ABSTRACT
Platelet-rich plasma (PRP) therapy is promising for treating skeletal muscle injuries in human athletes by promoting muscle regeneration. It might also be useful for treating muscle injuries in equine athletes. In the present study, muscle regeneration induced by injection of PRP into intact muscle of Thoroughbred was investigated. Autologous PRP and saline were injected twice into intact left and right gluteus medius muscles of seven clinically healthy Thoroughbreds. Muscle samples were collected from the injection sites by needle biopsy at 2 and 7 days after PRP injection. Immunohistochemical staining to identify the types of myosin heavy chains (MHCs) and satellite cells was performed to compare morphological changes among intact (pre-injection), saline-, and PRP-injected muscles. The expression of marker genes related to muscle regeneration (MHC-I, MHC-II, and embryonic MHC [MHC-e]), satellite cell activity (CK, Pax7, MyoD, and myogenin), and proinflammatory and promyogenic cytokines (IL-6, IGF-1, and HGF) was analyzed and compared between saline- and PRP-injected muscles. There were no obvious morphological differences among the three treatments. There were no significant differences in gene expression associated with satellite cell activity between saline and PRP injection at 7 days after injection. MHC genes showed significantly higher expression levels with PRP than with saline, including MHC-e at 2 days and MHC-I at 7 days after injection. It is suggested that injection of PRP into intact skeletal muscle does not induce specific morphological changes, but upregulate the expression of genes related to muscle regeneration.
Keywords: growth factor, muscle regeneration, myosin heavy chains, platelet-rich plasma, satellite cell
Platelet-rich plasma (PRP) therapy is widespread in human and animal medicine [7, 13, 16, 21, 28]. PRP is easily prepared from autologous whole blood by differential centrifugation and contains concentrated platelets [5,6,7, 25]. Therefore, PRP therapy is characterized by immunological safety and cost-effectiveness. Once the platelets are activated, their α-granules release various growth factors, including the transforming growth factor-β (TGF-β) and the platelet-derived growth factor (PDGF) [7]. The efficacy of PRP therapy is believed to be due to the growth factors enhancing tissue regeneration and promoting the healing of lesions [7]. In human medicine, PRP therapy has been developed together with regenerative cell therapy, especially in oral and dental surgery [10, 11, 18]. In orthopedic surgery, PRP is widely administered for the treatment of disorders including those of tendons, ligaments, bones, joints, and muscles, and favorable results in a clinical context have been reported [8, 21]. In equine medicine, PRP therapy is recognized as a technological innovation for the treatment of tendon and ligament injuries. In a study using culture explants, Schnabel et al. reported that PRP upregulated collagen gene expression in the superficial digital flexor tendon [23]. Recently, the administration of PRP in vivo has also been investigated [3, 27].
PRP therapy is now considered to be promising as a novel method of treating skeletal muscle injuries in human athletes [1, 21]. Hamid et al. reported the results of a randomized controlled trial in which a single autologous PRP injection induced earlier recovery of human acute hamstring injuries [1]. Mei-Dan et al. reviewed many case reports describing the effectiveness of PRP injection for human muscle injury [21]. In experimental animal injury models, it was reported that PRP injection promoted muscle regeneration morphologically [9]. Considering these findings, it is also possible that PRP therapy would be useful for muscle injury in equine athletes.
Although many studies on PRP for equine tendon and ligament injuries have been reported, to the best of our knowledge, no detailed reports regarding PRP therapy for equine skeletal muscles have been published. We hypothesized that the expression of genes associated with muscle regeneration would be upregulated and that the activation of satellite cell proliferation would be observed morphologically upon PRP injection, as is the case in injured rats [6, 9]. The purpose of this study was thus to investigate the changes in morphology and gene expression that are relevant to skeletal muscles and satellite cells induced by injection of PRP into healthy intact skeletal muscle.
Materials and Methods
Subjects
Seven clinically healthy Thoroughbreds (six males and one female, 6–12 years of age, weighing 435–493 kg) were used in this study. All experimental procedures were reviewed and approved by the Animal Welfare and Ethics Committee of the Equine Research Institute of the Japan Racing Association (authorization number, 2012-9).
Preparation of PRP
One hundred milliliters of equine whole blood was collected from the jugular vein with a disposable plastic syringe containing 10% sodium citrate anticoagulant (ACD-A injection, Terumo BCT Ltd., Tokyo, Japan). The blood was equally dispensed into 10 polypropylene tubes and then centrifuged at 400 × g for 7 min at 4°C. The plasma fraction of each tube was transferred into another polypropylene tube and then centrifuged at 2,000 × g for 7 min at 4°C. The supernatant was removed, and the remaining 1 ml of PRP in the bottom of each tube was resuspended and collected. The concentration of platelets in the PRP was determined using an automated blood cell counter (Sysmex K-4500, Sysmex Corp., Kobe, Japan). The obtained PRP was stored at −30°C until administered, and a single freeze-thaw cycle was used to induce platelet activation and the release of growth factors [22, 23].
Concentrations of PDGF isoform BB (PDGF-BB) and TGF-β isoform 1 (TGF-β1) in the freeze-thawed PRP were determined using ELISA kits (Quantikine Human PDGF-BB ELISA DBB00 and Quantikine Human TGF-β1 ELISA DB100B, R&D Systems, Minneapolis, MN, U.S.A.). These kits were designed for use in samples obtained from humans but have also been validated for use in horses [3, 23, 26].
Injection of PRP into gluteus medius muscle
All procedures were performed under sedation with medetomidine hydrochloride (Domitor, Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan; 5 µg/kg of body weight, IV). Three square patches (2 × 2 cm) on the right gluteus medius muscle were clipped and sterilized. These squares were set 5 cm apart from each other. First, intact muscle samples (PRE) were obtained from one patch by needle biopsy using a SuperCoreTM Biopsy Instrument (Argon Medical Devices, Inc., Plano, TX, U.S.A.) under local anesthesia with 2% lidocaine infiltration (Xylocaine, AstraZeneca K.K., Osaka, Japan). Subsequently, 4 ml of activated autologous PRP or 4 ml of saline was uniformly injected into the muscle at 2 to 3 cm under the skin around the remaining two patches. Five days after the first injection into the right side, the same procedure was performed for the left side symmetrically. At 7 days after the first injection, needle biopsy was performed from all of the injection sites on both sides of the gluteus medius muscle. The muscle samples obtained from the right side were named group 7D (i.e., 7 days after injection), and those from the left side were named group 2D (i.e., 2 days after injection). All biopsy samples were frozen quickly using liquid nitrogen and stored at −80°C until analysis. A prophylactic antibiotic (MYCILLIN inj. NZ, Nippon Zenyaku Kogyo Co., Ltd.) was given intramuscularly in the neck, and the wounds were covered with sterile dressings (PRIMAPORE, Smith & Nephew Wound Management K.K., Tokyo, Japan) after biopsy.
Immunohistochemical staining and morphological analysis
Serial sections of the biopsy specimens were made and stained according to the immunohistochemical staining method of Kawai et al. [15]. Briefly, serial 8 µm cross sections of the muscle were obtained on a cryostat (CM510, Leica Microsystems K.K., Tokyo, Japan) at −20°C and then dried on slides. Immunohistochemical staining was subsequently performed to identify the types of myosin heavy chains (MHCs). A primary monoclonal antibody was applied, namely, either (1) the mouse monoclonal antibody MY-32 (Sigma-Aldrich Japan, Tokyo, Japan), which specifically reacts with MHC-IIa and MHC-IIx; or (2) the mouse monoclonal antibody SC-71 (DSHB, Iowa City, IA, U.S.A.), which specifically reacts with MHC-IIa, followed by a reaction with a secondary antibody (goat anti-mouse IgG) conjugated with horseradish peroxidase (Bio-Rad Laboratories, Hercules, CA, U.S.A.). Diaminobenzidine tetrahydrochloride (DAB) was used as a chromogen.
To identify satellite cells, immunofluorescent staining was performed on another serial section from each biopsy, fixed in 4% paraformaldehyde. The primary monoclonal antibodies [a mouse anti-Pax7 (DSHB) and a rabbit anti-laminin L9393 (Sigma-Aldrich Co. LLC, St. Louis, MO, U.S.A.)] were applied, followed by the application of secondary antibodies: Cy3-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Inc., West Grove, PA, U.S.A.) and Alexa Fluor 488 goat anti-rabbit IgG (Molecular Probes, Tokyo, Japan), respectively. Cell nuclei were counterstained with DAPI.
Images of the stained muscle fibers were recorded with a photomicroscopic image-processing system (ACT-2U, Nikon, Tokyo, Japan). The fibers were classified as type I [MY-32 (−), SC-71 (−)], IIa [MY-32 (+), SC-71 (+)], or IIx [MY-32 (+), SC-71 (−)], and the fiber-type component ratio was determined according to Yamano et al. [29].
The images of the distributions of anti-Pax7, anti-laminin, and cell nuclei were merged using image processing software (Photoshop, Adobe Systems Inc., San Jose, CA, U.S.A.) and used for the identification of satellite cells. The cells positive for both DAPI and Pax7 at the periphery of each fiber beneath the basal lamina stained with the anti-laminin were identified as satellite cells [15].
Thereafter, about 25 fibers of type I and 50 fibers of type IIa and type IIx were randomly chosen, the numbers of myonuclei and satellite cells were counted, and the cross-sectional area of myofibers was then measured for each different type of fiber using the ImageJ software (NIH, Bethesda, MD, U.S.A.), followed by calculation of the mean number of myonuclei per fiber, the central nuclear ratio, the mean myonuclear domain size, and the mean number of satellite cells per fiber.
Real-time RT-PCR
Each muscle sample was analyzed for the expression of several mRNAs using a real-time RT-PCR system, in accordance with the method of Kawai et al. [15]. Total RNA was extracted using a TRIzol reagent (Invitrogen, Tokyo, Japan). The purity and yields of total RNA were determined by measuring the absorbance of the aliquots at 260 and 280 nm. Total RNA was then treated for 30 min at 37°C with TURBO DNase (Ambion, Tokyo, Japan) to remove genomic DNA from samples. DNase-treated RNA (0.5 µg) was used to synthesize first-strand cDNA with an ExscriptTM RT Reagent Kit (Takara, Shiga, Japan). Thereafter, the cDNA products were analyzed by real-time PCR using the SYBR Green PCR Master Mix protocol in a StepOne Real Time PCR System (Applied Biosystems Japan, Tokyo, Japan).
The amplification program included an initial denaturation step at 95°C for 10 min, and then 40 cycles of denaturation at 95°C for 30 sec and annealing/extension at 58°C for 1 min. The amount of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was estimated as an internal control. Each mRNA was normalized to GAPDH by subtracting the cycle threshold (Ct) value of GAPDH from the Ct value of the gene target. The relative expression of the target gene was calculated as the relative quantification (RQ) value for the PRE value. After determining the relative expression, dissociation-curve analysis detected no nonspecific amplification in cDNA samples.
Gene expression was analyzed, including for MHC-I, MHC-II, embryonic (i.e., undifferentiated) MHC (MHC-e), creatine kinase (CK), Pax7, myogenic determination factor (MyoD), myogenin, interleukin-6 (IL-6), insulin-like growth factor isoform 1 (IGF-1), and hepatocyte growth factor (HGF). The sequences of the specific primers used in this experiment are listed in the Appendix Table A1. Each PCR primer was designed using Primer Express software v3.0 (Kanagawa, Japan), and the oligonucleotides were purchased from FASMAC Co., Ltd. (Kanagawa, Japan).
Table A1. Real-time RT-PCR primer sequences used in the study.
| GAPDH | F | CAAGGCTGTGGGCAAGGT |
| R | GGAAGGCCATGCCAGTGA | |
| MHC-I | F | GGAATGACAACTCCTCTCGCTTT |
| R | TCAATATCAGCAGAAGCCAGTTTC | |
| MHC-II | F | CCCATGAACCCTCCCAAATA |
| R | GCAGGCTCATGCAGGTGAGT | |
| MHC-e | F | TCACTTTGTGCGCTGTATAATTCC |
| R | CTGGTGCAGGACAAGGCTATG | |
| CK | F | GGAATCAGCGCCGGAAA |
| R | ACCAATAGCACCCCCTGTCA | |
| Pax7 | F | CATCGGCGGCAGCAA |
| R | TCCTCGATCTTTTTCTCCACATC | |
| MyoD | F | ACGGCTCTCTCTGCAACTTTG |
| R | GAGTCGAAACACGGGTCATCA | |
| Myogenin | F | TCACGGCTGACCCTACAGATG |
| R | GGTGATGCTGTCCACAATGG | |
| IL-6 | F | AACAACTCACCTCATCCTTCGAA |
| R | CGAACAGCTCTCAGGCTGAAC | |
| IGF-1 | F | TGTCCTCCTCACATCTCTTCTACCT |
| R | CGTGGCAGAGCTGGTGAA | |
| HGF | F | GGTACGCTACGAAGTCTGTGACA |
| R | CCCATTGCAGGTCATGCAT |
Statistical analysis
The data obtained from the histochemical analysis were compared using one-way ANOVA. To compare the mRNA expression of each target gene between the groups injected with saline and those injected with PRP, the Wilcoxon signed-rank test was used. Analyses were performed using Microsoft Excel 2007 Macro applications (Statcel3, OMS Publishing Inc., Saitama, Japan). Statistical significance was set at P<0.05.
Results
PRP
The number of platelets collected from the seven horses ranged from 5.4 to 13.3 × 104/µl (mean ± SD, 9.8 ± 2.8 × 104/µl) in whole blood, and from 62.6 to 85.0 × 104/µl (72.3 ± 8.2 × 104/µl) in PRP. The concentration of platelets in PRP ranged from 5.4 to 12.1 times (7.9 ± 2.3 times) higher than that in whole blood. The freeze-thawed PRP contained 5.0 ± 1.6 ng/ml PDGF-BB and 15.4 ± 6.6 ng/ml TGF-β1. The concentrations of PDGF-BB and TGF-β1 in PRP were 5.0 ± 0.7 times and 3.7 ± 1.7 times higher than those in serum, respectively. There was no correlation among the concentrations of platelets, PDGF-BB, and TGF-β1 in PRP.
Morphological analysis of muscle
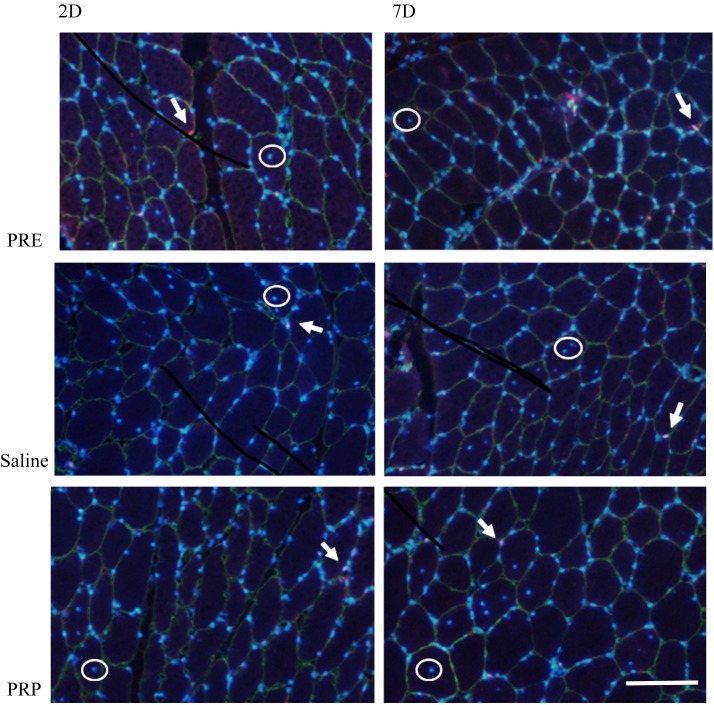
Typical images of immunohistochemical and immunofluorescent staining are shown in Fig. 1. The results of morphological measurements of muscle are shown in Table 1. There were no obvious morphological findings or significant differences among the PRE, saline, and PRP injection groups within the same type of myofiber and the same number of days after injection.
Fig. 1.
Typical merged images of triple-immunofluorescent staining for laminin (green), Pax7 (red), and nuclei (blue) before PRP or saline injection and at 2 and 7 days after it. All images contain some satellite cells (white arrow) and central nuclei (white circle). 2D, 2 days after injection, and 7D, 7 days after injection. Scale bar=100 µm.
Table 1. The morphological measurements of the muscle samples.
| Type I | Type IIa | Type IIx | |||
|---|---|---|---|---|---|
| Fiber-type component ratio (%) | 2D | PRE | 12.8 ± 4.1 | 47.7 ± 4.6 | 39.5 ± 5.4 |
| Saline | 10.0 ± 3.9 | 49.0 ± 5.4 | 41.0 ± 8.3 | ||
| PRP | 11.9 ± 2.7 | 46.4 ± 7.4 | 41.7 ± 8.5 | ||
| 7D | PRE | 11.7 ± 5.3 | 46.5 ± 5.2 | 41.8 ± 7.7 | |
| Saline | 12.1 ± 3.0 | 47.9 ± 6.3 | 40.0 ± 7.5 | ||
| PRP | 11.9 ± 3.3 | 44.6 ± 4.0 | 43.5 ± 6.5 | ||
| Cross-sectional area of fibers (×103µm2) | 2D | PRE | 2.61 ± 0.80 | 3.25 ± 0.50 | 4.22 ± 0.45 |
| Saline | 2.66 ± 0.83 | 3.18 ± 0.46 | 3.75 ± 0.43 | ||
| PRP | 2.61 ± 1.04 | 3.15 ± 1.15 | 3.85 ± 0.93 | ||
| 7D | PRE | 2.38 ± 0.77 | 3.10 ± 0.55 | 4.06 ± 0.78 | |
| Saline | 2.86 ± 0.81 | 3.06 ± 0.76 | 3.76 ± 1.01 | ||
| PRP | 2.69 ± 0.79 | 2.92 ± 0.58 | 3.97 ± 0.90 | ||
| Myonuclei/fiber | 2D | PRE | 2.63 ± 0.41 | 2.83 ± 0.42 | 2.92 ± 0.35 |
| Saline | 3.06 ± 0.45 | 2.87 ± 0.48 | 2.85 ± 0.30 | ||
| PRP | 2.83 ± 0.43 | 3.11 ± 0.55 | 3.02 ± 0.47 | ||
| 7D | PRE | 2.91 ± 0.35 | 3.00 ± 0.32 | 3.18 ± 0.33 | |
| Saline | 3.00 ± 0.49 | 3.13 ± 0.55 | 3.05 ± 0.61 | ||
| PRP | 3.14 ± 0.38 | 3.12 ± 0.37 | 3.20 ± 0.40 | ||
| Central nuclear ratio (%) | 2D | PRE | 4.00 ± 6.11 | 4.57 ± 7.09 | 7.42 ± 8.46 |
| Saline | 1.71 ± 4.53 | 2.57 ± 2.76 | 9.71 ± 11.10 | ||
| PRP | 0.57 ± 1.51 | 1.71 ± 1.80 | 4.57 ± 2.23 | ||
| 7D | PRE | 0 | 0 | 1.00 ± 2.00 | |
| Saline | 3.42 ± 6.29 | 5.42 ± 6.80 | 8.29 ± 6.68 | ||
| PRP | 1.14 ± 1.95 | 4.00 ± 4.47 | 8.57 ± 10.11 | ||
| Mean myonuclear domain (×103µm2) | 2D | PRE | 1.14 ± 0.41 | 1.23 ± 0.36 | 1.60 ± 0.35 |
| Saline | 0.93 ± 0.28 | 1.19 ± 0.28 | 1.50 ± 0.28 | ||
| PRP | 0.98 ± 0.35 | 1.07 ± 0.26 | 1.39 ± 0.28 | ||
| 7D | PRE | 0.89 ± 0.28 | 1.13 ± 0.21 | 1.45 ± 0.36 | |
| Saline | 1.00 ± 0.14 | 1.06 ± 0.19 | 1.36 ± 0.35 | ||
| PRP | 0.91 ± 0.25 | 1.04 ± 0.26 | 1.36 ± 0.25 | ||
| Mean number of satellite cells/fiber (×10−1) | 2D | PRE | 2.91 ± 1.84 | 3.86 ± 1.93 | 3.34 ± 2.09 |
| Saline | 3.63 ± 1.81 | 3.40 ± 1.33 | 3.49 ± 1.54 | ||
| PRP | 3.74 ± 1.54 | 3.74 ± 1.46 | 3.20 ± 1.97 | ||
| 7D | PRE | 2.10 ± 0.60 | 3.25 ± 1.35 | 2.70 ± 0.35 | |
| Saline | 3.23 ± 1.93 | 3.74 ± 1.68 | 3.17 ± 1.53 | ||
| PRP | 3.14 ± 1.95 | 3.87 ± 1.00 | 3.57 ± 1.00 | ||
There were no significant differences among the treatments (PRE, saline, and PRP) within the same type of myofiber and same number of days after injection (2D, 2 days after injection, and 7D, 7 days after injection; mean ± SD).
Real-time RT-PCR
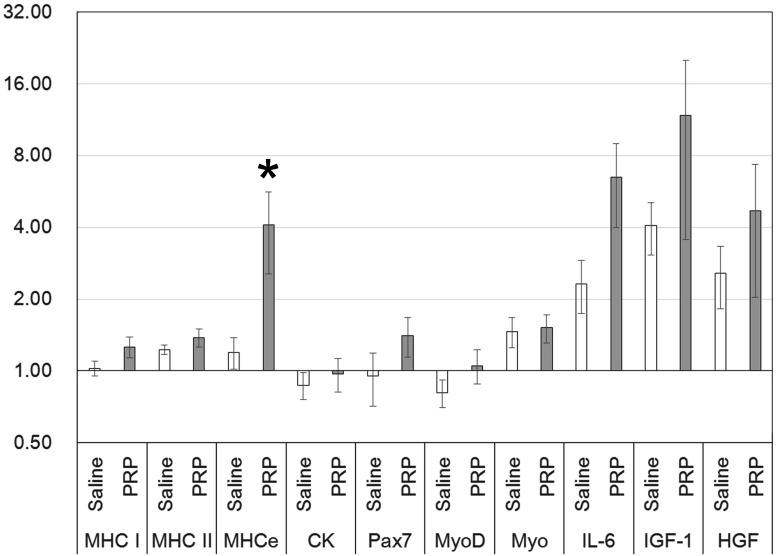
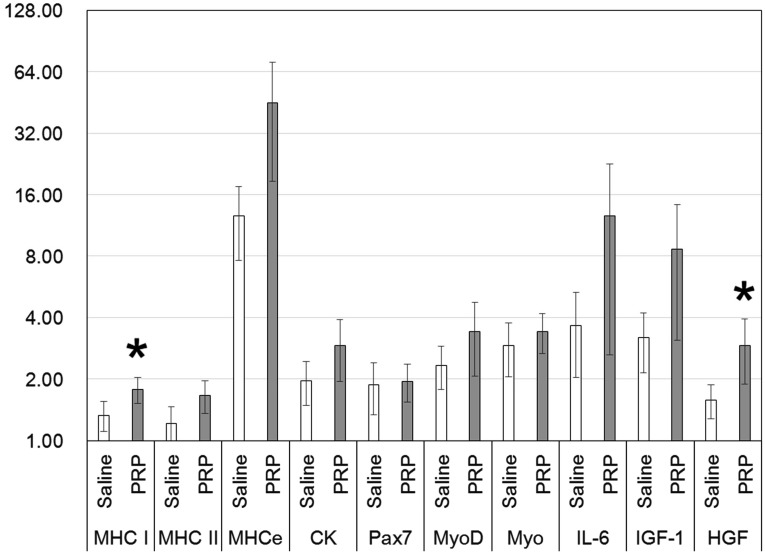
At 2 days after intramuscular injection, MHC-e gene expression was significantly higher in the case of treatment with PRP (mean ± SE, 4.09 ± 1.54) compared with treatment with saline (1.20 ± 0.18) (Fig. 2). At 7 days after intramuscular injection, MHC-I gene expression was significantly higher in the case of treatment with PRP (1.78 ± 0.26) compared with treatment with saline (1.33 ± 0.22) (Fig. 3).
Fig. 2.
The mRNA expression of the skeletal muscle samples obtained 2 days after intramuscular injection (n=7; mean ± SE; logarithmic scale). The value of the target gene is calculated as the relative quantification (RQ) value for the PRE value set at 1.00. The MHC-e expression level in the case of treatment with PRP was significantly higher than that in the case of treatment with saline (*P<0.05). Myo=myogenin.
Fig. 3.
The mRNA expression of the skeletal muscle samples obtained 7 days after intramuscular injection (n=7; mean ± SE; logarithmic scale). The value of the target gene is calculated as the relative quantification (RQ) value for the PRE value set at 1.00. MHC-I and HGF expression levels in the case of treatment with PRP were significantly higher than those in the case of treatment with saline (*P<0.05). Myo=myogenin.
At 7 days, CK, Pax7, MyoD, and myogenin expression did not differ significantly between saline and PRP injection, although their levels were low at 2 days after the injection of either PRP or saline (Figs. 2 and 3). The expression levels of other genes, namely, IL-6, IGF-1, and HGF, were similar at 2 and 7 days after injection of either PRP or saline (Figs. 2 and 3). A significant difference in the expression of HGF was detected upon comparison of PRP (2.91 ± 1.03) and saline (1.58 ± 0.29) at 7 days after injection (Fig. 3).
Discussion
Human studies have indicated that a therapeutic dose of platelets in PRP is approximately 1.0 × 106/µl, which is four to five times higher than the level in whole blood [7, 19]. Although the appropriate dose of platelets in PRP for equine practice remains unclear, the number of platelets and the growth factor concentrations of the PRP used in this study were similar to those in previous studies [23, 25]; therefore, we concluded that an effective level of PRP was administered in this study.
In the injured human muscle, satellite cell proliferation peaks at days 2–3 following injury, and significant numbers of centronucleated myofibers are observed at days 5–7 [24]. In our study, however, there was no difference in the histological findings among the three groups regardless of sampling time. In rodent injury models, Hammond et al. reported that PRP promoted muscle regeneration functionally and histologically [9]. By contrast, Delos et al. [5] reported that there was no difference functionally or morphologically between saline and PRP injection. In both reports, a common view was expressed that PRP may be of greater utility in treating multiple repetition injury, which relies on a muscle regeneration mechanism associated with satellite cell activity, rather than in treating a single contusion, which predominantly causes sarcolemmal injury. In our study, PRP was not injected into sites with severe injury, such as multiple repetition injury, but was instead injected into intact healthy muscle. Thus, the fact that no proliferation was observed in satellite cells and myofibers induced by PRP injection seems to be consistent with these suggestions.
Hammond et al. reported that PRP enhanced the expression of MyoD and myogenin genes in rodent injury models [9]. In this study, however, the same expression levels of marker genes related to myogenic satellite cell proliferation and differentiation, including Pax7, MyoD, myogenin, and CK [12, 14], were observed in both the PRP and saline groups at 7 days after injection. Our results were insufficient to prove that PRP upregulated satellite cell activity.
Injury causes the activation of inflammatory cells, and these cells release multiple cytokines including IL-6 [4, 30]. It is also known that IL-6 works as an activator of STAT3 protein, which activates satellite cells [14, 30]. In our study, the expression of the IL-6 gene was detected in muscle samples obtained around the injection sites despite using intact muscle. On comparing the PRP and saline injection groups, higher expression tended to be observed in the PRP group. However, no significant difference was observed at either sampling time points because of large standard errors. Dimauro et al. reported that PRP could not induce upregulation of the expression of the IL-6 gene by using a rat muscle injury model [6]. Our results support their finding despite the fact that we used intact muscle. These findings may suggest that the effect of PRP on the expression of the IL-6 gene could considerably vary between individuals. PRP injected into intact muscle seemed to have no apparent effect on satellite cell activity compared with saline injection.
Additionally, although the expression of growth factor genes such as HGF and IGF-1 was detected in the 2D and 7D samples, there was no significant difference in the expression of these genes between the PRP and saline injections, except that the expression of HGF in the PRP group was significantly higher than that in the saline group in the 7D sample. HGF and IGF-1 are known to be stored within the α-granules of platelets [3, 20, 23] and to promote skeletal muscle regeneration [14, 24]. It is also known that activated platelets induce the coagulation cascade and platelet degranulation with the release of various growth factors upon bleeding injury [2, 7]. If considerable bleeding was caused by the PRP or saline injection itself, it would be likely that activated platelets could release growth factors, including HGF and IGF-1, and equally upregulate the expression of these genes around the injection sites via a paracrine mechanism, regardless of the injected solution [2, 19]. Irrespective of this, no additional effect of PRP was observed in this study. Although the reason for the significantly higher expression of HGF in the PRP group in the 7D sample remains unclear, our results suggest that PRP has no effect on growth factor activity, as well as satellite cell activity and IL-6 expression.
Meanwhile, we detected significantly higher levels of MHC-e expression 2 days after PRP injection and significantly higher levels of MHC-I expression 7 days after PRP injection compared with the equivalent periods after saline injection. A number of studies have reported positive effects of PRP on myogenic proliferation and differentiation [6, 9, 21]. The elevation of the expression of MHC-e and MHC-I genes in this study might have been caused by satellite cell activation induced by PRP because activated satellite cells are essential for regenerating MHC [14, 17]. However, it was unclear why PRP injection had no significant effect on the expression of genes related to satellite cell activity, including Pax7, MyoD, myogenin, and CK. It is also necessary to mention that this preliminary study using intact muscle had a limitation in that the mechanism by which gene expression was influenced by the PRP or saline injection itself was not determined.
In summary, it is suggested that injection of PRP into intact skeletal muscle does not induce specific morphological changes, but upregulates the expression of genes related to muscle regeneration. Further studies such as on the administration of PRP into an experimentally induced severe wound in skeletal muscle without considerable bleeding would be needed to clarify the effects of PRP injection on muscle regeneration or repair.
Acknowledgments
The authors thank Drs. Minako Kawai and Yoko Imaoka for their technical assistance.
References
- 1.A Hamid M.S., Mohamed Ali M.R., Yusof A., George J., Lee L.P. 2014. Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial. Am. J. Sports Med. 42: 2410–2418. [DOI] [PubMed] [Google Scholar]
- 2.Barrientos S., Stojadinovic O., Golinko M.S., Brem H., Tomic-Canic M. 2008. Growth factors and cytokines in wound healing. Wound Repair Regen. 16: 585–601. [DOI] [PubMed] [Google Scholar]
- 3.Bosch G., van Schie H.T., de Groot M.W., Cadby J.A., van de Lest C.H., Barneveld A., van Weeren P.R. 2010. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: a placebo-controlled experimental study. J. Orthop. Res. 28: 211–217. [DOI] [PubMed] [Google Scholar]
- 4.Daley J.M., Brancato S.K., Thomay A.A., Reichner J.S., Albina J.E. 2010. The phenotype of murine wound macrophages. J. Leukoc. Biol. 87: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delos D., Leineweber M.J., Chaudhury S., Alzoobaee S., Gao Y., Rodeo S.A. 2014. The effect of platelet-rich plasma on muscle contusion healing in a rat model. Am. J. Sports Med. 42: 2067–2074. [DOI] [PubMed] [Google Scholar]
- 6.Dimauro I., Grasso L., Fittipaldi S., Fantini C., Mercatelli N., Racca S., Geuna S., Di Gianfrancesco A., Caporossi D., Pigozzi F., Borrione P. 2014. Platelet-rich plasma and skeletal muscle healing: a molecular analysis of the early phases of the regeneration process in an experimental animal model. PLoS One 9: e102993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everts P.A., Knape J.T., Weibrich G., Schönberger J.P., Hoffmann J., Overdevest E.P., Box H.A., van Zundert A. 2006. Platelet-rich plasma and platelet gel: a review. J. Extra Corpor. Technol. 38: 174–187. [PMC free article] [PubMed] [Google Scholar]
- 8.Gosens T., Peerbooms J.C., van Laar W., den Oudsten B.L. 2011. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am. J. Sports Med. 39: 1200–1208. [DOI] [PubMed] [Google Scholar]
- 9.Hammond J.W., Hinton R.Y., Curl L.A., Muriel J.M., Lovering R.M. 2009. Use of autologous platelet-rich plasma to treat muscle strain injuries. Am. J. Sports Med. 37: 1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang Y.J., Choi J.Y. 2010. Addition of mesenchymal stem cells to the scaffold of platelet-rich plasma is beneficial for the reduction of the consolidation period in mandibular distraction osteogenesis. J. Oral Maxillofac. Surg. 68: 1112–1124. [DOI] [PubMed] [Google Scholar]
- 11.Inchingolo F., Tatullo M., Marrelli M., Inchingolo A.M., Inchingolo A.D., Dipalma G., Flace P., Girolamo F., Tarullo A., Laino L., Sabatini R., Abbinante A., Cagiano R. 2012. Regenerative surgery performed with platelet-rich plasma used in sinus lift elevation before dental implant surgery: an useful aid in healing and regeneration of bone tissue. Eur. Rev. Med. Pharmacol. Sci. 16: 1222–1226. [PubMed] [Google Scholar]
- 12.Jaynes J.B., Chamberlain J.S., Buskin J.N., Johnson J.E., Hauschka S.D. 1986. Transcriptional regulation of the muscle creatine kinase gene and regulated expression in transfected mouse myoblasts. Mol. Cell. Biol. 6: 2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jee C.H., Eom N.Y., Jang H.M., Jung H.W., Choi E.S., Won J.H., Hong I.H., Kang B.T., Jeong D.W., Jung D.I. 2016. Effect of autologous platelet-rich plasma application on cutaneous wound healing in dogs. J. Vet. Sci. 17: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karalaki M., Fili S., Philippou A., Koutsilieris M. 2009. Muscle regeneration: cellular and molecular events. In Vivo 23: 779–796. [PubMed] [Google Scholar]
- 15.Kawai M., Aida H., Hiraga A., Miyata H. 2013. Muscle satellite cells are activated after exercise to exhaustion in Thoroughbred horses. Equine Vet. J. 45: 512–517. [DOI] [PubMed] [Google Scholar]
- 16.Kim J.H., Park C., Park H.M. 2009. Curative effect of autologous platelet-rich plasma on a large cutaneous lesion in a dog. Vet. Dermatol. 20: 123–126. [DOI] [PubMed] [Google Scholar]
- 17.Lepper C., Partridge T.A., Fan C.M. 2011. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138: 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marukawa E., Oshina H., Iino G., Morita K., Omura K. 2011. Reduction of bone resorption by the application of platelet-rich plasma (PRP) in bone grafting of the alveolar cleft. J. Craniomaxillofac. Surg. 39: 278–283. [DOI] [PubMed] [Google Scholar]
- 19.Marx R.E. 2004. Platelet-rich plasma: evidence to support its use. J. Oral Maxillofac. Surg. 62: 489–496. [DOI] [PubMed] [Google Scholar]
- 20.Matsuo R., Ohkohchi N., Murata S., Ikeda O., Nakano Y., Watanabe M., Hisakura K., Myronovych A., Kubota T., Narimatsu H., Ozaki M. 2008. Platelets strongly induce hepatocyte proliferation with IGF-1 and HGF in vitro. J. Surg. Res. 145: 279–286. [DOI] [PubMed] [Google Scholar]
- 21.Mei-Dan O., Lippi G., Sánchez M., Andia I., Maffulli N. 2010. Autologous platelet-rich plasma: a revolution in soft tissue sports injury management? Phys. Sportsmed. 38: 127–135. [DOI] [PubMed] [Google Scholar]
- 22.Schnabel L.V., Sonea H.O., Jacobson M.S., Fortier L.A. 2008. Effects of platelet rich plasma and acellular bone marrow on gene expression patterns and DNA content of equine suspensory ligament explant cultures. Equine Vet. J. 40: 260–265. [DOI] [PubMed] [Google Scholar]
- 23.Schnabel L.V., Mohammed H.O., Miller B.J., McDermott W.G., Jacobson M.S., Santangelo K.S., Fortier L.A. 2007. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J. Orthop. Res. 25: 230–240. [DOI] [PubMed] [Google Scholar]
- 24.Shi X., Garry D.J. 2006. Muscle stem cells in development, regeneration, and disease. Genes Dev. 20: 1692–1708. [DOI] [PubMed] [Google Scholar]
- 25.Textor J.A., Norris J.W., Tablin F. 2011. Effects of preparation method, shear force, and exposure to collagen on release of growth factors from equine platelet-rich plasma. Am. J. Vet. Res. 72: 271–278. [DOI] [PubMed] [Google Scholar]
- 26.Theoret C.L., Barber S.M., Moyana T.N., Gordon J.R. 2001. Expression of transforming growth factor beta(1), beta(3), and basic fibroblast growth factor in full-thickness skin wounds of equine limbs and thorax. Vet. Surg. 30: 269–277. [DOI] [PubMed] [Google Scholar]
- 27.Waselau M., Sutter W.W., Genovese R.L., Bertone A.L. 2008. Intralesional injection of platelet-rich plasma followed by controlled exercise for treatment of midbody suspensory ligament desmitis in Standardbred racehorses. J. Am. Vet. Med. Assoc. 232: 1515–1520. [DOI] [PubMed] [Google Scholar]
- 28.Xie X., Zhao S., Wu H., Xie G., Huangfu X., He Y., Zhao J. 2013. Platelet-rich plasma enhances autograft revascularization and reinnervation in a dog model of anterior cruciate ligament reconstruction. J. Surg. Res. 183: 214–222. [DOI] [PubMed] [Google Scholar]
- 29.Yamano S., Eto D., Kasashima Y., Hiraga A., Sugiura T., Miyata H. 2005. Evaluation of developmental changes in the coexpression of myosin heavy chains and metabolic properties of equine skeletal muscle fibers. Am. J. Vet. Res. 66: 401–405. [DOI] [PubMed] [Google Scholar]
- 30.Zhang C., Li Y., Wu Y., Wang L., Wang X., Du J. 2013. Interleukin-6/signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J. Biol. Chem. 288: 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]