Abstract
More than ten percent of people suffer from at least one episode of depression and related mental disorders in a lifetime, and depression and related mental disorders are one of the world's greatest public health problems. A multiple system theory holds that dysregulation of the multiple systems underlies the pathogenesis of depression and related mental disorders, and new therapies based on the multiple system dysregulation theory are urgently needed. In this study, the antidepressant effect of decoction from herb Cistanche deserticola Y.C.Ma and Cistanche tubulosa was examined. Herb Cistanche decoction reduced the immobility period significantly in the mouse tail suspension test. Mice treated with herb decoction showed an improved ability of spatial learning and memory in the Morris water maze test. Groups treated herb decoction displayed a downregulated monoamine oxidase (MAO) activity; the dopamine (DA) concentration in the brain was upregulated, indicating herb Cistanche decoction improved the nerve excitability; the serum concentration of corticosterone (CORT) was downregulated, showing that mice benefited from a reduced stress level. Hence, the antidepressant efficacy and mechanism of traditional Chinese herb Cistanche were explored in this study. Herb Cistanche showed a potential to be developed as a complementary and alternative therapy for depression.
1. Introduction
Depression and related mood disorders, with an estimate of 12~17% of the population experiencing at least one episode in a lifetime, are among the leading causes of mental disabilities and considered as one of the worldwide major public health problems [1]. Of particular relevance, a significant proportion of patients with depression suffer from memory loss, concentration problems, decreased energy or fatigue, loss of interest or pleasure in hobbies and activities, and difficulty in sleeping, early-morning awakening, or oversleeping. Although the neural substrates for abnormality in depression and related syndromes are unclear, it is generally accepted that the impairment of neuroplasticity and cellular resilience gives rise to the pathophysiology of depression and successful treatment may depend on the intervention of the neurotransmitter level [2]. The approach to depression medication is basically based on the classic monoamine system hypothesis, which claims that dysregulation of monoamines especially serotonin (5-HT) and norepinephrine (NE) contributes to the depression pathogenesis, and intervention of the neurotransmitter system with dopamine agonists and dopamine-noradrenergic modulators would alleviate the symptoms of major depression disorder [3]. Although widely prescribed, the antidepressants curative efficacy is questionable and the side-effect cannot be ignored because a significant proportion of the patients do not respond to the monoamine system manipulation [4]. Recently, novel theories of depression pathogenesis indicate that multiple systems, including inflammatory pathways, the oxidative stress pathway, the hypothalamic-pituitary-adrenal (HPA) axis, the metabolic and bioenergetics system, neurotrophic pathways, the glutamate system, the opioid system, and the cholinergic system, are closely associated with the depression occurrence [3]. Evidences show that multiple target intervention produced better efficacy than one single target intervention [5]. New therapies based on the multiple system dysregulation theory are urgently needed.
Herbal products reportedly consumed more than half of US population [6]; interest and demands for herb or herbal products as a supplemental way of treating depression are growing all over the world. Multiple medical plants are found to demonstrate depression-relieving beneficial effect. Herbs that are reported to possess the effect of suppressing depression and improving cognitive function include sea buckthorn [7], Ginkgo biloba [8], Piper nigrum [9], Hypericum perforatum [10], Griffonia simplicifolia [11]. A combination administration of ferulic acid and piperine displayed a synergistic antidepressant-like effect in mice by manipulating the monoaminergic system [7]. Albiflorin was characterized by a high selective affinity to the neurotransmitter receptors and transporters and significantly increased extracellular concentrations of 5-HT and NE in the hypothalamus of freely moving rats [12]. A medical herb Melissa officinalis L. relieved the depressive-behavior of mice by exerting the influence on the the serotonergic neurotransmitter level [13]. These herbs or decoctions, developed as the alternative remedies for depression, help alleviate the symptoms of depression and provide a new source for the antidepressant drug developing. Herb Cistanche species, distributed in arid lands and deserts northern part of China, are traditional Chinese medicine herbs and widely used for treating various diseases for more than 1,000 years in China. The multiple efficacy of herb Cistanche decoction ranges between the aphrodisiac [14], immune-promoting [15], antioxidative [16], and hepatoprotective [17] properties. C. deserticola decoctions induce the testicular steroidogenic enzymes [18], promote penis erectile response, and shorten the latency period of erectile reaction in castrated rats [19]. Herb Cistanche has been reported to inhibit the activities of dopaminergic neurons by regulating the inhibitory apoptosis genes and neurotrophic factors [20]. C. tubulosa decoctions amended the cognitive dysfunction caused by Aβ 1-42 via blocking amyloid deposition and revived the cholinergic and hippocampal dopaminergic neuronal function [21]. The neuroprotective property of herbal Cistanche implies the therapeutic potential in the cognitive-related diseases. Depression is a common mixed mode of emotion reactions with forgetfulness and cognitive decline as the common symptoms [22]. Herb Cistanche was commonly used as a traditional Chinese medicine to treat forgetfulness; moreover, a recent report shows that an early-adult outset administration of the cornmeal supplemented with C. tubulosa is beneficial for the longevity promotion and olfactory-associated learning and memory improvement in the nonvertebrate Drosophila model [23]. These facts indicate the cognitive improving property of herb Cistanche; here in this research, for the first time we characterized the antidepressant property and its relationship to cognitive improvement activities in the mammal model by regulating the monoamine system and HPA axis. Illuminating the antidepressant property and preliminary mechanism of herb Cistanche could expand the application spectrum of herb Cistanche, which could be developed as an alternative remedy for depression.
2. Materials and Methods
2.1. Plant Material and Decoction Preparation
C. deserticola Y.C.Ma was obtained from the Nei Mongol Autonomous Region, China; C. tubulosa was obtained from the Xinjiang Uygur Autonomous Region, China. Before decoction, the herb (C. deserticola Y.C.Ma or C. tubulosa) was inspected to detect the content of echinacoside and verbascoside using HPLC (high performance liquid chromatography) (Milford, MA, Waters, USA), and the eligible product (according to Pharmacopoeia of the People's Republic of China) was used for the animal experiments. The decoction was acquired based on the method of water vaporing developed by Food and Health Engineering Research Center of State Education Ministry, Sun Yat-sen University. Dried Cistanche deserticola Y.C.Ma of 125 g was smashed and sieved by 120-mesh screen and then powder was dissolved in 3 L ultrapure water, heated at 100°C for 2 h, then cooled down to room temperature, and sieved; the supernatant was recycled. The sediment was then dissolved in 2 L ultrapure water and then heated for 1.5 h and cooled down as mentioned above to recycle the supernatant; the sediment was then used for recycling the supernatant. The content of all the supernatant was enriched by rotary vacuum evaporators at 60°C; finally the crude drug concentration was set as 0.5 g/ml and the product was stored at −20°C.
2.2. Echinacoside and Verbascoside Assay by HPLC Analysis
HPLC (high performance liquid chromatography) was used to measure the qualitative and quantitative assay of echinacoside and verbascoside; verbascoside (purity > 93%) and echinacoside (purity > 93%) were purchased from the National Institutes for Food and Drug Control (Beijing, China) and utilized as a standard control.
2.3. The Animal Care and Experimental Conduct
The subjects were SPF (specific pathogen-free) male Kunming mice weighing 21~25 grams, supplied by Medical Experimental Animal Center of Guangdong Province. The animals were housed in plastic cages (ten per cage); the environment condition was set such as follows: humidity 40~70%, room temperature 21 ± 1°C, and a 12 h : 12 h light-dark period. All animal experiments were approved by the Animal Ethics Committee of Sun Yat-sen University.
2.4. Tail Suspension Test
The tail suspension test was done according to the protocol described by Cryan et al. [24] with modest changes. Briefly, on day 21, the mice were suspended by the tail using metallic gallows tethered by nylon catheter, with head at the height 50 mm from the floor. The mice were hung on the hook on adhesive tapes 10~20 mm from the extremity of its tail and were isolated acoustically and visually isolated by clapboards. The body movements of the mouse were recorded and the respiratory movements were ignored. Before the day of experiment, the mice were suspended for 8 min for adaptation.
2.5. The Morris Water Maze Test
Water maze test was done according to the description of Vorhees et al. [25–27] using the device developed by Chinese Academy of Medical Sciences. A circular pool (150 cm diameter) filled with water (26 ± 1°C; rim height (distance water surface to wall rim): 10 cm) was used, circular rescue platform (diameter: 11 cm; distance between platform center point and pool wall: 27 cm) was submerged 1–1.5 cm below the water surface, and the testing area was illuminated with indirect lighting (50 ± 10 lux) to avoid reflections. Four start locations were used, N, S, E, and W. For the spatial learning test, animals were trained daily on days 9–11 on the fixed start location. The escape latency period is measured to display the spatial memory and learning ability of the mice. If the mice could not find or climb onto the platform, the mice would be leaded to the platform and stay for 10 s. After taking a rest for 60 s, then next session of training would be started. Data obtained from the last spatial learning test were used as base value for molding. On days 23–28, place navigation test and spatial probe were done. The place navigation test lasted for 6 days and was taken on 1 o'clock p.m., animals were placed into the water with their head facing the pool wall clockwise, and the latency period (within 2 min) was recorded. After the place navigation test was done, the platform was removed. The mice were placed into water with their head facing the pool wall at a random location, and the motion trail and the frequency that mice swam across where the platform was within 2 min were recorded. The motion trail was recorded by multifunction autonomic recorder of mice motion (YLS-1A, Yiyan Technology, Jinan, China).
2.6. Detection of the MAO Activity
The MAO activity was measured using MAO detection kit (A034, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and the detection was done according to the manufacture's protocol. Homogenate from brain tissue was obtained; 500 μl was mixed with 300 μl of Agent 1 and 300 μl of Agent 2, incubated for 3 h at 37°C, and then successively added with 300 μl, of Agent 3, 3 ml of Agent 4, and vortex for 2 min. The reaction mixture was centrifuged 3,000 rpm for 10 min, and the optic density of the supernatant was determined by ultraviolet and visible spectrum spectrophotometer (Lambda 25, PerkinElmer, USA) using 242 nm excitation light.
2.7. Enzyme Linked Immunosorbent Assay of Neurotransmitters and ACTH and CORT
Enzyme Linked Immunosorbent Assay (ELISA) detection kit for dopamine (DA), norepinephrine (NE), and 5-hydroxytryptamine (5-HT) was bought from BLUE GENE, Shanghai. ELISA detection kits were bought from Qiyun Biotech, Guangzhou.
The procedures are such as follows: the experiment was conducted at room temperature. Coat the wells of a PVC microtiter plate with the capture antibody at 1–10 μg/mL concentration in carbonate/bicarbonate buffer (pH 9.6). Seal the plate and incubate overnight 4°C; wash the plate with PBS/tween 3 times, and the nonspecific binding sites are blocked by Block Solution; then wash the plate with PBS/tween 3 times. Standards and samples are diluted in Blocking Solution and 100 μl is added in the well of the plate, and the plate is sealed and incubated at 37°C for 1 h. Add 100 μl diluted detection antibody and incubate the plate at 37°C for 1 h; wash the plate with PBS/Tween 3 times. Add 50 μl color development solution and keep the plate away from the light and incubate it at room temperature for 15 min; 50 μl stop buffer is added to stop the reaction. Measure the optical density (OD) for each well with a microplate reader set to 405 nm in 30 min.
2.8. Statistical Analysis
All the data were represented as mean ± SEM (standard error of the mean), the significance difference was analyzed by t-test or one-way ANOVA followed by Duncan's multiple range test using SPSS 16.0, and differences between groups with P < 0.05 were considered statistically significant.
3. Results
3.1. Determining the Content of Main Bioactive Ingredients in the C. deserticola Y.C.Ma and C. tubulosa Samples
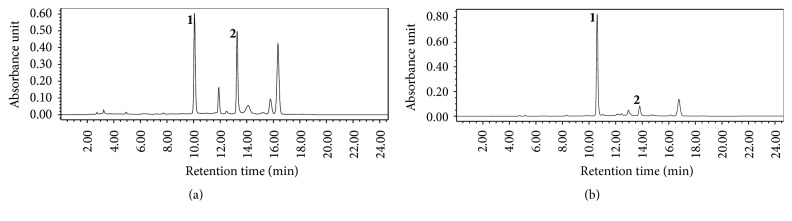
The phenylethanoid glycosides such as echinacoside and verbascoside are generally considered as the main bioactive ingredients; hence the content of echinacoside and verbascoside in both C. deserticola Y.C.Ma and C. tubulosa samples was determined by HPLC. The HPLC chromatograms were shown in Figure 1, and the results show that the total content of phenylethanoid glycosides from C. deserticola Y.C.Ma was approximately twice that from C. tubulosa. Echinacoside and verbascoside weights, respectively, account for 1.27 ± 0.009% and 0.52 ± 0.003% of powder prepared from C. deserticola Y.C.Ma and 19.81 ± 0.66% and 2.45 ± 0.12% of powder prepared from C. tubulosa. Echinacoside and verbascoside weights, respectively, account for 0.90 ± 0.001% and 0.20 ± 0.001% of C. deserticola Y.C.Ma decoction and 5.90 ± 0.12% and 0.54 ± 0.05% of powder prepared from C. tubulosa (Table 1).
Figure 1.
The HPLC chromatographic profile of phenylethanoid glycosides from the C. deserticola Y.C.Ma and C. tubulosa powder. Peaks 1 and 2 represent echinacoside and verbascoside, respectively.
Table 1.
The content of echinacoside and verbascoside in the powder and decoction of C. deserticola Y.C.Ma and C. tubulosa.
| Samples | PeGs% | |
|---|---|---|
| ECH% | VerBS% | |
| C. deserticola powder | 1.27 ± 0.009 | 0.52 ± 0.003 |
| C. tubulosa powder | 19.81 ± 0.66 | 2.45 ± 0.12 |
| C. deserticola decoction | 0.90 ± 0.001 | 0.20 ± 0.001 |
| C. tubulosa decoction | 5.90 ± 0.12 | 0.54 ± 0.05 |
Echinacoside, ECH; phenylethanoid glycosides, PeGs; verbascoside, VerBS.
3.2. Decoction of C. deserticola Y.C.Ma and C. tubulosa Alleviated the Stress and Depression Level
Tail suspension test (TST) is a quick and classic method to assess the antidepressant effect of drugs in mice [24]; in this research, the immobile status, caused by the short-term and inescapable stress of being suspended by the tail, was used as a trait to reflect the depression level of mice. We checked whether decoction of C. deserticola Y.C.Ma and C. tubulosa could reverse the immobility and promote the occurrence of escape-related behavior. Compared to control groups, the immobility period of the stress group increased by 27.4%; compared to the stress groups, the groups treated with medium, high concentration of C. deserticola Y.C.Ma herb decoction showed a statistically significant decrease of immobility period each by 44.1% and 56.1%; the groups treated with medium, high concentration of C. tubulosa herb decoction showed a statistically significant decrease of immobility period each by 41.9% and 47.7% (Figure 2). These results indicate that decoction of C. deserticola Y.C.Ma and C. tubulosa could decrease the stress and depression level of mice, and C. deserticola Y.C.Ma decoction exhibited a slightly higher efficacy than C. tubulosa decoction.
Figure 2.
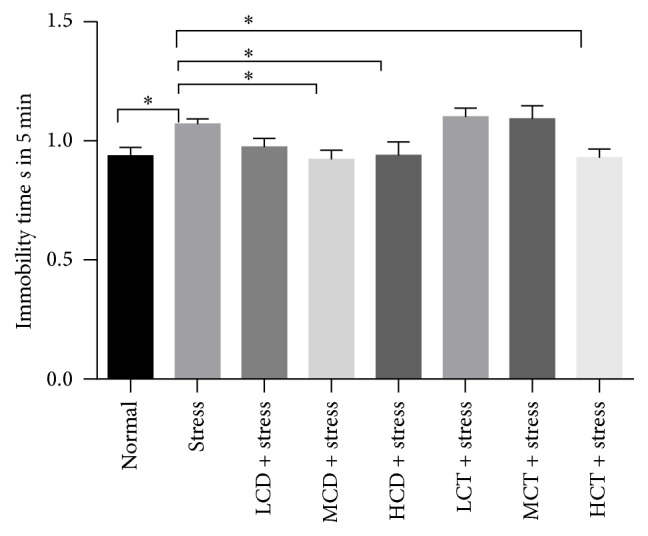
Effect of C. deserticola Y.C.Ma and C. tubulosa decoctions on immobility period of tail suspension mice in 5 min. After adaptation, the experimental groups were administered with herb Cistanche decoction by gavage for 21 days and then suspended by tail and the movement was monitored in 5 min after suspension; each group was comprised of 10 mice. L, M, and H, respectively, represent low, medium, and high; CD and CT, respectively, represent C. deserticola Y.C.Ma and C. tubulosa decoction. All the data were represented as mean ± SEM; P < 0.05 was considered as significantly different and was marked as “∗”.
3.3. Decoction of C. deserticola Y.C.Ma and C. tubulosa Improved the Spatial Learning and Memory of Mice
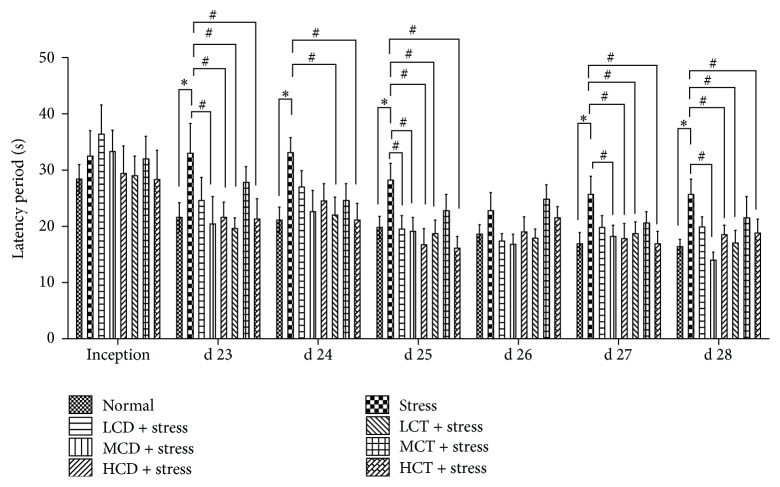
People (both adolescent and adult) afflicted to depression are prone to suffering learning disability [28]; decoction of C. deserticola Y.C.Ma and C. tubulosa was proved to alleviate the stress and depression level; then we chose to assess whether decoction of C. deserticola Y.C.Ma and C. tubulosa could affect the spatial learning and memory by using the Morris water maze model, which is widely applied in assessing the spatial learning and memory function of related brain area [29]. The motion trails are classified into four during the search latency; a straight line trajectory showed the mice pinpointed the rescue platform's position, a tendency trajectory showed that the mice exhibited a basically right orientation, and the edgy type trajectory showed that the mice either were in the early training period or suffered from dementia, trying to locate target place based on instinct; the random searching trajectory showed that mice had low ability of positioning and searched the target place aimlessly. Data were acquired according to the criteria.
Effect of C. deserticola Y.C.Ma and C. tubulosa decoctions on the space learning and memory capability of restraint stressed mice is shown in Figure 3. After tail suspension test, mice showed a significantly prolonged latency period. Compared to the stress groups, the mice treated moderate, high dosage C. deserticola Y.C.Ma and low, high dosage C. tubulosa decoction showed a shortened latency period; the frequency that mice swam the hidden platform was upregulated significantly in the groups treated with moderate, high dosage C. deserticola Y.C.Ma decoction. These results indicate that the stressed mice benefited from the administration of herb Cistanche decoction and displayed an improvement of the spatial learning and memory.
Figure 3.
The effect of C. deserticola Y.C.Ma and C. tubulosa decoctions on the space learning and memory capability of the restraint stressed mice. After adaptation, the experimental groups were administered with herb Cistanche decoction by gavage for 21 days and then tested with R. Morris water maze for determination of spatial learning and memory. L, M, and H, respectively, represent low, medium, and high; CD and CT, respectively, represent C. deserticola Y.C.Ma and C. tubulosa decoction. “#” and “∗” each represent P < 0.05 and P < 0.01.
3.4. Decoction of C. deserticola Y.C.Ma and C. tubulosa Downregulated the Monoamine Oxidase Activity
MAO catalyzes the oxidative deamination of bioamines such as tyramine, catecholamine, and 5-hydroxytryptamine (5-HT) in the brain and peripheral tissues; inhibition of MAO activity results in the antidepressant efficacy [30]. We sought to explain the antidepressant effect by C. deserticola Y.C.Ma and C. tubulosa; then the MAO activity in the brain was determined. After tail suspension test, the MAO activity was upregulated significantly; the MAO activity in the groups treated with tail suspension and then followed by moderate, high dosage decoction from C. deserticola Y.C.Ma showed a significant decline by approximately 13.9%~12.3%; the MAO activity in the groups treated with tail suspension and then high dosage decoction from C. tubulosa showed significant decline by about 13.2% (Figure 4). The data exhibited that decoction from both C. deserticola Y.C.Ma and C. tubulosa could inhibit the activity of MAO and may contribute to the antidepressant effect, and decoction of C. deserticola Y.C.Ma showed a stronger efficacy than that of C. tubulosa.
Figure 4.
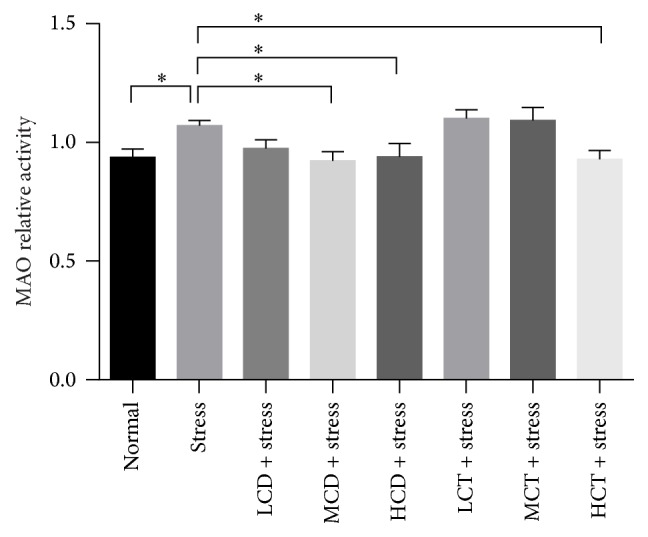
Effect of C. deserticola Y.C.Ma and C. tubulosa decoctions on brain monoamine oxidase (MAO) activity of tail suspension stressed mice. After adaptation, the experimental groups were administered with herb Cistanche decoction by gavage for 21 days and then suspended by tail for 5 min, each group was comprised of 10 mice, and the mice were sacrificed to harvest the brain tissues and used for MAO activity measurement. L, M, and H, respectively, represent low, medium, and high; CD and CT, respectively, represent C. deserticola Y.C.Ma and C. tubulosa decoction. n = 10; all the data were represented as mean ± SEM; P < 0.05 was considered as significantly different and was marked as “∗”.
3.5. The Administration of C. deserticola Y.C.Ma and C. tubulosa Decoction Resulted in the Upregulation of Central Dopamine Concentration and the Downregulation of Serum Corticosterone Concentration
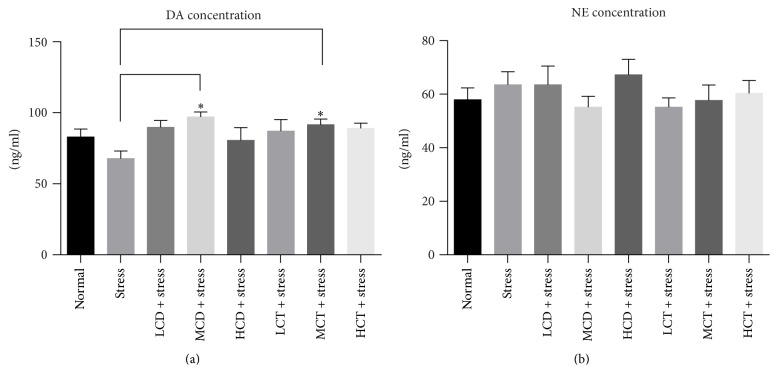
The level of DA and NE in the central nervous system was measured to determine the nerve excitability. After 4 weeks of tail suspension test, compared to the stress groups, the DA level of groups treated with moderate dosage C. deserticola Y.C.Ma was upregulated significantly each by 43.5%; the DA level of groups treated with moderate dosage C. tubulosa was upregulated significantly, too (Figure 5(a)). The level of NE was mildly upregulated in the groups treated with decoction of both herbs compared to the stress groups, and no statistical difference was found (Figure 5(b)). These results might explain the molecular mechanism of antidepressant effect of herb Cistanche.
Figure 5.
Effect of C. deserticola Y.C.Ma and C. tubulosa decoctions on brain neurotransmitter levels of tail suspension stressed mice. After adaptation, the experimental groups were administered with herb Cistanche decoction by gavage for 21 days and then suspended by tail for 5 min, each group was comprised of 10 mice, and the mice were sacrificed to harvest the brain tissues and used for brain neurotransmitter level measurement. L, M, and H, respectively, represent low, medium, and high; CD and CT, respectively, represent C. deserticola Y.C.Ma and C. tubulosa decoction. n = 10; all the data were represented as mean ± SEM; P < 0.05 was considered as significantly different and was marked as “∗”.
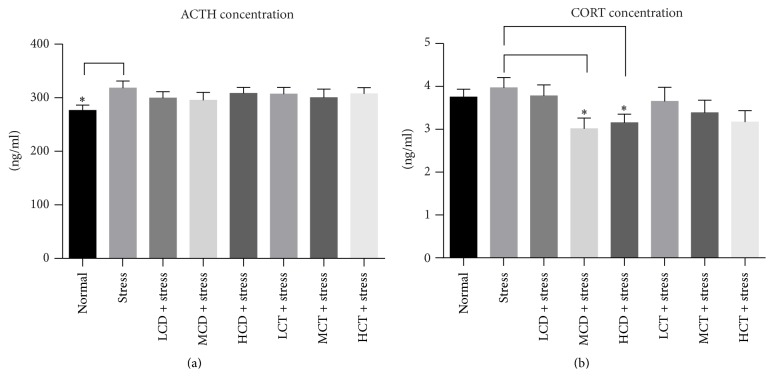
Adrenocorticotropic hormone (ACTH), a polypeptide hormone produced and secreted by the anterior pituitary gland, stimulates secretion of glucocorticosteroid by acting on the adrenal cortex, zona fasciculata [31]; corticosterone, a steroid hormone produced by the cortex of the adrenal glands, is involved in the regulation of the physiological processes such as energy boosting, immunity reaction, and stress responses [32], so we chose to study the effect of herb decoction on the HPA axis. And ACTH and corticosterone concentration in the serum were measured. After 4 weeks of tail suspension test, the ACTH and corticosterone concentration of the stress group were significantly upregulated (Figure 6). No significant changes were observed in the herb Cistanche decoction treatment groups compared to the stress group (Figure 6(a)). Compared to the stress group, the corticosterone serum concentration of groups treated with moderate, high dosage C. deserticola Y.C.Ma decoction was downregulated significantly; the corticosterone concentration of groups treated with high dosage C. tubulosa was downregulated but not to a significantly statistical difference (Figure 5(b)). The data showed that decoction from both C. deserticola Y.C.Ma and C. tubulosa could reduce the corticosterone serum concentration and might be involved in regulating the HPA axis, and decoction of C. deserticola Y.C.Ma showed a stronger efficacy than that of C. tubulosa.
Figure 6.
Effect of C. deserticola Y.C.Ma and C. tubulosa decoctions on serum hormone concentrations in hanging stressed mice. After adaptation, the experimental groups were administered with herb Cistanche decoction by gavage for 21 days and then suspended by tail for 5 min, each group was comprised of 10 mice, and the blood was harvested and used for determination of serum adrenocorticotrophic hormone (ACTH) and corticosterone (CORT) level. L, M, and H, respectively, represent low, medium, and high; CD and CT, respectively, represent C. deserticola Y.C.Ma and C. tubulosa decoction. n = 10; all the data were represented as mean ± SEM; P < 0.05 was considered as significantly different and was marked as “∗”.
4. Discussion
In this study, the antidepressant activities and improvement of memory and learning of herb Cistanche were defined, which might shed a new light on herb Cistanche function and give clues to expanding the therapeutic application range. Previous studies show that herb Cistanche is a multifunctional Chinese drug with biological activities of antiaging, antioxidant, estrogenic, antiosteoporotic, aphrodisiac, and anti-inflammation effects [33]. Herb Cistanche has been recognized as a treatment of kidney deficiency, infertility, and chronic constipation (Pharmacopoeia Committee of China, 2010). Herb Cistanche decoction is composed of volatile oils, nonvolatile phenylethanoid glycosides (PeGs), iridoids, lignans, alditols, oligosaccharides, and polysaccharides. Echinacoside and verbascoside are generally viewed as the main bioactive ingredients [34]. There are four main Cistanche species including C. deserticola Y.C.Ma, C. tubulosa, Cistanche sinensis G. Beck, and Cistanche salsa var. albiflorashi et al. [35]; we chose C. deserticola Y.C.Ma and C. tubulosa as our model to determine the PeGs content, and C. deserticola Y.C.Ma possessed a higher content of PeGs (Figure 1), which may explain the higher efficacy of C. deserticola Y.C.Ma. The results indicate that the content of the main bioactive ingredients determines the efficacy of herb Cistanche.
Previous studies indicate that herb Cistanche products are beneficial for treating neurodegenerative disorders by upregulating nerve growth factor (NGF) in the hippocampus of mice [36, 37], but no research has been reported on the antidepressant and cognitive learning activities of herb Cistanche in the mammal models. In this study, herb Cistanche decoction was proven to be effective to alleviate the stress level and substantially increase the survival behavior in tail suspension test (Figure 2) and improve the spatial learning and memory capacity in the water maze model (Figure 3). Lin et al. proved that herb Cistanche improved the olfactory-associated memory in the fruit-fly model [23]; we focused our research on the antidepressant effect and adopted a different animal model; the outcome proved that herb Cistanche improved a variant type of cognitive function, namely, the spatial learning ability in mice. According to the monoamine theory, the stress experience and depression are closely linked to the brain level of monoamine neurotransmitters, such as DA, NE, 5-HT, and epinephrine [38]. And we found that the herb Cistanche decoction could undermine the MAO activity (Figure 4) and augment the central DA concentration (Figure 5(a)). Surprisingly, the central 5-HT concentration was downregulated by herb Cistanche decoction treatment (data not shown), because previous research showed that the reduced central 5-HT activity is associated with depression [39]. Dysregulation of the hypothalamic-pituitary-adrenal axis is frequently associated with depression [40], intervention with the glucocorticoid receptor (GR) system successfully reversed the depressant phenotype in mice [41], and we found that herb Cistanche significantly alleviated the serum concentration of CORT (Figure 6(b)). Modulation of the monoamine system and HPA axis both contributes to the antidepressant effect of herb Cistanche. The echinacoside could cross the blood-brain barrier freely and act upon the central nervous system [42], which may explain why herb Cistanche decoction possessed an antidepressant function. For the first time, we characterized the antidepressant property and its relationship to cognitive improvement activities in the mouse model, and the preliminary mechanism was explored. Still, the mechanism of antidepressant property of Cistanche has not been clarified on the molecular level, and further research is needed to elucidate the comprehensive antidepressant mechanism of herb Cistanche.
Considering that herb Cistanche decoction is mixture of compounds, there might be multiple ways of herb Cistanche decoction exerting the antidepressant effect and boosting learning ability. Oxidative stress affects many cellular functions of neurons, and overproduction of reactive oxygen species (ROS) causes cell damage, apoptosis, and death [43]. Echinacoside extracted from the stems of C. salsa exhibits a neuroprotective effect by reducing production of ROS and mitochondria-mediated apoptosis [44]; acteoside, an extremely strong antioxidative compound, might be partly responsible for the neuroprotective effect [45]. Besides the traditional method of decoction, application of modern pharmaceutic methods such as controlled release glycoside capsules might boost the effect of herb decoction, because the bioactive ingredients could be digested and degraded by the gastrointestinal tract [37].
5. Conclusions
In this study, we found that decoctions of C. deserticola Y.C.Ma and C. tubulosa exhibited an antidepressant effect and improved the spatial learning and memory ability in the mouse model, and decoctions of C. deserticola Y.C.Ma and C. tubulosa had a significant impact on the HPA axis; C. deserticola Y.C.Ma showed a stronger pharmacological function. C. deserticola and C. tubulosa might hold the potential of being developed as alternative therapy drug for depression.
Acknowledgments
This project was supported by grants from National Natural Science Foundation (81503333) and the Science and Technology Support by Project in the Xinjiang Uygur Autonomous Region (China) (200840102-15).
Disclosure
The views expressed herein are those of the authors and do not necessarily reflect the views of any of these agencies.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Kessler R. C., McGonagle K. A., Zhao S., et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 2.Manji H. K., Moore G. J., Rajkowska G., Chen G. Neuroplasticity and cellular resilience in mood disorders. Molecular Psychiatry. 2000;5(6):578–593. doi: 10.1038/sj.mp.4000811. [DOI] [PubMed] [Google Scholar]
- 3.Rosenblat J. D., McIntyre R. S., Alves G. S., Fountoulakis K. N., Carvalho A. F. Beyond monoamines-novel targets for treatment-resistant depression: A comprehensive review. Current Neuropharmacology. 2015;13(5):636–655. doi: 10.2174/1570159X13666150630175044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson K., Mottram P. A comparison of side effects of selective serotonin reuptake inhibitors and tricyclic antidepressants in older depressed patients: A meta-analysis. International Journal of Geriatric Psychiatry. 2004;19(8):754–762. doi: 10.1002/gps.1156. [DOI] [PubMed] [Google Scholar]
- 5.Vezmar S., Miljković B., Vučićević K., et al. Pharmacokinetics and efficacy of fluvoxamine and amitriptyline in depression. Journal of Pharmacological Sciences. 2009;110(1):98–104. doi: 10.1254/jphs.09013FP. [DOI] [PubMed] [Google Scholar]
- 6.Halsted C. H. Dietary supplements and functional foods: 2 sides of a coin? American Journal of Clinical Nutrition. 2003;77:1001S–1007S. doi: 10.1093/ajcn/77.4.1001S. [DOI] [PubMed] [Google Scholar]
- 7.Tian J.-S., Liu C.-C., Xiang H., et al. Investigation on the antidepressant effect of sea buckthorn seed oil through the GC-MS-based metabolomics approach coupled with multivariate analysis. Food and Function. 2015;6(11):3585–3592. doi: 10.1039/c5fo00695c. [DOI] [PubMed] [Google Scholar]
- 8.Hou Y., Aboukhatwa M. A., Lei D.-L., Manaye K., Khan I., Luo Y. Anti-depressant natural flavonols modulate BDNF and beta amyloid in neurons and hippocampus of double TgAD mice. Neuropharmacology. 2010;58(6):911–920. doi: 10.1016/j.neuropharm.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wattanathorn J., Chonpathompikunlert P., Muchimapura S., Priprem A., Tankamnerdthai O. Piperine, the potential functional food for mood and cognitive disorders. Food and Chemical Toxicology. 2008;46(9):3106–3110. doi: 10.1016/j.fct.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Shirai M., Kawai Y., Yamanishi R., Terao J. Approach to novel functional foods for stress control 5. Antioxidant activity profiles of antidepressant herbs and their active components. Journal of Medical Investigation. 2005;52:249–251. doi: 10.2152/jmi.52.249. [DOI] [PubMed] [Google Scholar]
- 11.Kumar P. S., Praveen T., Jain N. P., Jitendra B. A review on Griffonia simplicifollia - an ideal herbal anti-depressant. International Journal of Pharmacy & Life Sciences. 2010 [Google Scholar]
- 12.Jin Z.-L., Gao N., Xu W., et al. Receptor and transporter binding and activity profiles of albiflorin extracted from Radix paeoniae Alba. Scientific Reports. 2016;6 doi: 10.1038/srep33793.33793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin S.-H., Chou M.-L., Chen W.-C., et al. A medicinal herb, Melissa officinalis L. ameliorates depressive-like behavior of rats in the forced swimming test via regulating the serotonergic neurotransmitter. Journal of Ethnopharmacology. 2015;175, article no. 9741:266–272. doi: 10.1016/j.jep.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Shimoda H., Tanaka J., Takahara Y., Takemoto K., Shan S.-J., Su M.-H. The hypocholesterolemic effects of Cistanche tubulosa extract, a chinese traditional crude medicine, in mice. American Journal of Chinese Medicine. 2009;37(6):1125–1138. doi: 10.1142/S0192415X09007545. [DOI] [PubMed] [Google Scholar]
- 15.Xia L. I., Yong-Qing M. A., Song Y. X., Shui J. F., Xiu-Wei L. I. Effect of different plant growth regulators on transplanting survival ratio of haloxylon ammodendron seedling and parasitic ratio of cistanche deserticola. Journal of Chinese Medicinal Materials. 2009;32:1651–1654. [PubMed] [Google Scholar]
- 16.Xiong Q., Kadota S., Tani T., Namba T. Antioxidative effects of phenylethanoids from Cistanche deserticola. Biological and Pharmaceutical Bulletin. 1996;19(12):1580–1585. doi: 10.1248/bpb.19.1580. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y., Li L., Wen T., Li Y.-Q. Protective effects of echinacoside on carbon tetrachloride-induced hepatotoxicity in rats. Toxicology. 2007;232(1-2):50–56. doi: 10.1016/j.tox.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Wang T., Chen C., Yang M., Deng B., Kirby G. M., Zhang X. Cistanche tubulosa ethanol extract mediates rat sex hormone levels by induction of testicular steroidgenic enzymes. Pharmaceutical Biology. 2016;54(3):481–487. doi: 10.3109/13880209.2015.1050114. [DOI] [PubMed] [Google Scholar]
- 19.Gu L., Xiong W. T., Zhuang Y. L., Zhang J. S., Liu X. Effects of Cistanche deserticola extract on penis erectile response in castrated rats. Pakistan Journal of Pharmaceutical Sciences. 2016;29(2):557–562. [PubMed] [Google Scholar]
- 20.Lin S. G., Ye S. F., Huang J. M., et al. How do Chinese medicines that tonify the kidney inhibit dopaminergic neuron apoptosis? Neural Regeneration Research. 2013;8(30):2820–2826. doi: 10.3969/j.issn.1673-5374.2013.30.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C.-R., Lin H.-C., Su M.-H. Reversal by aqueous extracts of Cistanche tubulosa from behavioral deficits in Alzheimer's disease-like rat model: relevance for amyloid deposition and central neurotransmitter function. BMC Complementary and Alternative Medicine. 2014;14:1–11. doi: 10.1186/1472-6882-14-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lara D. R., Bisol L. W., Munari L. R. Antidepressant, mood stabilizing and procognitive effects of very low dose sublingual ketamine in refractory unipolar and bipolar depression. International Journal of Neuropsychopharmacology. 2013;16(9):2111–2117. doi: 10.1017/S1461145713000485. [DOI] [PubMed] [Google Scholar]
- 23.Lin W., Yao C., Cheng J., Kao S., Tsai F., Liu H. Molecular pathways related to the longevity promotion and cognitive improvement of Cistanche tubulosa in Drosophila. Phytomedicine. 2017;26:37–44. doi: 10.1016/j.phymed.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Cryan J. F., Mombereau C., Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neuroscience and Biobehavioral Reviews. 2005;29(4-5):571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Vorhees C. V., Williams M. T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature Protocols. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of Neuroscience Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 27.Wolf A., Bauer B., Abner E. L., Ashkenazy-Frolinger T., Hartz A. M. S. A comprehensive behavioral test battery to assess learning and memory in 129S6/Tg2576 mice. PLoS ONE. 2016;11(1) doi: 10.1371/journal.pone.0147733.e0147733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rourke B. P., Young G. C., Leenaars A. A. A childhood learning disability that predisposes those afflicted to adolescent and adult depression and suicide risk. Journal of Learning Disabilities. 1989;22(3):169–175. doi: 10.1177/002221948902200305. [DOI] [PubMed] [Google Scholar]
- 29.D'Hooge R., De Deyn P. P. Applications of the Morris water maze in the study of learning and memory. Brain Research Reviews. 2001;36(1):60–90. doi: 10.1016/S0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 30.Shih J. C., Chen K., Ridd M. J. Monoamine oxidase: from genes to behavior. Annual Review of Neuroscience. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bornstein S. R., Engeland W. C., Ehrhart-Bornstein M., Herman J. P. Dissociation of ACTH and glucocorticoids. Trends in Endocrinology and Metabolism. 2008;19(5):175–180. doi: 10.1016/j.tem.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Whirledge S., Cidlowski J. A. Glucocorticoids, stress, and fertility. Minerva Endocrinologica. 2010;35(2):109–125. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L., Ding H., Yu H., et al. Cistanches Herba: Chemical Constituents and Pharmacological Effects. Chinese Herbal Medicines. 2015;7(2):135–142. doi: 10.1016/S1674-6384(15)60017-X. [DOI] [Google Scholar]
- 34.Li Z., Lin H., Gu L., Gao J., Tzeng C.-M. Herba Cistanche (Rou Cong-Rong): One of the best pharmaceutical gifts of traditional Chinese medicine. Frontiers in Pharmacology. 2016;7, article 41 doi: 10.3389/fphar.2016.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi H. M., Wang J., Wang M. Y., Tu P. F., Li X. B. Identification of Cistanche species by chemical and inter-simple sequence repeat fingerprinting. Biological and Pharmaceutical Bulletin. 2009;32(1):142–146. doi: 10.1248/bpb.32.142. [DOI] [PubMed] [Google Scholar]
- 36.Choi J. G., Moon M., Jeong H. U., Kim M. C., Kim S. Y., Oh M. S. Cistanches Herba enhances learning and memory by inducing nerve growth factor. Behavioural Brain Research. 2011;216(2):652–658. doi: 10.1016/j.bbr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Guo Q., Zhou Y., Wang C.-J., et al. An open-label, nonplacebo-controlled study on cistanche tubulosa glycoside capsules (Memoregain®) for treating moderate alzheimer's disease. American Journal of Alzheimer's Disease and other Dementias. 2013;28(4):363–370. doi: 10.1177/1533317513488907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popoli M., Gennarelli M., Racagni G. Modulation of synaptic plasticity by stress and antidepressants. Bipolar Disorders. 2002;4(3):166–182. doi: 10.1034/j.1399-5618.2002.01159.x. [DOI] [PubMed] [Google Scholar]
- 39.Cleare A. J., McGregor A., O'Keane V. Neuroendocrine evidence for an association between hypothyroidism, reduced central 5-HT activity and depression. Clinical Endocrinology. 1995;43(6):713–719. doi: 10.1111/j.1365-2265.1995.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Duran N. L., Kovacs M., George C. J. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrinology. 2009;34(9):1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. Journal of Psychiatry and Neuroscience. 2004;29(3):185–193. [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu M., Lu C., Li W. Transient exposure to echinacoside is sufficient to activate Trk signaling and protect neuronal cells from rotenone. Journal of Neurochemistry. 2013;124(4):571–580. doi: 10.1111/jnc.12103. [DOI] [PubMed] [Google Scholar]
- 43.Lu T.-H., Su C.-C., Tang F.-C., et al. Chloroacetic acid triggers apoptosis in neuronal cells via a reactive oxygen species-induced endoplasmic reticulum stress signaling pathway. Chemico-Biological Interactions. 2015;225:1–12. doi: 10.1016/j.cbi.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y.-H., Xuan Z.-H., Tian S., Du G.-H. Echinacoside protects against 6-hydroxydopamine-induced mitochondrial dysfunction and inflammatory responses in PC12 cells via reducing ROS production. Evidence-Based Complementary and Alternative Medicine. 2015;2015:9. doi: 10.1155/2015/189239.189239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiou W. F., Lin L. C., Chen C. F. Acteoside protects endothelial cells against free radical-induced oxidative stress. Journal of Pharmacy and Pharmacology. 2004;56(6):743–748. doi: 10.1211/0022357023501. [DOI] [PubMed] [Google Scholar]