in T cells present in the female genital tract.30 Reduced frequencies of susceptible HIV target cells in blood could therefore influence the availability of these cells at the genital mucosa.
Exposed‐uninfected individuals in this study had lower concentrations of GM‐CSF, IFN‐γ and IL‐2 in plasma than HIV‐unexposed individuals. Both in vivo 47 and in vitro 48 studies have shown that IL‐2 increases CCR5 expression on T cells. Interferon‐γ influences the ability of macrophages to present antigens, so could modulate cell‐to‐cell spread of HIV.49 Furthermore, both IFN‐γ and IL‐2 increase the expression of HLA class II (including HLA‐DR) and enhance antigen presentation, so lower concentrations of these cytokines in exposed–uninfected individuals could contribute to lower cell surface expression of HLA class II.50 GM‐CSF promotes activation, maturation and differentiation of several immune cell subsets, so reduced GM‐CSF concentrations in exposed–uninfected individuals compared with unexposed individuals could also contribute directly to the lower T‐cell activation that we observed in this study. In a study by Schramm et al.51, the cord blood plasma GM‐CSF levels were lower in exposed–uninfected infants who had HIV‐specific responses compared with exposed–uninfected infants who did not have such responses. These levels were also lower in exposed–uninfected infants when compared with infants who became infected intrapartum or unexposed infants.
In conclusion, this study showed lower levels of systemic immune activation, CCR5 expression and lower levels of plasma cytokines in HIV‐exposed–uninfected individuals, compared with HIV‐unexposed individuals. Lower availability of susceptible HIV target cells could explain the apparent resistance of these individuals to HIV infection, despite exposure. Elucidating the biological characteristics underlying resistance to or protection against HIV infection could provide valuable insight on the protective mechanisms that may be harnessed for the development of new treatments and HIV prevention strategies.
Funding
The original study (ALW PI) was funded by Poliomyelitis Research Fund, Medical Research Council, Swedish International Development Cooperation Agency, Swedish Cancer Foundation, Cancer Association of South Africa and National Health Laboratory Services. This work was also partially based upon research supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (ALW, CTT). Initial recruitment of couples who participated in this study was funded by the Bill and Melinda Gates Foundation. Participants were recruited for a study funded by the Bill and Melinda Gates Foundation (DC PI). JAP, LM, SZJ all received funding from the Department of Science and Technology‐National Research Foundation (NRF) of South Africa Centres of Excellence in HIV prevention, CAPRISA to conduct this research. In addition, SZJ received post‐graduate funding from the Carnegie Foundation and the Poliomyelitis Research Foundation. LM receives funding from the NRF South Africa Research Career Advancement program.
Author's contribution
SZJ performed the experiments, analysis and wrote the paper. AP, MP, HG and LM performed some of the experiments and analysis. CTT performed analysis and contributed to writing the paper. HBJ contributed to writing the paper. DC and ALW designed the study. FL contributed to analysis. PG performed some of the experiments and contributed to writing the paper. JSP designed the study and contributed to analysis and writing the paper.
Disclosure
None of the authors reported any conflict of interest.
Supporting information
Table S1 Cytokine concentrations and quality assessment.
Acknowledgements
We thank all the individuals who kindly participated in the study and Sister Ntombizonke Makhonza for collecting the specimens.
References
- 1. Jennes W, Vuylsteke B, Borget M‐Y, Traore‐Ettiegne V, Maurice C, Nolan M et al HIV‐specific T helper responses and frequency of exposure among HIV‐exposed seronegative female sex workers in Abidjan, Cote d'Ivoire. J Infect Dis 2004; 189:602–10. [DOI] [PubMed] [Google Scholar]
- 2. Bernard NF, Yannakis CM, Lee JS, Tsoukas CM. Human immunodeficiency virus (HIV)‐specific cytotoxic T lymphocyte activity in HIV‐exposed seronegative persons. J Infect Dis 1999; 179:538–47. [DOI] [PubMed] [Google Scholar]
- 3. Biasin M, Caputo S Lo, Speciale L, Colombo F, Racioppi L, Zagliani A et al Mucosal and systemic immune activation is present in human immunodeficiency virus‐exposed seronegative women. J Infect Dis 2000; 182:1365–74. [DOI] [PubMed] [Google Scholar]
- 4. Suy A, Castro P, Nomdedeu M, Garcia F, Lopez A, Fumero E et al Immunological profile of heterosexual highly HIV‐exposed uninfected individuals: predominant role of CD4 and CD8 T‐cell activation. J Infect Dis 2007; 196:1191–201. [DOI] [PubMed] [Google Scholar]
- 5. Makedonas G, Bruneau J, Lin H, Sékaly R‐P, Lamothe F, Bernard NF. HIV‐specific CD8 T‐cell activity in uninfected injection drug users is associated with maintenance of seronegativity. AIDS 2002; 16:1595–602. [DOI] [PubMed] [Google Scholar]
- 6. Kuhn L, Meddows‐Taylor S, Gray G, Tiemessen C. Human immunodeficiency virus (HIV)–specific cellular immune response in newborns exposed to HIV in utero. Clin Infect Dis 2002; 34:267–76. [DOI] [PubMed] [Google Scholar]
- 7. Clerici M, Levin JM, Kessler HA, Harris A, Berzofsky JA, Landay AL et al HIV‐specific T‐helper activity in seronegative health care workers exposed to contaminated blood. JAMA 1994; 271:42–6. [PubMed] [Google Scholar]
- 8. Hladik F, Desbien A, Lang J, Wang L, Ding Y, Holte S, et al Most highly exposed seronegative men lack HIV‐1‐specific, IFN‐γ‐secreting T cells. J Immunol 2003; 171:2671–83. [DOI] [PubMed] [Google Scholar]
- 9. Paxton WA, Liu R, Kang S, Wu L, Gingeras TR, Landau NR et al Reduced HIV‐1 infectability of CD4+ lymphocytes from exposed‐uninfected individuals: association with low expression of CCR5 and high production of β‐chemokines. Virology 1998; 244:66–73. [DOI] [PubMed] [Google Scholar]
- 10. Furci L, Lopalco L, Loverro P, Sinnone M, Tambussi G, Lazzarin A et al Non‐cytotoxic inhibition of HIV‐1 infection by unstimulated CD8+ T lymphocytes from HIV‐exposed‐uninfected individuals. AIDS 2002; 16:1003–8. [DOI] [PubMed] [Google Scholar]
- 11. Wichukchinda N, Kitamura Y, Rojanawiwat A, Nakayama EE, Song H, Pathipvanich P et al The polymorphisms in DC‐SIGNR affect susceptibility to HIV type 1 infection. AIDS Res Hum Retroviruses 2007; 23:686–92. [DOI] [PubMed] [Google Scholar]
- 12. Iqbal SM, Ball TB, Kimani J, Kiama P, Thottingal P, Embree JE et al Elevated T cell counts and RANTES expression in the genital mucosa of HIV‐1‐resistant Kenyan commercial sex workers. J Infect Dis 2005; 192:728–38. [DOI] [PubMed] [Google Scholar]
- 13. Hirbod T, Reichard C, Hasselrot K, Soderlund J, Kimani J, Bwayo JJ et al HIV‐1 neutralizing activity is correlated with increased levels of chemokines in saliva of HIV‐1‐exposed uninfected individuals. Curr HIV Res 2008; 6:28–33. [DOI] [PubMed] [Google Scholar]
- 14. Kebba A, Kaleebu P, Rowland S, Ingram R, Whitworm J, Imami N et al Distinct patterns of peripheral HIV‐1‐specific interferon‐γ responses in exposed HIV‐1‐seronegative individuals. J Infect Dis 2004; 189:1705–13. [DOI] [PubMed] [Google Scholar]
- 15. Pallikkuth S, Wanchu A, Bhatnagar A, Sachdeva RK, Sharma M. Human Immunodeficiency Virus (HIV) gag antigen‐specific T‐helper and granule‐dependent CD8 T‐cell activities in exposed but uninfected heterosexual partners of HIV type 1‐infected individuals in North India. Clin Vaccine Immunol 2007; 14:1196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koning FA, Otto SA, Hazenberg MD, Dekker L, Prins M, Miedema F et al Low‐level CD4+ T cell activation is associated with low susceptibility to HIV‐1 infection. J Immunol 2005; 175:6117–22. [DOI] [PubMed] [Google Scholar]
- 17. Bégaud E, Chartier L, Marechal V, Ipero J, Léal J, Vermisse P et al Reduced CD4 T cell activation and in vitro susceptibility to HIV‐1 infection in exposed uninfected Central Africans. Retrovirology 2006; 3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jennes W, Evertse D, Borget MY, Vuylsteke B, Maurice C, Nkengasong JN et al Suppressed cellular alloimmune responses in HIV‐exposed seronegative female sex workers. Clin Exp Immunol 2006; 143:435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gornalusse GG, Mummidi S, Gaitan AA, Jimenez F, Ramsuran V, Picton A et al Epigenetic mechanisms, T‐cell activation, and CCR5 genetics interact to regulate T‐cell expression of CCR5, the major HIV‐1 coreceptor. Proc Natl Acad Sci USA 2015; 112:E4762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R et al Homozygous defect in HIV‐1 coreceptor accounts for resistance of some multiply‐exposed individuals to HIV‐1 infection. Cell 1996; 86:367–77. [DOI] [PubMed] [Google Scholar]
- 21. Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. Molecular cloning and functional expression of a new human CC‐chemokine receptor gene. Biochemistry 1996; 35:3362–7. [DOI] [PubMed] [Google Scholar]
- 22. Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte‐derived macrophages. Proc Natl Acad Sci USA 1999; 96:5215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quillent C, Oberlin E, Braun J, Rousset D, Gonzalez‐Canali G, Métais P et al HIV‐1‐resistance phenotype conferred by combination of two separate inherited mutations of CCR5 gene. Lancet 1998; 351:14–8. [DOI] [PubMed] [Google Scholar]
- 24. Gonzalez E, Bamshad M, Sato N, Mummidi S, Dhanda R, Catano G et al Race‐specific HIV‐1 disease‐modifying effects associated with CCR5 haplotypes. Proc Natl Acad Sci USA 1999; 96:12004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Howard OMZ, Shirakawa AK, Turpin JA, Maynard A, Tobin GJ, Carrington M et al Naturally occurring CCR5 extracellular and transmembrane domain variants affect HIV‐1 co‐receptor and ligand binding function. J Biol Chem 1999; 274:16228–34. [DOI] [PubMed] [Google Scholar]
- 26. Mangano A, Gonzalez E, Dhanda R, Catano G, Bamshad M, Bock A et al Concordance between the CC chemokine receptor 5 genetic determinants that alter risks of transmission and disease progression in children exposed perinatally to human immunodeficiency virus. J Infect Dis 2001; 183:1574–85. [DOI] [PubMed] [Google Scholar]
- 27. Card CM, McLaren PJ, Wachihi C, Kimani J, Plummer FA, Fowke KR. Decreased immune activation in resistance to HIV‐1 infection is associated with an elevated frequency of CD4+CD25+FOXP3+ regulatory T cells. J Infect Dis 2009; 199:1318–22. [DOI] [PubMed] [Google Scholar]
- 28. Mbulawa ZZA, Coetzee D, Marais DJ, Kamupira M, Zwane E, Allan B et al Genital human papillomavirus prevalence and human papillomavirus concordance in heterosexual couples are positively associated with human immunodeficiency virus coinfection. J Infect Dis 2009; 199:1514–24. [DOI] [PubMed] [Google Scholar]
- 29. Gumbi PP, Jaumdally SZ, Salkinder AL, Burgers WA, Mkhize NN, Hanekom W et al CD4 T cell depletion at the cervix during HIV infection is associated with accumulation of terminally differentiated T cells. J Virol 2011; 85:13333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jaspan HB, Liebenberg L, Hanekom W, Burgers W, Coetzee D, Williamson A‐L et al Immune activation in the female genital tract during HIV infection predicts mucosal CD4 depletion and HIV shedding. J Infect Dis 2011; 204:1550–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Picton ACP, Paximadis M, Tiemessen CT. Genetic variation within the gene encoding the HIV‐1 CCR5 coreceptor in two South African populations. Infect Genet Evol 2010; 10:487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niu T, Qin ZS, Xu X, Liu JS. Bayesian haplotype inference for multiple linked single‐nucleotide polymorphisms. Am J Hum Genet 2002; 70:157–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire‐Mangen F et al Viral load and heterosexual transmission of human immunodeficiency virus type 1, Rakai Project Study Group. NEJM 2000; 342:921–9. [DOI] [PubMed] [Google Scholar]
- 34. Picton ACP, Paximadis M, Tiemessen CT. CCR5 promoter haplotypes differentially influence CCR5 expression on natural killer and T cell subsets in ethnically divergent HIV‐1 uninfected South African populations. Immunogenetics 2012; 64:795–806. [DOI] [PubMed] [Google Scholar]
- 35. Gonzalez E, Kulkarni H, Bolivar H, Mangano A, Sanchez R, Catano G et al The influence of CCL3L1 gene‐containing segmental duplications on HIV‐1/AIDS susceptibility. Science 2005; 307:1434–40. [DOI] [PubMed] [Google Scholar]
- 36. Malhotra R, Hu L, Song W, Brill I, Mulenga J, Allen S et al Association of chemokine receptor gene (CCR2‐CCR5) haplotypes with acquisition and control of HIV‐1 infection in Zambians. Retrovirology 2011; 8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M et al Identification of a major co‐receptor for primary isolates of HIV‐1. Nature 1996; 381:661–6. [DOI] [PubMed] [Google Scholar]
- 38. Pandrea I, Apetrei C, Gordon S, Barbercheck J, Dufour J, Bohm R et al Paucity of CD4+ CCR5+ T cells is a typical feature of natural SIV hosts. Blood 2007; 109:1069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu L, Paxton WA, Kassam N, Ruffing N, Rottman JB, Sullivan N et al CCR5 levels and expression pattern correlate with infectability by macrophage‐tropic HIV‐1, in vitro . J Exp Med 1997; 185:1681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blaak H, Ran LJ, Rientsma R, Schuitemaker H. Susceptibility of in vitro stimulated PBMC to infection with NSI HIV‐1 is associated with levels of CCR5 expression and β‐chemokine production. Virology 2000; 267:237–46. [DOI] [PubMed] [Google Scholar]
- 41. Huang Y, Paxton WA, Wolinsky SM, Neumann AU, Zhang L, He T et al The role of a mutant CCR5 allele in HIV‐1 transmission and disease progression. Nat Med 1996; 2:1240–3. [DOI] [PubMed] [Google Scholar]
- 42. Picton ACP, Shalekoff S, Paximadis M, Tiemessen CT. Marked differences in CCR5 expression and activation levels in two South African populations. Immunology 2012; 136:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mummidi S, Bamshad M, Ahuja SS, Gonzalez E, Feuillet PM, Begum K et al Evolution of human and non‐human primate CC chemokine receptor 5 gene and mRNA: potential roles for haplotype and mRNA diversity, differential haplotype‐specific transcriptional activity, and altered transcription factor binding to polymorphic nucleotides. J Biol Chem 2000; 275:18946–61. [DOI] [PubMed] [Google Scholar]
- 44. Fowke KR, Dong T, Rowland‐Jones SL, Oyugi J, Rutherford WJ, Kimani J et al HIV type 1 resistance in Kenyan sex workers is not associated with altered cellular susceptibility to HIV type 1 infection or enhanced β‐chemokine production. AIDS Res Hum Retroviruses 1998; 14:1521–30. [DOI] [PubMed] [Google Scholar]
- 45. Clerici M, Butto S, Lukwiya M, Saresella M, Declicj S, Trabattoni D et al Immune activation in Africa is environmentally‐driven and is associated with upregulation of CCR5. Italian‐Ugandan AIDS Project. AIDS 2000; 14:2083–92. [DOI] [PubMed] [Google Scholar]
- 46. Cohen CR, Moscicki A‐B, Scott ME, Ma Y, Shiboski S, Bukusi E et al Increased levels of immune activation in the genital tract of healthy young women from sub‐Saharan Africa. AIDS 2011; 24:2069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weissman D, Dybul M, Daucher MB, Davey RT, Walker RE, Kovacs JA. Interleukin‐2 up‐regulates expression of the human immunodeficiency virus fusion coreceptor CCR5 by CD4+ lymphocytes in vivo . J Infect Dis 2000; 181:933–8. [DOI] [PubMed] [Google Scholar]
- 48. Yang Y‐F, Tomura M, Iwasaki M, Mukai T, Gao P, Ono S et al IL‐12 as well as IL‐2 upregulates CCR5 expression on T cell. Cell 2001; 21:116–25. [DOI] [PubMed] [Google Scholar]
- 49. Gowda SD, Stein B, Mohagheghpour N, Benike CJ, Engleman EG. Evidence that T cell activation is required for HIV‐1 entry in CD4+ Lymphocytes. J Immunol 1989; 142:773–80. [PubMed] [Google Scholar]
- 50. Paxton WA, Martin SR, Tse D, O'Brien TR, Skurnick J, VanDevanter NL et al Relative resistance to HIV‐1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high‐risk sexual exposure. Nat Med 1996; 2:412–7. [DOI] [PubMed] [Google Scholar]
- 51. Schramm DB, Meddows‐Taylor S, Gray GE, Kuhn L, Tiemessen CT. Low maternal viral loads and reduced granulocyte–macrophage colony‐stimulating factor levels characterize exposed, uninfected infants who develop protective human immunodeficiency virus type 1‐specific responses. Clin Vaccine Immunol 2007; 14:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Cytokine concentrations and quality assessment.


