Abstract
Purpose of Review
Chronic Kidney Disease (CKD) affects nearly 10% of the population. The incidence of fractures in population studies demonstrate an increase with worsening stages of kidney disease suggesting specific CKD related causes of fracture.
Recent Findings
The increase in fractures with CKD most likely represents disordered bone quality due to the abnormal bone remodeling from renal osteodystrophy. There is also an increase in fractures with age in patients with CKD, suggesting that patients with CKD also have many fracture risk factors common to patients without known CKD. Osteoporosis is defined by the National Institutes of Health as “A skeletal disorder characterized by compromised bone strength predisposing to an increased risk of fracture. Bone strength reflects the integration of two main features: bone quantity and bone quality”.
Summary
Thus, CKD related fractures can be considered a type of osteoporosis- where the bone quality is additionally impaired above that of age/hormonal related osteoporosis. Perhaps using the term CKD induced osteoporosis, similar to steroid induced osteoporosis, will allow patients with CKD to be studied in trials investigating therapeutic agents. In this series we will examine how CKD induced osteoporosis may be diagnosed and treated.
Keywords: renal osteodystrophy, CKD, osteoporosis
Introduction
Chronic Kidney Disease (CKD) affects between 8 and 10% of the world population[1] with an increasing prevalence due to the rise in diabetes, the leading cause of kidney disease. Bone disease is a major complication of CKD and is one manifestation of CKD-Mineral Bone Disorder (CKD-MBD) (Table 1). Renal osteodystrophy is an alteration of bone morphology in patients with CKD and is one measure of the skeletal component of the systemic disorder of CKD-MBD that is quantifiable by histomorphometry assessed on bone biopsy[2]. The pathogenesis of renal osteodystrophy is complex and, as described elsewhere in this series by Susan Ott, the nomenclature has evolved as we slowly unravel the complex physiology. There is also an inverse relationship between arterial calcification and bone calcification (density or area) in CKD[3–5] implying system disordered metabolism and the uremic milieu may alter bone. Traditionally, the primary focus on bone health has been to control parathyroid hormone (PTH) with calcitriol or other vitamin D analogs and more recently with calcimimetics. Clearly secondary and tertiary hyperparathyroidism has a major role in stimulating bone remodeling in CKD. However, studies have demonstrated the incidence of age adjusted hip fracture has actually increased over the past decades despite an intensive focus on treatments for renal osteodystrophy such as PTH and phosphate lowering therapies[6,7]. Thus, there is more to abnormal bone in patients with CKD than just PTH.
Table 1.
Kidney Disease Improving Global Outcomes (KDIGO) classification of CKD-MBD and Renal Osteodystrophy
| Definition of CKD-MBD |
|---|
A systemic disorder of mineral and bone metabolism due to CKD manifested by either one or a combination of the following:
|
Definition of Renal Osteodystrophy
|
From: Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006; 69(11):1945–53. Used with permission from Elsevier.
Fractures are very common in CKD, and the incidence increases with progressive CKD (Figure 1). The progressive rise in incidence of fracture with more advanced CKD suggests risk factors caused by CKD, further supported by a 2 to 3 fold increased fracture risk in children with CKD[8]. However, the progressive rise in incidence with age at all stages of CKD suggests that other factors, perhaps those common to advanced aging, also play a role. The average age of patients starting dialysis has progressively risen over time, and thus many patients will already have risk factors for fracture prior to developing kidney disease. Known risk factors for fracture in the general population include family history of fracture, previous fracture, ethnicity and bone structure, peak bone mass, hormone deficiency, smoking, alcohol use, and falls. These risk factors are clearly independent of the presence or absence of CKD yet may be exacerbated by CKD. For example, hormone deficiency is nearly universal in both male and female patients with CKD[9,10]. Falls are more common in CKD due to peripheral neuropathy of diabetes and uremia[11] and a high prevalence of sarcopenia[12] and have been associated with fracture in patients with CKD.
Figure 1. Hip Fracture Incidence Increases with Progressive CKD.
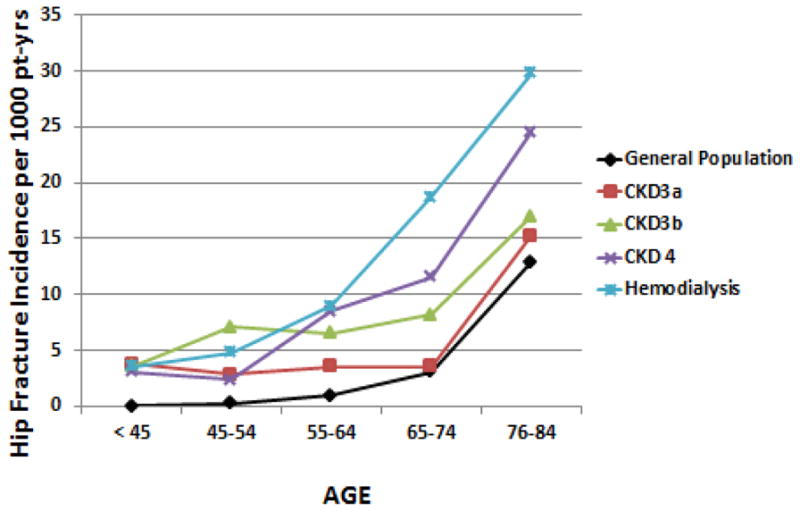
As patients age in the general population there is an increased incidence of hip fracture. This incidence increases with progression of CKD. Data from Alem et al for dialysis patients and the general population from Olmstead Minnesota[28], Naylor et al for CKD stages 3–4[29] courtesy of the Canadian Institute for Clinical Evaluative Sciences (ICES). Pt-yrs = patient years. From: Moe SM, Nickolas TL. Fractures in patients with CKD: Time for Action. Clin J Am Soc Nephrol. 2016 Nov 7;11(11):1929–1931. Epub 2016 Oct 24. Used with permission from the American Society of Nephrology.
Operationally, the World Health Organization considers low bone mineral density (BMD) assessed by dual X-ray absorptiometry (DXA) to be synonymous with osteoporosis. However, the improvement in BMD in response to treatments is small compared to fracture risk reduction in therapeutic trials, indicating there is more to bone than bone mineral density[13]. In 2000, the NIH defined osteoporosis as “A skeletal disorder characterized by compromised bone strength predisposing to an increased risk of fracture. Bone strength reflects the integration of two main features: bone quantity and bone quality”[14]. Thus bone quantity (assessed by DXA to give 2-dimensional areal bone density or with quantified computed tomography (CT) for area) is only one component. Bone quality is a second major component and includes bone remodeling abnormalities, collagen cross linking and mineralization properties. Remodeling abnormalities are generally evaluated with bone biopsy and dynamic histomorphometry (tetracycline labeling) and such abnormalities are nearly universally abnormal in patients with CKD[15]. Thus, renal osteodystrophy is one component of abnormal bone quality, not the only cause of fractures. Treatments only focused on the abnormal bone remodeling of CKD are thus not likely to be efficacious in preventing fractures due to the many other risk factors for low bone mass and other factors adversely affecting bone quality.
The organization Kidney Disease Improving Global Outcomes (KDIGO) recommended using the term renal osteodystrophy to define abnormalities in turnover, mineralization, and volume assessed by bone biopsy as “Renal osteodystrophy”[2]. But in the definition of CKD-MBD (Table 1), strength (or increased fragility) was also a component of abnormal bone indicating that fractures, regardless of the underlying histology, also are a manifestation of CKD-MBD. Indeed volume is a key characteristic of both underlying abnormal bone histology (a.k.a. renal osteodystrophy) and a major component contributing to fragility (low impact) fractures. Thus, the assessment of bone volume by DXA or CT based methods may be helpful in management of patients with high risk of fracture and may provide additive information to that obtained with bone biopsy.
Unfortunately, data supporting that BMD testing is relevant in CKD has lagged behind that generated in the general population. In the 2003 Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines, BMD testing was only recommended in post-transplant patients as there were no studies in patients with CKD demonstrating that a low BMD by DXA predicted subsequent fracture. In other words, its utility as a screening tool to identify those patients at risk was not yet proven.[16]. There was also concern that DXA may be artificially elevated in the setting of arterial calcification, especially at the lumbar spine[16]. In the 2009 KDIGO CKD-MBD guidelines there were studies demonstrating that low BMD predicted fractures in patients with all stages of CKD[17–21]. However, the consensus was that the risk of treating patients with the commonly used class of drugs, bisphosphonates, was high and the benefit uncertain despite secondary analyses of common anti-osteoporosis treatments in post-menopausal women. Unfortunately, these secondary analyses included generally normal creatinine and normal PTH levels and it was felt the results were not generalizable to more advanced CKD (See article by Paul Miller). Therefore the guideline did not recommend screening because there was no treatment[22]. In 2016 KDIGO guideline update (currently out for public comment www.kdigo.org), BMD is now recommended “if the results will change clinical management”. The committee felt that although treatment options remain ill-defined and under-studied, in some patients, the treatment outweighs risk and clinicians must individualize treatments.
In the general population, clinical risk factors have been combined into a fracture prediction algorithm called Fracture Risk Assessment Tool or FRAX®, developed by the World Health Organization[23]. The goal of FRAX is to evaluate fracture risk of patients on an individual basis. The various FRAX tools have been refined in different countries to take into account the genetics of bone fracture. There are many risk factors for fractures utilized in FRAX models, including age, sex, body mass index, family history, alcohol use, smoking, glucocorticoids and rheumatoid arthritis. There is also an option to say ‘yes’ or ‘no’ to a question asking for the presence of secondary osteoporosis (including diabetes, osteogenesis imperfecta, long standing hyperthyroidism, hypogonadism, premature menopause, chronic malnutrition, or malabsorption and chronic liver disease). Although CKD is not on this list, it probably should be given the obvious increase incidence of fracture with progressive CKD shown in Figure 1. Studies have demonstrated that the use of FRAX discriminates those with and without fracture in both patients with advanced CKD[24] and transplant recipients[25]. The addition of BMD to the FRAX score improves the prediction of fracture risk.
Thus, assessments of bone health in the general population with tools such as BMD and FRAX risk assessment appear to be relevant in patients with CKD. The presence of CKD likely increases the fracture risk above that assessed by these tools and should be regarded as an independent risk factor for fracture, similar to how CKD is now considered an independent risk factor for cardiovascular disease. Fractures are one of many ‘premature aging’ consequences observed in patients with CKD. Mortality doubles in patients on dialysis who sustain a major fracture that requires hospitalization[26,27] and thus fractures have major consequences. Unfortunately, patients with CKD are uniformly excluded from clinical trials evaluating pharmacotherapies. While post hoc analyses have identified low GFR in many patients enrolled in these trials as discussed in another article in this series by Paul Miller, the presence of a single measure of low GFR does not define CKD[1]. The first step is to consider Renal Osteodystrophy a form of osteoporosis, or at least a major risk factor. Perhaps we should coin a new term- “CKD induced osteoporosis” as a parallel term to corticosteroid induced osteoporosis. Studies must examine the utility of currently available and new therapies specifically in patients with CKD. It is likely that concomitant therapy to treat the abnormal bone remodeling through control of PTH will also be needed, but only prospective studies can determine the optimal treatment. In this series, we will focus on renal osteodystrophy and the similarities and differences with other forms of osteoporosis. Our hope is that CKD becomes a recognized major risk factor for fracture.
Acknowledgments
The authors wish to thank Dr. Naylor and the other authors of the original work[29] for the adaptation of their original work in the construction of Figure 1. The author is funded by the Veterans Administration and the National Institute of Health DK11087 and DK100306.
Thank you to Isidoro Salusky for reviewing this paper.
Footnotes
Compliance with Ethical Guidelines
Conflict of Interest
Sharon Moe reports other from Sanofi, grants from chugai, other from Lilly, outside the submitted work. The author reports no disclosures relevant to the current article.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.KDIGO. Kdigo 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Supplement. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- **2.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G Kidney Disease. Improving Global O: Definition, evaluation, and classification of renal osteodystrophy: A position statement from kidney disease: Improving global outcomes (kdigo) Kidney Int. 2006;69(11):1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Qureshi AR, Ripsweden J, Wennberg L, Heimburger O, Lindholm B, Barany P, Haarhaus M, Brismar TB, Stenvinkel P. Vertebral bone density associates with coronary artery calcification and is an independent predictor of poor outcome in end-stage renal disease patients. Bone. 2016;92:50–57. doi: 10.1016/j.bone.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Cejka D, Weber M, Diarra D, Reiter T, Kainberger F, Haas M. Inverse association between bone microarchitecture assessed by hr-pqct and coronary artery calcification in patients with end-stage renal disease. Bone. 2014;64:33–38. doi: 10.1016/j.bone.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe R, Lemos MM, Carvalho AB, Rochitte CE, Santos RD, Draibe SA, Canziani ME. The association between coronary artery calcification progression and loss of bone density in non-dialyzed ckd patients. Clin Nephrol. 2012;78(6):425–431. doi: 10.5414/CN107515. [DOI] [PubMed] [Google Scholar]
- **6.Wagner J, Jhaveri KD, Rosen L, Sunday S, Mathew AT, Fishbane S. Increased bone fractures among elderly united states hemodialysis patients. Nephrol Dial Transplant. 2014;29(1):146–151. doi: 10.1093/ndt/gft352. [DOI] [PubMed] [Google Scholar]
- 7.Wakasugi M, Kazama JJ, Taniguchi M, Wada A, Iseki K, Tsubakihara Y, Narita I. Increased risk of hip fracture among japanese hemodialysis patients. J Bone Miner Metab. 2013;31(3):315–321. doi: 10.1007/s00774-012-0411-z. [DOI] [PubMed] [Google Scholar]
- 8.Denburg MR, Kumar J, Jemielita T, Brooks ER, Skversky A, Portale AA, Salusky IB, Warady BA, Furth SL, Leonard MB. Fracture burden and risk factors in childhood ckd: Results from the ckid cohort study. J Am Soc Nephrol. 2016;27(2):543–550. doi: 10.1681/ASN.2015020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weisinger JR, Bellorin-Font E. Outcomes associated with hypogonadism in women with chronic kidney disease. Adv Chronic Kidney Dis. 2004;11(4):361–370. [PubMed] [Google Scholar]
- 10.Carrero JJ, Qureshi AR, Nakashima A, Arver S, Parini P, Lindholm B, Barany P, Heimburger O, Stenvinkel P. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant. 2011;26(1):184–190. doi: 10.1093/ndt/gfq397. [DOI] [PubMed] [Google Scholar]
- *11.West SL, Jamal SA, Lok CE. Tests of neuromuscular function are associated with fractures in patients with chronic kidney disease. Nephrol Dial Transplant. 2012;27(6):2384–2388. doi: 10.1093/ndt/gfr620. [DOI] [PubMed] [Google Scholar]
- 12.Fahal IH. Uraemic sarcopenia: Aetiology and implications. Nephrol Dial Transplant. 2014;29(9):1655–1665. doi: 10.1093/ndt/gft070. [DOI] [PubMed] [Google Scholar]
- 13.Turner CH. Biomechanics of bone: Determinants of skeletal fragility and bone quality. Osteoporos Int. 2002;13(2):97–104. doi: 10.1007/s001980200000. [DOI] [PubMed] [Google Scholar]
- 14.Nih consensus development panel on osteoporosis prevention, diagnosis, and therapy, march 7–29, 2000 Highlights of the conference. South Med J. 2001;94(6):569–573. [PubMed] [Google Scholar]
- 15.Sprague SM, Bellorin-Font E, Jorgetti V, Carvalho AB, Malluche HH, Ferreira A, D'Haese PC, Drueke TB, Du H, Manley T, Rojas E, et al. Diagnostic accuracy of bone turnover markers and bone histology in patients with ckd treated by dialysis. Am J Kidney Dis. 2015 doi: 10.1053/j.ajkd.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 16.K/DOQI NKF. Clinical practice guidelines for bone metabolism and disease in chronic kidney disease. American Journal of Kidney Diseases. 2003;42(Supplement):S1–S201. [PubMed] [Google Scholar]
- 17.West SL, Lok CE, Langsetmo L, Cheung AM, Szabo E, Pearce D, Fusaro M, Wald R, Weinstein J, Jamal SA. Bone mineral density predicts fractures in chronic kidney disease. J Bone Miner Res. 2015;30(5):913–919. doi: 10.1002/jbmr.2406. [DOI] [PubMed] [Google Scholar]
- 18.Nickolas TL, Leonard MB, Shane E. Chronic kidney disease and bone fracture: A growing concern. Kidney Int. 2008;74(6):721–731. doi: 10.1038/ki.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **19.Nickolas TL, Cremers S, Zhang A, Thomas V, Stein E, Cohen A, Chauncey R, Nikkel L, Yin MT, Liu XS, Boutroy S, et al. Discriminants of prevalent fractures in chronic kidney disease. J Am Soc Nephrol. 2011;22(8):1560–1572. doi: 10.1681/ASN.2010121275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.Naylor KL, Garg AX, Zou G, Langsetmo L, Leslie WD, Fraser LA, Adachi JD, Morin S, Goltzman D, Lentle B, Jackson SA, et al. Comparison of fracture risk prediction among individuals with reduced and normal kidney function. Clin J Am Soc Nephrol. 2015;10(4):646–653. doi: 10.2215/CJN.06040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **21.Fried LF, Biggs ML, Shlipak MG, Seliger S, Kestenbaum B, Stehman-Breen C, Sarnak M, Siscovick D, Harris T, Cauley J, Newman AB, et al. Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol. 2007;18(1):282–286. doi: 10.1681/ASN.2006050546. [DOI] [PubMed] [Google Scholar]
- **22.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2009;113:S1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 23.[Internet] FWHOFRAT. World Health Organization; 2011. [Accessed October 10, 2016]. Availabe from http://www.shef.ac.uk/FRAX/index.aspx. [Google Scholar]
- *24.Jamal SA, West SL, Nickolas TL. The clinical utility of frax to discriminate fracture sta tus in men and women with chronic kidney disease. Osteoporos Int. 2014;25(1):71–76. doi: 10.1007/s00198-013-2524-1. [DOI] [PubMed] [Google Scholar]
- 25.Naylor KL, Leslie WD, Hodsman AB, Rush DN, Garg AX. Frax predicts fracture risk in kidney transplant recipients. Transplantation. 2014;97(9):940–945. doi: 10.1097/01.TP.0000438200.84154.1a. [DOI] [PubMed] [Google Scholar]
- 26.Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115–1121. doi: 10.1053/ajkd.2000.19812. [DOI] [PubMed] [Google Scholar]
- 27.Beaubrun AC, Kilpatrick RD, Freburger JK, Bradbury BD, Wang L, Brookhart MA. Temporal trends in fracture rates and postdischarge outcomes among hemodialysis patients. J Am Soc Nephrol. 2013;24(9):1461–1469. doi: 10.1681/ASN.2012090916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, Wong C, Stehman-Breen C. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58(1):396–399. doi: 10.1046/j.1523-1755.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- *29.Naylor K, McArthur E, Leslie W, Fraser L, Jamal S, Cadarette S, Pouget J, Lok C, Hodsman A, Adachi J, Garg A. 3-year incidence of fracture in chronic kidney disease. Kidney Int. 2013 doi: 10.1038/ki.2013.547. [DOI] [PubMed] [Google Scholar]


