Summary
As it was established that aging is not associated with massive neuronal loss, as was believed in the mid‐20th Century, scientific interest has addressed the influence of aging on particular neuronal subpopulations and their synaptic contacts, which constitute the substrate for neural plasticity. Inhibitory neurons represent the most complex and diverse group of neurons, showing distinct molecular and physiological characteristics and possessing a compelling ability to control the physiology of neural circuits. This review focuses on the aging of GABAergic neurons and synapses. Understanding how aging affects synapses of particular neuronal subpopulations may help explain the heterogeneity of aging‐related effects. We reviewed the literature concerning the effects of aging on the numbers of GABAergic neurons and synapses as well as aging‐related alterations in their presynaptic and postsynaptic components. Finally, we discussed the influence of those changes on the plasticity of the GABAergic system, highlighting our results concerning aging in mouse somatosensory cortex and linking them to plasticity impairments and brain disorders. We posit that aging‐induced impairments of the GABAergic system lead to an inhibitory/excitatory imbalance, thereby decreasing neuron's ability to respond with plastic changes to environmental and cellular challenges, leaving the brain more vulnerable to cognitive decline and damage by synaptopathic diseases.
Keywords: ageing, aging, GABA, GABAergic, postsynaptic, presynaptic, synapse
Introduction
Aging is a physiological process that alters brain function, thereby resulting in behavioural changes, memory decline and cognitive impairments. Those changes depend on many factors and are related to structural, neurochemical and physiological processes in the brain (Burke & Barnes, 2006; Ouda et al., 2015). The age‐related cognitive impairments include numerous functions, such as learning, attention, working memory and executive functions (Burke & Barnes, 2006; Mattay et al., 2006).
Cognitive aging is a consequence of molecular and biochemical aging, which results in metabolic, hormonal and immune dysregulation, increased oxidative stress and inflammation, altered neurotransmission and reduced neurotrophic support of neural cells (Sibille, 2013). Alterations in gene expression, influencing the levels of proteins in many biological pathways, can be regarded as a hallmark of molecular aging. Changes in the biochemical composition of neural cells, which affect the efficiency of their synapses and whole circuits, impair the plasticity of the brain, that is the ability to reorganize, learn and remember. In this way, the disturbances of synaptic machinery profoundly contribute to the cognitive impairments as well as to the age‐related brain disorders.
The majority of studies concerning the plasticity of neural circuits have focused on excitatory synapses. However, the role of inhibitory interactions in neuroplastic changes has recently been widely recognized (Letzkus et al., 2011; Castillo et al., 2011; Kullmann et al., 2012). The most basic role of inhibitory neurons is to control the excitability of the principal cells, ensuring a proper homoeostatic balance and preventing runaway excitation (Karmarkar & Buonomano, 2006). In sensory systems, responses of cortical neurons are shaped by the temporal interplay between excitatory thalamocortical input and local cortical inhibition (Miller et al., 2010). Rapid reduction in excitation restricts the window that is available for temporal summation, enabling neurons to act as coincidence detectors and thus increasing temporal precision (Pouille & Scanziani, 2001). Interneurons are also involved in the phenomenon called the inhibitory sharpening of receptive fields (Foeller et al., 2005; Froemke et al., 2007; Carvalho & Buonomano, 2009). Strong network inhibition suppresses the excitatory population response to tonic input, providing the circuit with an intrinsic mechanism enabling precise contrast‐gain control. Therefore, even though excitatory neurons are a large majority of cortical neurons, local inhibitory interneurons shape their firing and timing. There is increasing support for the hypothesis that disruption of inhibitory circuits is responsible for some of the clinical features of many neuropsychiatric and neurodegenerative disorders, such as schizophrenia, autism, depression, epilepsy, Alzheimer's disease and Parkinson's disease. Many of them have been proposed to be synaptopathies – diseases related to the dysfunction of synapses (Brose et al., 2010). Brain aging is, in this context, considered a phenomenon promoting biological alterations associated with the above‐mentioned disorders, resulting in so‐called late‐onset diseases.
The difficulty in understanding the mechanisms of interneurons aging, along with its relationship to plasticity impairments, cognitive decline and brain disorders, lies in the tremendous diversity of inhibitory neurons. Inhibition can be performed by perisomatically, dendritically or axonally targeting interneurons, which can be devoted to different inhibitory tasks (Royer et al., 2012). Interneurons differ in their connectivity, input/output efficacy, membrane properties and firing pattern, and all those features determine their postsynaptic impact on target cells (Gupta et al., 2000; Beierlein et al., 2003; Markram et al., 2004; Tremblay et al., 2016). Furthermore, over 20 subtypes of neurons using GABA as a neurotransmitter have been recognized based on their anatomical, physiological and molecular features, such as the presence of characteristic additional markers like other neurotransmitters, cell surface markers, transcription factors, neuropeptides and calcium‐binding proteins (Ascoli et al., 2008).
Nevertheless, this diversity makes interneurons a potent and complex regulatory machinery controlling the physiology of neural circuits (Maffei, 2011), and their molecular and biochemical aging can significantly contribute to the cognitive deficits observed in the aged brain. The role of neuroplasticity is to compensate for those age‐related changes and to maintain the proper function of inhibitory circuits, supporting the balance between excitation and inhibition and the correct cognitive performance.
In this review, we collected information regarding age‐related alterations in GABAergic neurons and synapses, trying to relate them to the impairments of plasticity that are observed in old age.
Numbers of GABAergic neurons and synapses
Originally, it was postulated that inhibitory deficits observed in aging and neurological disorders are due to decreases in the number of interneurons, and many studies have supported this hypothesis. In aging, Hua et al. (2008) observed that although the total density of neurons remained unaltered in visual cortex of cats, the density of GABA‐immunoreactive (GABA‐IR) neurons was significantly lower. A loss of GABAergic interneurons was shown in the aged rat hippocampus together with reduced inhibition of dendritic input from the entorhinal cortex (Stanley et al., 2012). Other studies have shown loss of inhibitory synaptic contacts. Gradual loss of symmetric synapses was confirmed, for example in layer 2 and 3 of the monkey prefrontal cortex by Peters et al. (2008), and decreased density of GABAergic boutons was found in the frontal and parietal cortices of aged rats (Majdi et al., 2007). Declines in the number and complexity of GABAergic terminals unrelated to neuronal loss were observed in the hippocampus of a mouse model of Alzheimer's disease (Rubio et al., 2012; Soler et al., 2017). Recent studies suggest that inhibitory deficits may also be related to the dysfunction of particular interneuronal subpopulations and their circuits (Marín, 2012). In accordance with this hypothesis, Akbarian et al. (1995) demonstrated reduced expression of GAD in the absence of significant cell loss in schizophrenic brains. However, dysfunction and pruning of synaptic endings may occur simultaneously, preceding neuronal loss as observed in Alzheimer disease (Shankar & Walsh, 2009).
To conclude, age‐related regression, loss and dysfunction of inhibitory synapses can be observed in many brain structures (Dickstein et al., 2007; Morrison & Baxter, 2012).
Parvalbumin (PV)‐containing interneurons
Fast‐spiking PV interneurons are the most numerous GABAergic cells, and they not only execute feedforward and feedback inhibition but also are responsible for generating gamma‐frequency oscillations. In aging, loss of PV‐containing neurons has been shown in the somatosensory, auditory and motor cortices of rats (Miettinen et al., 1993; Ouda et al., 2008) as well as in the hippocampus. On the other hand, an unchanged number of PV cells was detected in the tissue of aged healthy human cerebral cortex and hippocampus (Bu et al., 2003). Nevertheless, decreases in the number of PV neurons and their dysfunction have been associated with the loss of gamma oscillations in schizophrenic patients who manifested deficits in working memory and executive functions (Torrey et al., 2005; Sohal et al., 2009). The link between altered function of PV neurons and neurological/psychiatric disorders has also been confirmed for epilepsy, autism, Alzheimer's disease and depression (Rossignol, 2011).
Somatostatin (SOM)‐containing interneurons
The number of SOM neurons has been reported to be decreased in the somatosensory and motor cortices of aged rats (Miettinen et al., 1993), as well as in the human auditory cortex beginning in midlife (Ouellet & de Villers‐Sidani, 2014). Selective loss of SOM cells has also been shown in rat hippocampus (Stanley et al., 2012). Moreover, lower levels of mRNA for SOM have been observed in the frontal, temporal, motor, visual, and somatosensory cortices and hippocampus of primates (Hayashi et al., 1997).
Many pathological conditions are associated with alterations in SOM interneurons (Lin & Sibille, 2013). These cells are vulnerable to seizure‐induced death, and a decrease in their number is considered a hallmark of epileptic hippocampus (Clynen et al., 2014). Alterations in the somatostatinergic system have been reported in depression, schizophrenia, bipolar disorder and Alzheimer's disease (see Liguz‐Lecznar et al., 2016). Saito et al. (2005) postulated that a decrease in SOM expression can act as a trigger for amyloid β accumulation, thereby contributing to late‐onset Alzheimer's disease.
Calbindin (CB)‐ and Calretinin (CCR)‐containing interneurons
Bu et al. (2003) reported an age‐related decline in CB‐immunopositive cell density in the human visual cortex and parahippocampal gyrus and a decline in CCR neuronal density in the auditory cortex (see also Ouda et al. 2012). Loss of CB‐positive interneurons was reported by Potier et al. (2006) in the aged rat hippocampus together with lower GABAergic inhibition.
Alterations in CB‐positive interneurons were detected in a mouse model of autism (Rossignol, 2011). Coincident age‐related loss of CB neurons and tangle formation in the basal forebrain in Alzheimer's disease were shown by Ahmadian et al. (2015).
Other GABAergic subpopulations
Roles of vasointestinal peptide (VIP)‐containing interneurons have been confirmed in the pathology and treatment of such neurological disorders as Alzheimer's disease, Parkinson's disease and autism spectrum disorders (White et al., 2010). Decreased GABAergic inhibition due to deficits in forebrain neuropeptide Y (NPY)‐containing, SOM‐containing and PV‐containing neurons has been proposed as a possible pathogenic mechanism of autism spectrum disorder (Sgadò et al., 2013). Further, NPY neurons have been shown to degenerate selectively in a mouse model of amyloidosis and tauopathy (Loreth et al., 2012).
In aging, Cha et al. (1997) observed a substantial loss of VIP interneurons in sensory cortex of aged rats. An age‐related loss of NPY neurons was observed in the rat retrosplenial, frontal, occipital and temporal cortical areas, as well as in the hippocampus (Cha et al., 1997). Additionally, a decline in NPY cells has been reported in the auditory cortex of rats (Ouellet & de Villers‐Sidani, 2014).
Notably, the impact of aging on the expression of distinct GABAergic markers can be not only species‐specific but also strain‐specific, and such differences have been reported for parvalbumin in the rat strains Long–Evans vs. Fischer 344 (Ouda et al., 2008) as well as for CB and CCR in mice when comparing the strains CBA/CaJ and C57BL/6J (Zettel et al., 1997).
Together with decreased neurotransmitter release and reduced responsiveness of postsynaptic neurons, the depletion of GABAergic neurons and their synaptic contacts can result in loss of the excitatory/inhibitory balance. This prompts plasticity impairment, and if compensatory mechanisms are ineffective, such imbalance can generate cognitive impairments or even trigger pathophysiological pathways leading to disease.
Functional changes
The dysregulation of GABAergic signalling in aging is a widely accepted phenomenon. However, there is no single universal scheme for age‐related alterations of intrinsic neuronal properties, and the direction of changes depends on the structure and neuronal population. In contrast to the prefrontal cortex, for which experimental data support increased inhibition with age (Luebke et al., 2004; Bories et al., 2013; Bañuelos et al., 2014), there is strong evidence for decreased intracortical inhibition in sensory systems and the hippocampus. Increased spontaneous activity and a decreased signal‐to‐noise ratio were observed in the visual system of aged cats and rats (Wang et al., 2006; Lehmann et al., 2012). Using a paired‐pulse stimulation paradigm, Schmidt et al. (2010) confirmed an age‐dependent reduction in functional inhibition in the parietal cortex, and David‐Jürgens & Dinse (2010) confirmed this in rat somatosensory cortex. Furthermore, the same phenomenon has been observed in the human somatosensory system (Cheng & Lin, 2013), where it was associated with impaired tactile acuity (Lenz et al., 2012), as well as in primary motor cortex, where weaker resting‐state inhibition was associated with poorer manual motor performance (Heise et al., 2013). Several studies using the whole‐cell patch‐clamp method have demonstrated a reduced frequency of spontaneous inhibitory postsynaptic potentials (IPSPs), as well as reduced amplitude and frequency of GABA receptor (GABAR)‐mediated currents, in the aged hippocampus (Potier et al., 2006; McQuail et al., 2015). This altered balance of excitatory and inhibitory pathways in local brain circuits influences the neuroplastic potential of the aging nervous system.
Aged GABAergic synapses and plasticity
There is ample evidence that GABAergic neurons and synapses undergo plastic changes. The significance of GABAergic synapse plasticity is highlighted by the number of experiments on this topic (Gubellini et al., 2001; Kawaguchi & Hirano, 2002; Patenaude et al., 2003; Lien et al., 2006; Maffei et al., 2006). Furthermore, it has been shown that the learning process is associated with modification of inhibitory GABAergic neurotransmission and reorganization of cortical activation (Froemke et al., 2007; Tokarski et al., 2007; Brosh & Barkai, 2009; Jasinska et al., 2010; Urban‐Ciecko et al., 2010).
Animal studies investigating the impact of aging on the GABAergic system have mainly demonstrated reduced inhibition with age, but in some specific areas (such as the prefrontal cortex), inhibition has been reported to increase with age (Potier et al., 2006; Stanley et al., 2012; Bories et al., 2013). Regardless of whether inhibition in the particular brain area was attenuated or augmented, changes in specific inhibitory interneurons functioning have been found to contribute to cognitive, memory and behavioural changes (Stanley et al., 2012; Bories et al., 2013).
Aging is associated with peripheral deafferentation, resulting in compensatory mechanisms in the appropriate CNS pathways. This phenomenon, described as adaptive plasticity, can be found in different sensory systems and leads to a selective downregulation of inhibition (Caspary et al., 2008), which is considered a type of ‘negative plasticity’ (Mahncke et al., 2006). Consequences of reduced inhibition include impaired tactile acuity and diminished visual and auditory signal‐to‐noise coding (Leventhal et al., 2003; Yu et al., 2006; Hua et al., 2008; David‐Jürgens & Dinse, 2010; Kamal et al., 2013). Moreover, an association between weaker inhibition and lower manual motor performance was confirmed in the human motor system (Heise et al., 2013). All the above‐mentioned changes are indications of homoeostatic plasticity that serves to re‐establish the balance between the excitatory and inhibitory systems.
The situation becomes even more complicated when an upregulation of inhibitory system is necessary to induce plasticity or to accomplish some cognitive task (Scelfo et al., 2008; Petrini et al., 2014; Wang & Maffei, 2014). Plasticity of cortical maps induced by sensory training based on classical conditioning paradigms is a type of plasticity that requires upregulation of the GABAergic system (Siucinska, 2006; Tokarski et al., 2007; Jasinska et al., 2010; Liguz‐Lecznar et al., 2011; Posluszny et al., 2015). Following the training in which stroking a row of whiskers is paired with a tail shock for three consecutive daily sessions, functional cortical representation of stimulated whiskers expands in young animals (Siucinska, 2006) (Fig. 1). This plastic change is accompanied by an increase in the number of SOM neurons in the representation of the trained row (Cybulska‐Klosowicz et al., 2013), increased inhibitory synaptogenesis, increased tonic inhibition of fast‐spiking neurons and increased IPSC frequency. We found that in aged mouse somatosensory cortex, the same training is ineffective, and learning‐induced plasticity of cortical whisker representations could not be observed (Liguz‐Lecznar et al., 2015), In young mice, the training causes an increase in the GABA content in the barrel cortex, as measured by HPLC, while in aged mice, no such effect was found (Liguz‐Lecznar et al., 2015). However, the plastic change could be induced with a longer training paradigm (Fig. 1), and then, the increase in GABA level was observed. These data led us to postulate that the decrement in plasticity observed in aged animals was not due to neurodegeneration but rather due to an ineffectiveness of mechanisms governing plasticity: specifically, the upregulation of the GABAergic system in response to increased demands on the inhibitory drive (Liguz‐Lecznar et al., 2015) (Fig. 1).
Figure 1.
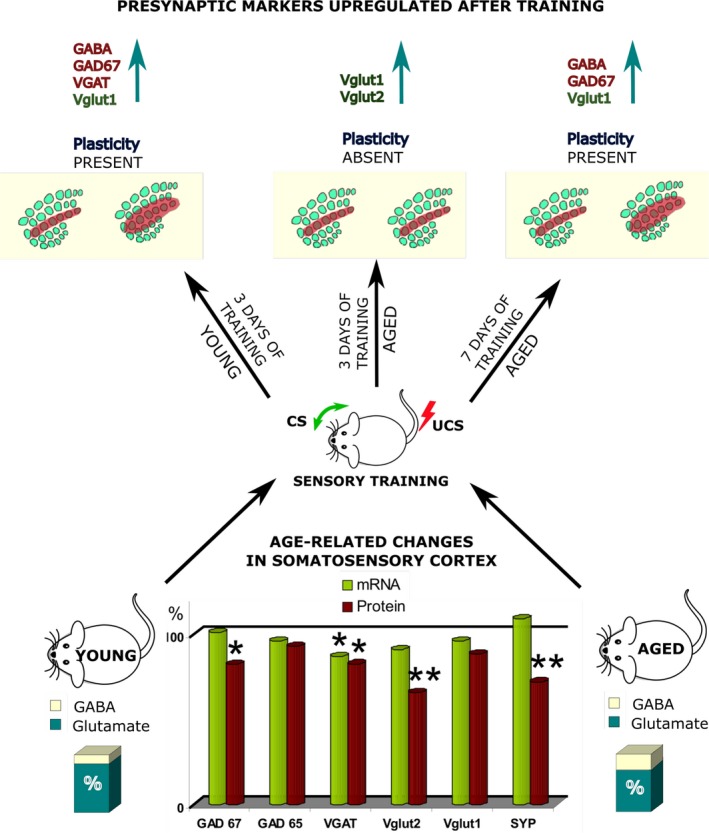
Age‐related changes influence learning‐dependent plasticity in the mouse somatosensory cortex. From the bottom: Aging alters the level of several proteins related to glutamatergic and GABAergic neurotransmission in the somatosensory (SI) cortex of mice. mRNA for those proteins, with the exception of VGAT, is unaltered. Due to the decreased level of glutamate with an unchanged level of GABA in aged mouse SI, a lower glutamate‐to‐GABA ratio can be observed, suggesting an imbalance between excitation and inhibition. When submitted to short (3‐day) sensory training based on a classical conditioning paradigm (conditioned tactile stimulus paired with an unconditioned aversive stimulus), young mice elaborate functional cortical plasticity. This plastic change is visible, after mapping brain activity using the 2‐DG method, as an enlargement of the functional cortical representation of the trained row of vibrissae. Aged (1‐year‐old) mice do not demonstrate such plasticity after short training, even though they present the conditioned response similarly to the young mice. After longer (7‐day) conditioning, aged animals also elaborate the plastic change in SI. Training‐induced plasticity is associated with increased levels of GAD67 and GABA in SI. This may serve to attenuate the increased activity suggested by upregulation of Vglut1 and Vglut2 after training. In young animals, training also upregulates VGAT, which can further support GABAergic neurotransmission. In aged animals, probably due to a significant reduction in the VGAT mRNA level, the upregulation of VGAT expression after training is impossible, and that decreases the ability of the GABAergic system to effectively respond to increased demands on the inhibitory drive. Thus, aged mice require more time to elaborate the plastic change. GAD65 and GAD67 – glutamic acid decarboxylases; SYP – synaptophysin; VGAT – vesicular GABA transporter; Vglut1 and Vglut2 – vesicular glutamate transporters; CS – conditioned stimulus; UCS – unconditioned stimulus, 100% – expression level in young animals.
In aging, two plasticity‐related phenomena interfere with each other: adaptive changes and experience‐dependent plasticity. Some of these adaptive mechanisms may also be shared with degenerative cascades, and once activated, they may lead to cognitive impairment and disease (Cotman & Anderson, 2000). Thus, the strong plastic potential and involvement of experience‐dependent plasticity may allow the brain to overcome the negative effects of aging. This was confirmed in numerous experiments that demonstrated the beneficial result of a stimulating environment and physical/cognitive training on cognitive functions such as memory (Sale et al., 2014; Consortium T.t.B., 2017) and also based on the effects of enriched environment on Aβ accumulation during aging (Li et al., 2013), hippocampal neurogenesis and the level of neurotransmitters (Segovia et al., 2006).
Synapse – the space where aging acts
The structure of the synapse is complex, and maintaining the correct composition of presynaptic and postsynaptic terminals as well as precise coordination of presynaptic and postsynaptic activities is essential for cognitive functions (including learning and memory consolidation) and for synaptic plasticity (Abraham, 2008). In fact, synaptic dysfunction has been documented to be an early event in the course of many neurodegenerative diseases such as Huntington's disease (Li et al., 2001), temporal lobe epilepsy (Ratté & Lacaille, 2006) and Alzheimer's disease (Masliah et al., 2006). In physiological aging, the decline in synaptic density, pruning of the dendritic tree, loss of dendritic spines, structural changes within the presynaptic active zone and alteration of receptors for different neurotransmitters have been detected across the nervous system (Burke & Barnes, 2006). To maintain stable neurotransmission and enable optimal synaptic performance, synapses are under the control of homoeostatic mechanisms that act through compensatory adjustments in synaptic strength and cellular excitability (Turrigiano et al., 1998; Burrone et al., 2002). Aging‐related changes can be detected in excitatory and inhibitory synapses and often lead to an imbalance of excitation and inhibition, which can cause further instability in neuronal networks and gating defects related to cognitive impairments (Marín, 2012).
Many recent genomewide human and animal studies show that the proportion of genes undergoing an age‐dependent regulation is relatively small, representing no more than 5% of the entire genome. However, because research by Loerch et al. (2008) using species‐specific genome‐scale microarrays revealed that genes associated with GABAergic inhibitory function are significantly more strongly age‐downregulated in humans than in rhesus monkeys or mice, results regarding gene alterations with aging should be interpreted cautiously.
The postulated pattern is that neuron‐related transcripts involved in signalling and cellular communication are downregulated, while the opposite can be observed for glial‐related genes engaged in inflammation and cellular defence (see Sibille, 2013). Despite the relatively stable transcriptome, the brain shows many age‐related protein alterations, which indicates mRNA–protein decoupling (Wei et al., 2015). Our analysis of aging‐related changes in the expression of several presynaptic markers also revealed that they were downregulated with age, mainly at the protein level (Liguz‐Lecznar et al., 2015) (Fig. 1).
Inside the aged GABAergic synapse
Presynaptic impact
Existing data support the idea that synaptic dysfunction resulting from altered neurotransmission is a significant contributor to aging‐related impairments of nervous system functioning (Morrison & Baxter, 2012). Several conditions must be met to maintain synaptic functionality and unaltered neurotransmission: preservation of proper synaptic structure, coordination of synaptic vesicle release and membrane excitability, and effective integration of retrograde signals from the postsynaptic terminal (Azpurua & Eaton, 2015).
Presynaptic terminals serve to release neurotransmitters and contain an extensive array of proteins that are expressed in precise quantities, located in specific areas and designated for particular functions (Garner et al., 2000; Fritschy et al., 2012). Together, they constitute a dedicated system to ensure accurate, efficient and reliable synaptic transmission.
Consistent with age‐related decreases in inhibition, many studies have demonstrated decreases in the synthesis and level of GABA. GABA is synthesized from glutamate by its decarboxylation, catalysed by the two isoforms of the enzyme glutamate decarboxylase (GAD65 and GAD67) (Erlander et al., 1991). Many studies have reported aging‐associated alterations in the levels of both GAD isoforms in different brain areas. In most cases, the number of GAD‐IR neurons, the amount of protein or the mRNA level decreased with age (Gold & Bajo, 2014). Ling et al. (2005) reported reduced levels of GAD65 and GAD67 mRNAs, and Burianova et al. (2009) reported an age‐related decrease in the protein levels of GAD65 and GAD67 in the rat auditory cortex. They suggested that the observed changes may contribute significantly to the deterioration of hearing function. In the aging mouse barrel cortex on the other hand, age‐related decreases in the protein levels of both GAD isoforms were observed without significant changes in mRNA expression (Liguz‐Lecznar et al., 2015). Loss of GAD level in interneurons was also observed in aging visual cortex and the hippocampus (Shi et al., 2004; Stanley & Shetty, 2004; Liao et al., 2016). Such results are not always accompanied by alterations in the GABA levels in aging tissue. In our experiments, despite decreased levels of GAD65 and 67 in aging mice, we did not detect a significant decrement in the GABA level in the somatosensory cortex (Liguz‐Lecznar et al., 2015). Nevertheless, although GAD expression may not directly translate to basic GABA level, its depletion may play a restrictive role during the induction of plastic changes.
GABA transport
After release from presynaptic terminals, GABA is quickly removed from the extracellular space by a system of transporters, which are transmembrane proteins with four distinct subclasses: GAT‐1, GAT‐2, GAT‐3 and BGT‐1. They regulate the extracellular concentration of GABA and prevent excessive activation of GABARs (Scimemi, 2014). However, before it can be released, GABA must first be packed into synaptic vesicles. This step is mediated by the vesicular GABA transporter (VGAT) (Chaudhry et al., 1998).
Information about the role of GATs and changes in their expression related to brain aging is sparse. A few studies reported a significant reduction in GAT‐1 expression in specific brain areas, such as the rat medial prefrontal cortex (Bañuelos et al., 2014) and the human frontal cortex (Sundman‐Eriksson & Allard, 2006). Similarly, little is known about how VGAT expression or activity changes during aging. A study by Canas et al. (2009) presented an age‐related continuous decrease in the VGAT level in the rat hippocampus. Additionally, our experiments on the barrel cortex of aged mice demonstrated decreased VGAT mRNA and protein levels (Liguz‐Lecznar et al., 2015) (Fig. 1).
Postsynaptic impact
GABA receptors
The main and potentially most important postsynaptic components that may influence the action of inhibitory synapses are GABA receptors (GABARs). They are divided into two classes: fast‐acting ionotropic GABAA and slower acting metabotropic GABAB receptors (GABAARs and GABABRs, respectively) (Pinto et al., 2010).
GABAARs
GABAARs are ligand‐gated chloride‐ion channels that regulate fast inhibitory neurotransmission in the brain (Bowery et al., 2002). Biophysical properties of the receptor are determined by its subunit composition (Koksma et al., 2005), which can be regionally specific in the brain (Sieghart & Sperk, 2002). The number of postsynaptic GABAARs plays a crucial role in shaping synaptic plasticity because it is proportional to the functional strength of GABAergic synapses (Nusser et al., 1997; Luscher et al., 2011). Thus, even a slight decrease in the number of GABAARs (by 5–35%) can result in noticeable behavioural changes (Shen et al., 2010; Luscher et al., 2011). It has been shown that age‐related changes in the composition of GABAAR subunits that affect channel kinetics, ligand binding and ion specificity contribute to cognitive impairments (Rissman & Mobley, 2011). In rat auditory cortex, Schmidt et al. (2010) showed a decline in the α5 subunit of GABAAR, and in the auditory thalamus, Richardson et al. (2013) demonstrated reduced activation and expression of high‐affinity GABAARs, mediating tonic inhibition. Caspary et al. (2013) showed that in the aged rat auditory cortex, some subunits of GABAAR were downregulated (α1, β1, β2, γ1 and γ2 subunits) while the α3 subunit was upregulated, and this effect was specific for particular cortical layers. Yu et al. (2006) revealed changes in the regional expression of GABAAR subunits, with decreases in the α3 and α5 subunits in rat motor and somatosensory cortex, a transient decrease in the γ2 subunit and no alterations in the expression levels of α1 and α2. Gutiérrez et al. (1997) showed age‐related changes in the protein and mRNA levels of the α1 and γ2 subunits in the rat cortex. In the hippocampus, an age‐related upregulation of the α1 subunit of GABAAR was reported in rats, while a decrease was observed in monkeys (Gutiérrez et al., 1996; Rissman et al., 2007). Moreover, the γ2 subunit was downregulated in the aged rat hippocampus (Yu et al., 2006).
Those results suggest that aging, through quite subtle changes in the subunit composition of GABAAR, can change its physiology and alter inhibitory synaptic transmission.
GABABRs
GABABRs constitute a group of heterodimeric G protein‐coupled receptors. Those transmembrane proteins mediate slow inhibitory responses and can be expressed on the presynaptic or postsynaptic side. Presynaptically localized (on GABAergic and glutamatergic terminals – autoreceptors) GABABR regulate neurotransmitter release, while receptors localized postsynaptically or extrasynaptically on pyramidal neurons mediate tonic inhibition (Wang et al., 2010). Depending on the brain region, aging induced different alterations in GABABRs, thereby causing different changes in behaviour and learning. Binding of GABABRs was reduced in the inferior colliculus and cortex of aged rats (Milbrandt et al., 1994; Turgeon & Albin, 1994; Caspary et al., 1995). In the hippocampus, a selective loss of the GABABR1 subunit was observed in aged rats with a spatial learning impairment (McQuail et al., 2012). On the other hand, Bañuelos et al. (2014) reported that GABABR expression was negatively correlated with working memory performance. It was further shown that administration of a GABABR antagonist enhanced working memory in aged rats and ameliorated deficits in olfactory discrimination learning (Lasarge et al., 2009). However, the possible effects of age‐related GABABR alterations can be complex, because its presynaptic action inhibits neurotransmitter release and thus contributes to a net effect of enhancing excitatory signalling, while its postsynaptic activation induces postsynaptic inhibitory currents and activates CREB2, a transcription factor involved in the regulation of memory formation suppressor genes (Helm et al., 2005; Emson, 2007).
Although GABARs have a substantial impact on synaptic plasticity by controlling LTP, synaptic strength and maturation of dendritic spines, many other components of the inhibitory postsynaptic density (PSD) in GABAergic synapses contribute to controlling the function of postsynaptic terminals.
Gephyrin and other proteins of the inhibitory PSD
Gephyrin, initially characterized as a tubulin‐binding protein, is described as a neuronal assembly protein that anchors other proteins and is the major inhibitory scaffolding component. It is a membrane‐associated protein that co‐localizes with both glycine and GABA receptors (Wang et al., 1999; Craig & Boudin, 2001), and its level can be used as an indicator of the total amount of GABAAR (Pinto et al., 2010). Gephyrin functioning is regulated by phosphorylation, and gephyrin can be added or removed from the postsynaptic scaffold (Fritschy et al., 2012). Clustering and functioning of gephyrin is regulated by interactions with many other proteins, including neuroligin‐2 (NL2), which is a postsynaptic adhesion molecule (Maćkowiak et al., 2014), and collybistin, which regulates the aggregation of gephyrin and GABAAR (Poulopoulos et al., 2009). Little is known about the age‐related changes in those proteins. A decrease in gephyrin expression with age was observed in the human visual cortex (Pinto et al., 2010). On the other hand, an increase in gephyrin expression was detected in the parietal cortex of cognitively impaired aged rats (Majdi et al., 2009).
Similarities between age‐related and pathology‐related changes within GABAergic synapses
Alterations similar to those seen in aging can be observed in neurological and psychiatric diseases. In schizophrenia, GAD67 mRNA expression in prefrontal cortex is downregulated in a subset of parvalbumin‐containing interneurons (Volk & Lewis, 2005). Reduced levels of GAD65 and 67 can be observed in parietal and cerebellar cortices in autism (Fatemi et al., 2002). A diminished number of GABAARs was confirmed in the cell membranes of Alzheimer's disease brains (Bernareggi et al., 2007). Analysis of mRNA expression and the proportions of GABA receptor subunits revealed downregulation of the α1 and γ2 subunits with concomitant upregulation of α2, β1 and γ1 transcripts in AD brains (Limon et al., 2012).
Supporting this similarity, the reinforcement of the GABAergic system is regarded as a therapeutic strategy in age‐related cognitive decline (McQuail et al., 2015) as well as in several neurodegenerative diseases and neurological disorders (Stan & Lewis, 2012). Leventhal et al. (2003) have shown that stimulation of the GABAergic system with the GABAAR agonist muscimol resulted in improved properties of neurons in aged monkey visual cortex. In schizophrenia, a clinical trial with a benzodiazepine‐like drug specific for particular subtype of GABAAR revealed its effectiveness in memory improvement (Lewis et al., 2008). The therapeutic potential of GABABR ligands has also been suggested for depression, epilepsy and Alzheimer's disease (see Kumar et al., 2013). In addition to the benefits of drugs attenuating amyloid‐induced synaptic dysfunction, several studies have demonstrated protection against Aβ‐induced neurotoxicity with a selective GABAA receptor agonist (i.e. muscimol) in retinal, hippocampal and cortical neurons in rodents (see Nava‐Mesa et al., 2014). Additionally, more recent studies in preclinical models show that GABAergic cell grafting can be beneficial for treating schizophrenia, neuropathic pain, Alzheimer's disease and Parkinson's disease (Chohan & Moore, 2016; Shetty & Bates, 2016). Interestingly, such interneuron‐based transplantation (PV and SST neurons) was also demonstrated to be sufficient to induce cortical plasticity (Tang et al., 2014).
The connection between brain aging and late‐life or late‐onset diseases can be explained by the age‐by‐disease biological interaction model. According to this model, the common direction of many genes alterations in aging and diseases suggests that the ‘brain progressively moves with advancing age towards a state that is biologically more consistent with those observed in the context of neuropsychiatric and neurological disorders’ (Sibille, 2013). To this hypothesis, we add the role of synaptic plasticity, which, if sufficient, can support the neural circuits in resisting the detrimental effect of aging.
Compensatory mechanisms in aging synapses
Our experiments revealed that despite the impairment of learning‐induced plasticity in aged animals, it was still possible to evoke but required longer training. Moreover, in the somatosensory cortex of those animals, we observed a distinct pattern of proteins with changed level after training, different from the pattern found in young animals (Liguz‐Lecznar et al., 2015) (Fig. 1). This suggests that young and aged animals can engage different mechanisms to accomplish the same process and that even aged synapses with some protein deficits can support plasticity (Fig. 1). This can most probably be achieved by some compensatory or adaptive changes in the synapse, and such changes have been seen in many aged brain regions. For example, it was suggested that the loss of inhibitory synapses in the aged prefrontal cortex may be offset by the upregulation of GABA synthesis (Luebke et al., 2004; Bories et al., 2013). In the hippocampus, an age‐related increase in synaptophysin expression has been suggested to compensate for deficits in spatial learning and memory (Benice et al., 2006; Kumar & Thakur, 2015). Experimental data confirmed that a loss of GABAergic input in the hippocampus during aging can be compensated for at the postsynaptic level by upregulation of GABAAR expression with higher sensitivity to GABA (Ruano et al., 2000; Vela et al., 2003).
Such compensational changes, if sufficient, would help to preserve, to some extent, plastic potential and cognitive flexibility. Moreover, de Villers‐Sidani et al. (2010) have shown, in the rat auditory cortex, that age‐related auditory processing deficits (i.e. temporal coding and cortical desynchronization) and structural changes in parvalbumin‐containing interneurons can be largely reversed with behavioural training. This demonstrates that sensory experience strongly influences the cognitive capabilities in aging and has the potential to reverse the alterations in inhibitory interneuron morphology and cognitive decline in the aged brain.
Conclusions
Age‐related loss of synaptic contacts, decreased neurotransmitter release and reduced postsynaptic responsiveness to neurotransmitters result in a decline in synaptic strength, contributing to age‐related cognitive decline. Molecular aging, defined as age‐related transcriptome changes, and biochemical protein‐related alterations within synapses weaken the plastic potential of neurons. Inhibitory neurons, despite being in the minority, are powerful regulators of neuronal excitability and, being particularly susceptible to aging‐related alterations, are involved in many aging‐induced cognitive impairments and brain disorders.
In the model of aged mouse somatosensory cortex, we have shown that although potential for learning‐related plasticity is preserved there, the corresponding mechanisms are weakened and need longer stimulation to trigger plastic changes. We have postulated that the decreased effectiveness of the GABAergic system in the aged mouse somatosensory cortex contributes to the deficits in learning‐induced plasticity.
Based on the age‐by‐disease molecular interaction model, stating that aging of the brain overlaps with biological pathways implicated in multiple brain disorders, we suggest that biochemical alterations in synapses, leading GABAergic neurons to be unable to respond quickly to the increased demand on the inhibitory drive, are a cause of age‐related learning‐induced plasticity impairment, which is an intermediate stage of the transition from healthy aging to age‐related cognitive decline and then to disease (See Box 1). Pharmacological and/or environmental reinforcement of the GABAergic system seems to be a promising therapeutic target for aging‐related brain disorders.
Box 1. Progressive age‐related alterations in inhibitory neurons resulting in a decline in synaptic plasticity and compensatory mechanisms are determining factors for the transition between healthy and pathological aging.
Molecular aging (transcriptome alterations) and biochemical aging (protein alterations) result in synaptic alterations and deficits of inhibition, which generate an imbalance between excitation and inhibition. This results in deregulation of excitatory cell input/output, local neuronal circuit processing and induction of negative adaptive changes; together with decreased plasticity, these changes cause further dysfunction of synapses and finally synaptic loss. Weakened compensatory mechanisms are unable to cope with those alterations, and pathological neurochemical and neurophysiological processes are triggered, directing neurons towards disease.
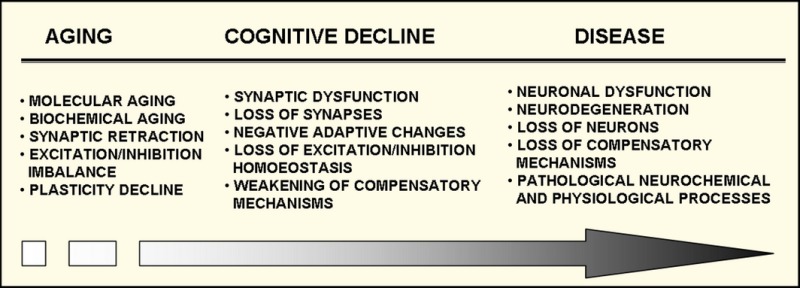
The consequences of age‐induced alterations in the synapse are difficult to interpret and predict. They are often specific for a given brain region or even a particular cell type, and the knowledge concerning the impact of aging on particular components of the synapse is fragmentary. While the extensive array of proteins present in presynaptic and postsynaptic terminals provides the possibility of functional compensation in age‐related deficits, it would be of particular importance to discover synaptic components and mechanisms that are altered in aging. Such knowledge would enable old synapses to be supported, thus maintaining proper neuronal signalling and counteracting the detrimental effects of aging.
Author contributions
Both authors contributed equally to the data collection and to writing the manuscript.
Funding
Work leading to this review was supported in part by a National Science Centre Grant (2013/09/B/NZ3/00540) and by Statutable Funds of the Nencki Institute of Experimental Biology PAS.
Conflict of interest
None declared.
References
- Abraham WC, et al (2008) Metaplasticity: tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 9, 387. [DOI] [PubMed] [Google Scholar]
- Ahmadian SS, Rezvanian A, Peterson M, Weintraub S, Bigio EH, Mesulam MM, Geula C (2015) Loss of calbindin‐D28K is associated with the full range of tangle pathology within basal forebrain cholinergic neurons in Alzheimer's disease. Neurobiol. Aging 36, 3163–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jones EG (1995) Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch. Gen. Psychiatry 52, 258–266. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Alonso‐Nanclares L, Anderson SA, Barrionuevo G, Benavides‐Piccione R, Burkhalter A, Buzsáki G, Cauli B, Defelipe J, Fairén A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvárday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Muñoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo‐Rodriguez M, Wang Y, West DC, Yuste R. Group P. I. N. (2008) Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 9, 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpurua J, Eaton BA (2015) Neuronal epigenetics and the aging synapse. Front. Cell Neurosci. 9, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bañuelos C, Beas BS, McQuail JA, Gilbert RJ, Frazier CJ, Setlow B, Bizon JL (2014) Prefrontal cortical GABAergic dysfunction contributes to age‐related working memory impairment. J. Neurosci. 34, 3457–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW (2003) Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J. Neurophysiol. 90, 2987–3000. [DOI] [PubMed] [Google Scholar]
- Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J (2006) Sex‐differences in age‐related cognitive decline in C57BL/6J mice associated with increased brain microtubule‐associated protein 2 and synaptophysin immunoreactivity. Neuroscience 137, 413–423. [DOI] [PubMed] [Google Scholar]
- Bernareggi A, Dueñas Z, Reyes‐Ruiz JM, Ruzzier F, Miledi R (2007) Properties of glutamate receptors of Alzheimer's disease brain transplanted to frog oocytes. Proc. Natl Acad. Sci. USA 104, 2956–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bories C, Husson Z, Guitton MJ, De Koninck Y (2013) Differential balance of prefrontal synaptic activity in successful versus unsuccessful cognitive aging. J. Neurosci. 33, 1344–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Enna SJ (2002) International Union of Pharmacology. XXXIII. Mammalian gamma‐aminobutyric acid(B) receptors: structure and function. Pharmacol. Rev. 54, 247–264. [DOI] [PubMed] [Google Scholar]
- Brose N, O'Connor V, Skehel P (2010) Synaptopathy: dysfunction of synaptic function? Biochem. Soc. Trans. 38, 443–444. [DOI] [PubMed] [Google Scholar]
- Brosh I, Barkai E (2009) Learning‐induced enhancement of feedback inhibitory synaptic transmission. Learn Mem. 16, 413–416. [DOI] [PubMed] [Google Scholar]
- Bu J, Sathyendra V, Nagykery N, Geula C (2003) Age‐related changes in calbindin‐D28k, calretinin, and parvalbumin‐immunoreactive neurons in the human cerebral cortex. Exp. Neurol. 182, 220–231. [DOI] [PubMed] [Google Scholar]
- Burianova J, Ouda L, Profant O, Syka J (2009) Age‐related changes in GAD levels in the central auditory system of the rat. Exp. Gerontol. 44, 161–169. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA (2006) Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 7, 30–40. [DOI] [PubMed] [Google Scholar]
- Burrone J, O'Byrne M, Murthy VN (2002) Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature 420, 414–418. [DOI] [PubMed] [Google Scholar]
- Canas PM, Duarte JM, Rodrigues RJ, Köfalvi A, Cunha RA (2009) Modification upon aging of the density of presynaptic modulation systems in the hippocampus. Neurobiol. Aging 30, 1877–1884. [DOI] [PubMed] [Google Scholar]
- Carvalho TP, Buonomano DV (2009) Differential effects of excitatory and inhibitory plasticity on synaptically driven neuronal input‐output functions. Neuron 61, 774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Milbrandt JC, Helfert RH (1995) Central auditory aging: GABA changes in the inferior colliculus. Exp. Gerontol. 30, 349–360. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF (2008) Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J. Exp. Biol. 211(Pt 11), 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Hughes LF, Ling LL (2013) Age‐related GABAA receptor changes in rat auditory cortex. Neurobiol. Aging 34, 1486–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Chiu CQ, Carroll RC (2011) Long‐term plasticity at inhibitory synapses. Curr. Opin. Neurobiol. 21, 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha CI, Lee YI, Lee EY, Park KH, Baik SH (1997) Age‐related changes of VIP, NPY and somatostatin‐immunoreactive neurons in the cerebral cortex of aged rats. Brain Res. 753, 235–244. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm‐Mathisen J (1998) The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J. Neurosci. 18, 9733–9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CH, Lin YY (2013) Aging‐related decline in somatosensory inhibition of the human cerebral cortex. Exp. Brain Res. 226, 145–152. [DOI] [PubMed] [Google Scholar]
- Chohan MO, Moore H (2016) Interneuron progenitor transplantation to treat CNS dysfunction. Front. Neural. Circuits. 10, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynen E, Swijsen A, Raijmakers M, Hoogland G, Rigo JM (2014) Neuropeptides as targets for the development of anticonvulsant drugs. Mol. Neurobiol. 50, 626–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium, T. t. B (2017) Randomized trial on the effects of a combined physical/cognitive training in aged MCI subjects: the Train the Brain study. Sci. Rep. 7, 39471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Anderson AJ (2000) The brain's microenvironment, early functional loss, and the conversion to Alzheimer's disease. Ann. N. Y. Acad. Sci. 924, 112–116. [DOI] [PubMed] [Google Scholar]
- Craig AM, Boudin H (2001) Molecular heterogeneity of central synapses: afferent and target regulation. Nat. Neurosci. 4, 569–578. [DOI] [PubMed] [Google Scholar]
- Cybulska‐Klosowicz A, Posluszny A, Nowak K, Siucinska E, Kossut M, Liguz‐Lecznar M (2013) Interneurons containing somatostatin are affected by learning‐induced cortical plasticity. Neuroscience 254, 18–25. [DOI] [PubMed] [Google Scholar]
- David‐Jürgens M, Dinse HR (2010) Effects of aging on paired‐pulse behavior of rat somatosensory cortical neurons. Cereb. Cortex 20, 1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR (2007) Changes in the structural complexity of the aged brain. Aging Cell 6, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emson PC (2007) GABA(B) receptors: structure and function. Prog. Brain Res. 160, 43–57. [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ (1991) Two genes encode distinct glutamate decarboxylases. Neuron 7, 91–100. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR (2002) Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol. Psychiatry 52, 805–810. [DOI] [PubMed] [Google Scholar]
- Foeller E, Celikel T, Feldman DE (2005) Inhibitory sharpening of receptive fields contributes to whisker map plasticity in rat somatosensory cortex. J. Neurophysiol. 94, 4387–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Panzanelli P, Tyagarajan SK (2012) Molecular and functional heterogeneity of GABAergic synapses. Cell. Mol. Life Sci. 69, 2485–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE (2007) A synaptic memory trace for cortical receptive field plasticity. Nature 450, 425–429. [DOI] [PubMed] [Google Scholar]
- Garner CC, Kindler S, Gundelfinger ED (2000) Molecular determinants of presynaptic active zones. Curr. Opin. Neurobiol. 10, 321–327. [DOI] [PubMed] [Google Scholar]
- Gold JR, Bajo VM (2014) Insult‐induced adaptive plasticity of the auditory system. Front. Neurosci. 8, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubellini P, Ben‐Ari Y, Gaïarsa JL (2001) Activity‐ and age‐dependent GABAergic synaptic plasticity in the developing rat hippocampus. Eur. J. Neurosci. 14, 1937–1946. [DOI] [PubMed] [Google Scholar]
- Gupta A, Wang Y, Markram H (2000) Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science 287, 273–278. [DOI] [PubMed] [Google Scholar]
- Gutiérrez A, Khan ZU, Ruano D, Miralles CP, Vitorica J, De Blas AL (1996) Aging‐related subunit expression changes of the GABAA receptor in the rat hippocampus. Neuroscience 74, 341–348. [DOI] [PubMed] [Google Scholar]
- Gutiérrez A, Khan ZU, Miralles CP, Mehta AK, Ruano D, Araujo F, De Blas AL (1997) GABAA receptor subunit expression changes in the rat cerebellum and cerebral cortex during aging. Brain Res. Mol. Brain Res. 45, 59–70. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Yamashita A, Shimizu K (1997) Somatostatin and brain‐derived neurotrophic factor mRNA expression in the primate brain: decreased levels of mRNAs during aging. Brain Res. 749, 283–289. [DOI] [PubMed] [Google Scholar]
- Heise KF, Zimerman M, Hoppe J, Gerloff C, Wegscheider K, Hummel FC (2013) The aging motor system as a model for plastic changes of GABA‐mediated intracortical inhibition and their behavioral relevance. J. Neurosci. 33, 9039–9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm KA, Haberman RP, Dean SL, Hoyt EC, Melcher T, Lund PK, Gallagher M (2005) GABAB receptor antagonist SGS742 improves spatial memory and reduces protein binding to the cAMP response element (CRE) in the hippocampus. Neuropharmacology 48, 956–964. [DOI] [PubMed] [Google Scholar]
- Hua T, Kao C, Sun Q, Li X, Zhou Y (2008) Decreased proportion of GABA neurons accompanies age‐related degradation of neuronal function in cat striate cortex. Brain Res. Bull. 75, 119–125. [DOI] [PubMed] [Google Scholar]
- Jasinska M, Siucinska E, Cybulska‐Klosowicz A, Pyza E, Furness DN, Kossut M, Glazewski S (2010) Rapid, learning‐induced inhibitory synaptogenesis in murine barrel field. J. Neurosci. 30, 1176–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal B, Holman C, de Villers‐Sidani E (2013) Shaping the aging brain: role of auditory input patterns in the emergence of auditory cortical impairments. Front. Syst. Neurosci. 7, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmarkar UR, Buonomano DV (2006) Different forms of homeostatic plasticity are engaged with distinct temporal profiles. Eur. J. Neurosci. 23, 1575–1584. [DOI] [PubMed] [Google Scholar]
- Kawaguchi SY, Hirano T (2002) Signaling cascade regulating long‐term potentiation of GABA(A) receptor responsiveness in cerebellar Purkinje neurons. J. Neurosci. 22, 3969–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koksma JJ, Fritschy JM, Mack V, Van Kesteren RE, Brussaard AB (2005) Differential GABAA receptor clustering determines GABA synapse plasticity in rat oxytocin neurons around parturition and the onset of lactation. Mol. Cell Neurosci. 28, 128–140. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Moreau AW, Bakiri Y, Nicholson E (2012) Plasticity of inhibition. Neuron 75, 951–962. [DOI] [PubMed] [Google Scholar]
- Kumar D, Thakur MK (2015) Age‐related expression of Neurexin1 and Neuroligin3 is correlated with presynaptic density in the cerebral cortex and hippocampus of male mice. Age 37, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K, Sharma S, Kumar P, Deshmukh R (2013) Therapeutic potential of GABA(B) receptor ligands in drug addiction, anxiety, depression and other CNS disorders. Pharmacol. Biochem. Behav. 110, 174–184. [DOI] [PubMed] [Google Scholar]
- Lasarge CL, Bañuelos C, Mayse JD, Bizon JL (2009) Blockade of GABA(B) receptors completely reverses age‐related learning impairment. Neuroscience 164, 941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann K, Schmidt KF, Löwel S (2012) Vision and visual plasticity in ageing mice. Restor. Neurol. Neurosci. 30, 161–178. [DOI] [PubMed] [Google Scholar]
- Lenz M, Tegenthoff M, Kohlhaas K, Stude P, Höffken O, Gatica Tossi MA, Dinse HR (2012) Increased excitability of somatosensory cortex in aged humans is associated with impaired tactile acuity. J. Neurosci. 32, 1811–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Lüthi A (2011) A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331–335. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y (2003) GABA and its agonists improved visual cortical function in senescent monkeys. Science 300, 812–815. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D (2008) Subunit‐selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am. J. Psychiatry 165, 1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li SH, Yu ZX, Shelbourne P, Li XJ (2001) Huntingtin aggregate‐associated axonal degeneration is an early pathological event in Huntington's disease mice. J. Neurosci. 21, 8473–8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jin M, Zhang D, Yang T, Koeglsperger T, Fu H, Selkoe DJ (2013) Environmental novelty activates β2‐adrenergic signaling to prevent the impairment of hippocampal LTP by Aβ oligomers. Neuron 77, 929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C, Han Q, Ma Y, Su B (2016) Age‐related gene expression change of GABAergic system in visual cortex of rhesus macaque. Gene 590, 227–233. [DOI] [PubMed] [Google Scholar]
- Lien CC, Mu Y, Vargas‐Caballero M, Poo MM (2006) Visual stimuli‐induced LTD of GABAergic synapses mediated by presynaptic NMDA receptors. Nat. Neurosci. 9, 372–380. [DOI] [PubMed] [Google Scholar]
- Liguz‐Lecznar M, Siucinska E, Zakrzewska R, Kossut M (2011) Impairment of experience‐dependent cortical plasticity in aged mice. Neurobiol. Aging 32, 1896–1905. [DOI] [PubMed] [Google Scholar]
- Liguz‐Lecznar M, Lehner M, Kaliszewska A, Zakrzewska R, Sobolewska A, Kossut M (2015) Altered glutamate/GABA equilibrium in aged mice cortex influences cortical plasticity. Brain Struct. Funct. 220, 1681–1693. [DOI] [PubMed] [Google Scholar]
- Liguz‐Lecznar M, Urban‐Ciecko J, Kossut M (2016) Somatostatin and somatostatin‐containing neurons in shaping neuronal activity and plasticity. Front. Neural. Circuits. 10, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limon A, Reyes‐Ruiz JM, Miledi R (2012) Loss of functional GABA(A) receptors in the Alzheimer diseased brain. Proc. Natl Acad. Sci. USA 109, 10071–10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LC, Sibille E (2013) Reduced brain somatostatin in mood disorders: a common pathophysiological substrate and drug target? Front. Pharmacol. 4, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling LL, Hughes LF, Caspary DM (2005) Age‐related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience 132, 1103–1113. [DOI] [PubMed] [Google Scholar]
- Loerch PM, Lu T, Dakin KA, Vann JM, Isaacs A, Geula C, Yankner BA (2008) Evolution of the aging brain transcriptome and synaptic regulation. PLoS ONE 3, e3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreth D, Ozmen L, Revel FG, Knoflach F, Wetzel P, Frotscher M, …, Kretz O (2012) Selective degeneration of septal and hippocampal GABAergic neurons in a mouse model of amyloidosis and tauopathy. Neurobiol. Dis. 47, 1–12. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Chang YM, Moore TL, Rosene DL (2004) Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience 125, 277–288. [DOI] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL (2011) GABAA receptor trafficking‐mediated plasticity of inhibitory synapses. Neuron 70, 385–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maćkowiak M, Mordalska P, Wędzony K (2014) Neuroligins, synapse balance and neuropsychiatric disorders. Pharmacol. Rep. 66, 830–835. [DOI] [PubMed] [Google Scholar]
- Maffei A (2011) The many forms and functions of long term plasticity at GABAergic synapses. Neural. Plast. 2011, 254724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG (2006) Potentiation of cortical inhibition by visual deprivation. Nature 443, 81–84. [DOI] [PubMed] [Google Scholar]
- Mahncke HW, Bronstone A, Merzenich MM (2006) Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog. Brain Res. 157, 81–109. [DOI] [PubMed] [Google Scholar]
- Majdi M, Ribeiro‐da‐Silva A, Cuello AC (2007) Cognitive impairment and transmitter‐specific pre‐ and postsynaptic changes in the rat cerebral cortex during ageing. Eur. J. Neurosci. 26, 3583–3596. [DOI] [PubMed] [Google Scholar]
- Majdi M, Ribeiro‐da‐Silva A, Cuello AC (2009) Variations in excitatory and inhibitory postsynaptic protein content in rat cerebral cortex with respect to aging and cognitive status. Neuroscience 159, 896–907. [DOI] [PubMed] [Google Scholar]
- Marín O (2012) Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 13, 107–120. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo‐Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C (2004) Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793–807. [DOI] [PubMed] [Google Scholar]
- Masliah E, Crews L, Hansen L (2006) Synaptic remodeling during aging and in Alzheimer's disease. J. Alzheimers Dis. 9(Suppl. 3), 91–99. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, …, Weinberger DR (2006) Neurophysiological correlates of age‐related changes in working memory capacity. Neurosci. Lett. 392, 32–37. [DOI] [PubMed] [Google Scholar]
- McQuail JA, Bañuelos C, LaSarge CL, Nicolle MM, Bizon JL (2012) GABA(B) receptor GTP‐binding is decreased in the prefrontal cortex but not the hippocampus of aged rats. Neurobiol. Aging 33, 1124.e1121–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Frazier CJ, Bizon JL (2015) Molecular aspects of age‐related cognitive decline: the role of GABA signaling. Trends Mol. Med. 21, 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen R, Sirviö J, Riekkinen P, Laakso MP, Riekkinen M (1993) Neocortical, hippocampal and septal parvalbumin‐ and somatostatin‐containing neurons in young and aged rats: correlation with passive avoidance and water maze performance. Neuroscience 53, 367–378. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Albin RL, Caspary DM (1994) Age‐related decrease in GABAB receptor binding in the Fischer 344 rat inferior colliculus. Neurobiol. Aging 15, 699–703. [DOI] [PubMed] [Google Scholar]
- Miller EJ, Saint Marie LR, Breier MR, Swerdlow NR (2010) Pathways from the ventral hippocampus and caudal amygdala to forebrain regions that regulate sensorimotor gating in the rat. Neuroscience 165, 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG (2012) The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat. Rev. Neurosci. 13, 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava‐Mesa MO, Jiménez‐Díaz L, Yajeya J, Navarro‐Lopez JD (2014) GABAergic neurotransmission and new strategies of neuromodulation to compensate synaptic dysfunction in early stages of Alzheimer's disease. Front. Cell Neurosci. 8, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Cull‐Candy S, Farrant M (1997) Differences in synaptic GABA(A) receptor number underlie variation in GABA mini amplitude. Neuron 19, 697–709. [DOI] [PubMed] [Google Scholar]
- Ouda L, Druga R, Syka J (2008) Changes in parvalbumin immunoreactivity with aging in the central auditory system of the rat. Exp. Gerontol. 43, 782–789. [DOI] [PubMed] [Google Scholar]
- Ouda L, Burianova J, Syka J (2012) Age‐related changes in calbindin and calretinin immunoreactivity in the central auditory system of the rat. Exp. Gerontol. 47, 497–506. [DOI] [PubMed] [Google Scholar]
- Ouda L, Profant O, Syka J (2015) Age‐related changes in the central auditory system. Cell Tissue Res. 361, 337–358. [DOI] [PubMed] [Google Scholar]
- Ouellet L, de Villers‐Sidani E (2014) Trajectory of the main GABAergic interneuron populations from early development to old age in the rat primary auditory cortex. Front. Neuroanat. 8, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude C, Chapman CA, Bertrand S, Congar P, Lacaille JC (2003) GABAB receptor‐ and metabotropic glutamate receptor‐dependent cooperative long‐term potentiation of rat hippocampal GABAA synaptic transmission. J. Physiol. 553(Pt 1), 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Sethares C, Luebke JI (2008) Synapses are lost during aging in the primate prefrontal cortex. Neuroscience 152, 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini EM, Ravasenga T, Hausrat TJ, Iurilli G, Olcese U, Racine V, Barberis A (2014) Synaptic recruitment of gephyrin regulates surface GABAA receptor dynamics for the expression of inhibitory LTP. Nat. Commun. 5, 3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto JG, Hornby KR, Jones DG, Murphy KM (2010) Developmental changes in GABAergic mechanisms in human visual cortex across the lifespan. Front. Cell Neurosci. 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posluszny A, Liguz‐Lecznar M, Turzynska D, Zakrzewska R, Bielecki M, Kossut M (2015) Learning‐dependent plasticity of the barrel cortex is impaired by restricting GABA‐ergic transmission. PLoS ONE 10, e0144415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier B, Jouvenceau A, Epelbaum J, Dutar P (2006) Age‐related alterations of GABAergic input to CA1 pyramidal neurons and its control by nicotinic acetylcholine receptors in rat hippocampus. Neuroscience 142, 187–201. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M (2001) Enforcement of temporal fidelity in pyramidal cells by somatic feed‐forward inhibition. Science 293, 1159–1163. [DOI] [PubMed] [Google Scholar]
- Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Varoqueaux F (2009) Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron 63, 628–642. [DOI] [PubMed] [Google Scholar]
- Ratté S, Lacaille JC (2006) Selective degeneration and synaptic reorganization of hippocampal interneurons in a chronic model of temporal lobe epilepsy. Adv. Neurol. 97, 69–76. [PubMed] [Google Scholar]
- Richardson BD, Ling LL, Uteshev VV, Caspary DM (2013) Reduced GABA(A) receptor‐mediated tonic inhibition in aged rat auditory thalamus. J. Neurosci. 33, 1218–1227a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman RA, Mobley WC (2011) Implications for treatment: GABAA receptors in aging, Down syndrome and Alzheimer's disease. J. Neurochem. 117, 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman RA, De Blas AL, Armstrong DM (2007) GABA(A) receptors in aging and Alzheimer's disease. J. Neurochem. 103, 1285–1292. [DOI] [PubMed] [Google Scholar]
- Rossignol E (2011) Genetics and function of neocortical GABAergic interneurons in neurodevelopmental disorders. Neural. Plast. 2011, 649325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC, Buzsáki G (2012) Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat. Neurosci. 15, 769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruano D, Araujo F, Revilla E, Vela J, Bergis O, Vitorica J (2000) GABAA and alpha‐amino‐3‐hydroxy‐5‐methylsoxazole‐4‐propionate receptors are differentially affected by aging in the rat hippocampus. J. Biol. Chem. 275, 19585–19593. [DOI] [PubMed] [Google Scholar]
- Rubio SE, Vega‐Flores G, Martínez A, Bosch C, Pérez‐Mediavilla A, del Río J, …, Pascual M (2012) Accelerated aging of the GABAergic septohippocampal pathway and decreased hippocampal rhythms in a mouse model of Alzheimer's disease. FASEB J. 26, 4458–4467. [DOI] [PubMed] [Google Scholar]
- Saito T, Iwata N, Tsubuki S, Takaki Y, Takano J, Huang SM, Saido TC (2005) Somatostatin regulates brain amyloid beta peptide Abeta42 through modulation of proteolytic degradation. Nat. Med. 11, 434–439. [DOI] [PubMed] [Google Scholar]
- Sale A, Berardi N, Maffei L (2014) Environment and brain plasticity: towards an endogenous pharmacotherapy. Physiol. Rev. 94, 189–234. [DOI] [PubMed] [Google Scholar]
- Scelfo B, Sacchetti B, Strata P (2008) Learning‐related long‐term potentiation of inhibitory synapses in the cerebellar cortex. Proc. Natl Acad. Sci. USA 105, 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Redecker C, Bruehl C, Witte OW (2010) Age‐related decline of functional inhibition in rat cortex. Neurobiol. Aging 31, 504–511. [DOI] [PubMed] [Google Scholar]
- Scimemi A (2014) Structure, function, and plasticity of GABA transporters. Front. Cell Neurosci. 8, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia G, Yagüe AG, García‐Verdugo JM, Mora F (2006) Environmental enrichment promotes neurogenesis and changes the extracellular concentrations of glutamate and GABA in the hippocampus of aged rats. Brain Res. Bull. 70, 8–14. [DOI] [PubMed] [Google Scholar]
- Sgadò P, Genovesi S, Kalinovsky A, Zunino G, Macchi F, Allegra M, …, Bozzi Y (2013) Loss of GABAergic neurons in the hippocampus and cerebral cortex of Engrailed‐2 null mutant mice: implications for autism spectrum disorders. Exp. Neurol. 247, 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Walsh DM (2009) Alzheimer's disease: synaptic dysfunction and Abeta. Mol. Neurodegener. 4, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS (2010) A critical role for alpha4betadelta GABAA receptors in shaping learning deficits at puberty in mice. Science 327, 1515–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Bates A (2016) Potential of GABA‐ergic cell therapy for schizophrenia, neuropathic pain, and Alzheimer's and Parkinson's diseases. Brain Res. 1638(Pt A), 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Argenta AE, Winseck AK, Brunso‐Bechtold JK (2004) Stereological quantification of GAD‐67‐immunoreactive neurons and boutons in the hippocampus of middle‐aged and old Fischer 344 x Brown Norway rats. J. Comp. Neurol. 478, 282–291. [DOI] [PubMed] [Google Scholar]
- Sibille E (2013) Molecular aging of the brain, neuroplasticity, and vulnerability to depression and other brain‐related disorders. Dialogues Clin. Neurosci. 15, 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Sperk G (2002) Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr. Top. Med. Chem. 2, 795–816. [DOI] [PubMed] [Google Scholar]
- Siucinska E (2006) GAD67‐positive puncta: contributors to learning‐dependent plasticity in the barrel cortex of adult mice. Brain Res. 1106, 52–62. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K (2009) Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler H, Dorca‐Arévalo J, González M, Rubio SE, Ávila J, Soriano E, Pascual M (2017) The GABAergic septohippocampal connection is impaired in a mouse model of tauopathy. Neurobiol. Aging 49, 40–51. [DOI] [PubMed] [Google Scholar]
- Stan AD, Lewis DA (2012) Altered cortical GABA neurotransmission in schizophrenia: insights into novel therapeutic strategies. Curr. Pharm. Biotechnol. 13, 1557–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley DP, Shetty AK (2004) Aging in the rat hippocampus is associated with widespread reductions in the number of glutamate decarboxylase‐67 positive interneurons but not interneuron degeneration. J. Neurochem. 89, 204–216. [DOI] [PubMed] [Google Scholar]
- Stanley EM, Fadel JR, Mott DD (2012) Interneuron loss reduces dendritic inhibition and GABA release in hippocampus of aged rats. Neurobiol. Aging 33, 431.e431–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundman‐Eriksson I, Allard P (2006) Age‐correlated decline in [3H]tiagabine binding to GAT‐1 in human frontal cortex. Aging Clin. Exp. Res. 18, 257–260. [DOI] [PubMed] [Google Scholar]
- Tang Y, Stryker MP, Alvarez‐Buylla A, Espinosa JS (2014) Cortical plasticity induced by transplantation of embryonic somatostatin or parvalbumin interneurons. Proc. Natl Acad. Sci. USA 111, 18339–18344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarski K, Urban‐Ciecko J, Kossut M, Hess G (2007) Sensory learning‐induced enhancement of inhibitory synaptic transmission in the barrel cortex of the mouse. Eur. J. Neurosci. 26, 134–141. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador‐Woodruff JH, Knable MB (2005) Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol. Psychiatry 57, 252–260. [DOI] [PubMed] [Google Scholar]
- Tremblay R, Lee S, Rudy B (2016) GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91, 260–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon SM, Albin RL (1994) GABAB binding sites in early adult and aging rat brain. Neurobiol. Aging 15, 705–711. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB (1998) Activity‐dependent scaling of quantal amplitude in neocortical neurons. Nature 391, 892–896. [DOI] [PubMed] [Google Scholar]
- Urban‐Ciecko J, Kossut M, Mozrzymas JW (2010) Sensory learning differentially affects GABAergic tonic currents in excitatory neurons and fast spiking interneurons in layer 4 of mouse barrel cortex. J. Neurophysiol. 104, 746–754. [DOI] [PubMed] [Google Scholar]
- Vela J, Gutierrez A, Vitorica J, Ruano D (2003) Rat hippocampal GABAergic molecular markers are differentially affected by ageing. J. Neurochem. 85, 368–377. [DOI] [PubMed] [Google Scholar]
- de Villers‐Sidani E, Alzghoul L, Zhou X, Simpson KL, Lin RC, Merzenich MM (2010) Recovery of functional and structural age‐related changes in the rat primary auditory cortex with operant training. Proc. Natl Acad. Sci. USA 107, 13900–13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Lewis DA (2005) GABA targets for the treatment of cognitive dysfunction in schizophrenia. Curr. Neuropharmacol. 3, 45–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Maffei A (2014) Inhibitory plasticity dictates the sign of plasticity at excitatory synapses. J. Neurosci. 34, 1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW (1999) GABA(A)‐receptor‐associated protein links GABA(A) receptors and the cytoskeleton. Nature 397, 69–72. [DOI] [PubMed] [Google Scholar]
- Wang H, Xie X, Li X, Chen B, Zhou Y (2006) Functional degradation of visual cortical cells in aged rats. Brain Res. 1122, 93–98. [DOI] [PubMed] [Google Scholar]
- Wang Y, Neubauer FB, Lüscher HR, Thurley K (2010) GABAB receptor‐dependent modulation of network activity in the rat prefrontal cortex in vitro . Eur. J. Neurosci. 31, 1582–1594. [DOI] [PubMed] [Google Scholar]
- Wei YN, Hu HY, Xie GC, Fu N, Ning ZB, Zeng R, Khaitovich P (2015) Transcript and protein expression decoupling reveals RNA binding proteins and miRNAs as potential modulators of human aging. Genome Biol. 16, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CM, Ji S, Cai H, Maudsley S, Martin B (2010) Therapeutic potential of vasoactive intestinal peptide and its receptors in neurological disorders. CNS Neurol. Disord Drug Targets 9, 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C (2006) Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res. 1099, 73–81. [DOI] [PubMed] [Google Scholar]
- Zettel ML, Frisina RD, Haider SE, O'Neill WE (1997) Age‐related changes in calbindin D‐28k and calretinin immunoreactivity in the inferior colliculus of CBA/CaJ and C57Bl/6 mice. J. Comp. Neurol. 386, 92–110. [DOI] [PubMed] [Google Scholar]


