Abstract
We have developed a microplate reader that records a complete high-quality fluorescence emission spectrum on a well-by-well basis under true high-throughput screening (HTS) conditions. The read time for an entire 384-well plate is less than 3 minutes. This instrument is particularly well suited for assays based on fluorescence resonance energy transfer (FRET). Intramolecular protein biosensors with genetically encoded GFP donor and RFP acceptor tags at positions sensitive to structural changes were stably expressed and studied in living HEK cells. Accurate quantitation of FRET was achieved by decomposing each observed spectrum into a linear combination of four component (basis) spectra (GFP emission, RFP emission, water Raman, and cell autofluorescence). Excitation and detection are both conducted from the top, allowing for thermoelectric control of the sample temperature from below. This spectral unmixing plate-reader (SUPR) delivers an unprecedented combination of speed, precision, and accuracy for studying ensemble-averaged FRET in living cells. It complements our previously reported fluorescence lifetime plate reader, which offers the feature of resolving multiple FRET populations within the ensemble. The combination of these two direct waveform-recording technologies greatly enhances the precision and information content for HTS in drug discovery.
Keywords: Fluorescence lifetime, biosensor, drug screening, high-throughput, SERCA
Introduction
Numerous live-cell FRET biosensors based on genetically encoded fluorescent fusion proteins have been developed, but their application in high-throughput screening (HTS) assays is uncommon. An intramolecular FRET sensor for sarco/endoplasmic reticulum calcium ATPase (SERCA) 1–3 is a rare exception. 3 This two-color SERCA (2CS) biosensor, expressed in HEK293 cells, employs eGFP and tagRFP (further referred to as GFP and RFP) fluorescent proteins.4 Fluorescent proteins were engineered at carefully selected locations on SERCA’s cytoplasmic headpiece domains.. The headpiece domains are known to undergo large-scale structural changes as ATP hydrolysis fuels the active pumping of calcium from the cytosol into the endoplasmic reticulum. The interactions of small molecules with SERCA produce measurable changes in intramolecular FRET that correlate with function, making this structure-based biosensor a powerful tool for the discovery of novel drugs related to calcium homeostasis.
The previous development of a novel fluorescence lifetime readout in the nanosecond time domain was shown to enable rapid and reliable identification of small-molecules that affected FRET in the two-color SERCA (2CS) biosensor. 3,5–7. Conventional microplate readers, which measure fluorescence intensity, lack the precision required for reliable observation of the typically small FRET changes associated with allosteric effectors in live-cell assays. Similarly, the throughput of related fluorescence technologies such as microscopy is too low, and the precision of flow cytometry is currently too low for large-scale library screening 8,9. The technological advances of direct waveform recording (DWR) led to the development of a novel fluorescence lifetime plate reader in the time domain5 and now have been applied to the wavelength-domain. This new spectral unmixing microplate reader rapidly and accurately records the entire fluorescence emission spectrum.
The quality of the acquired spectral data enables a simple and direct decomposition of the observed spectra into linear combinations of component spectra (spectral unmixing), yielding accurate and precise FRET efficiency values. The benefits are critically evaluated by coupling fluorescence lifetime detection with the complementary spectral recording to further optimize the precision of FRET measurements for high-throughput screening. The combination of the spectral and fluorescence lifetime readouts represents a promising screening platform for detecting small changes in FRET.
This study emphasizes live-cell biosensors, but the same approach is equally applicable to purified proteins labeled with dyes.10 In addition to drug discovery activities, the spectral recording technology has high potential for a wide range of biological applications, such as binding studies, analytical biochemistry, and molecular diagnostics.
Materials and Methods
Cell Culture
HEK293 cells were maintained in phenol-red-free DMEM from Gibco (Waltham, MA) supplemented with 2 mM GlutaMAX (Gibco), 10% fetal bovine serum (FBS) from Atlanta Biologicals (Laweranceville, GA), and 1 IU/mL penicillin/streptomycin (Gibco) and grown at 37°C with 5% CO2. Stable clones expressing the FRET biosensors and corresponding donor- and acceptor-labeled control cell lines were established as described previously 3. Briefly, cells were transfected with the recombinant DNA following established protocols lipofectaimine 3000 from Invitrogen (Carlsbad, CA). 48 hours post transfection the cells were placed under G418 (500 μg/mL) from Sigma (St. Louis, MO), antibiotic selection and plated to allow for the growth of single colonies. Clones were isolated 2–3 weeks after transfection and antibiotic selection. The stability of each clone was assessed by flow cytometry and confocal microscopy (data not shown). The cell lines were expanded in T225 flasks from Corning (Corning, NY), harvested by treatment with Tryple (Invitrogen), washed three times in phosphate buffer solution (PBS) with no magnesium or calcium Thermo Fischer (Waltham, MA)) and centrifuged at 300 g, filtered using 70 μm cell strainers (Corning), and diluted to 106 cells/mL using an automated countess cell counter from Invitrogen. In studies with cyan and yellow fluorescent protein biosensors, transient transfections were performed. In these studies, cells were harvested and prepared as described above, 48 hours after transfection. Cell viability was assessed using trypan blue.
Cell and drug liquid dispensing
Cells were dispensed using a Multidrop Combi liquid dispenser from Thermo Fischer (Pittsburg, PA) at a density of 106/mL. Compounds were diluted in DMSO and dispensed either using a Mosquito liquid handeler from TTP Labtech (Melbourn, UK) or a Mantis liquid dispenser from Formulatrix (Bedford, MA). The known SERCA inhibitors thapsigargin (TG, Sigma), 1,4-dihydroxy-2,5-di-tert-butylbenzene (BHQ) from Tocris (Minneapolis, MN), and cyclopiazonic acid (CPA, Tocris) were diluted at 50X concentrations and subsequently serially diluted in 96-well mother plates prior to liquid dispensing. Cells and drug mixtures were dispensed into 384-well flat, black-bottom polypropylene plates from Greiner (Kremsmünste, Austria) and incubated for 20 min at room temperature (20–23°C), unless otherwise noted.
Instrumentation overview
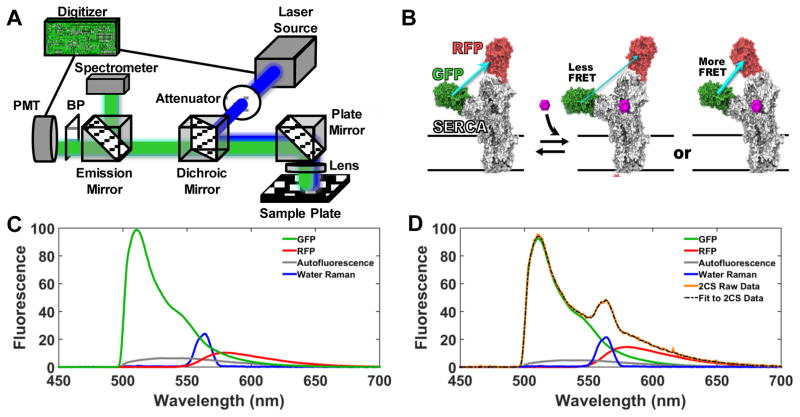
Figure 1A is a schematic drawing depicting key features of the fluorescence plate-reader platform. The lifetime and spectral readout experiments presented here were conducted with separate instruments at 20°C, but both modes can be incorporated into a single instrument, and work along these lines is in progress. Simultaneous acquisition of the lifetime and spectral data is also feasible. The photomultiplier tube (PMT) and digitizer are as described previously5, but this new instrument employs epi-illumination excitation and detection from above, which facilitates implementation of temperature control.
Figure 1.
(A) Diagram of the instrument, which provides high-throughput detection of the emission spectrum or lifetime (time-dependent decay). A 473 nm laser (continuous wavelength for spectrum, microchip pulsed for lifetime) excites the sample, and the spectral (fluorescence vs. wavelength) or lifetime (fluorescence vs. time) waveform is recorded directly using a photomultiplier tube (PMT) coupled to a proprietary digitizer or spectrograph, respectively. (B) The two-color SERCA (2CS) FRET biosensor, expressed in HEK293 cells, enables measurement of FRET between green (GFP) and red (RFP) fluorescent proteins, positioned at optimized locations on SERCA1–3. As depicted, FRET efficiency depends on the structural status of SERCA’s domains. (C) Reference fluorescence emission (basis) spectra corresponding to one-color SERCA (GFP or RFP), cellular autofluorescence, and water Raman (inelastic light scattering), normalized to GFP. These basis spectra were used to analyze the spectra of cells expressing 2CS (D), to decompose the observed spectrum of 2CS (orange) into a linear combination of component spectra (best fit shown in dashed black, components shown with same color scheme as in C), thus permitting accurate quantitation of the GFP (donor) and RFP (acceptor) fluorescence needed to calculate FRET (13.7% for data shown in D, with SD = 0.11%), using (Eq. 4. Complete emission spectra from 500–700 nm were acquired in less than 3 minutes for a full 384-well plate.
In its spectral unmixing plate reader (SUPR) mode, the instrument provides direct high-throughput detection of the complete fluorescence emission spectrum, (emission vs wavelength), with excitation provided by a 473 nm continuous wave laser. Spectra are recorded using a grating-based fiber optic input spectrograph equipped with linear-array CCD detector (Sony ILX511B). The recorded wavelength range in these experiments spanned the entire visible spectrum, but only the 500–700nm range was used in the data analysis. In lifetime mode, the full nanosecond-resolved fluorescence emission waveform is acquired following excitation with a 473 nm pulsed microchip laser. The acquisition time per well is typically 200 ms in either spectral or lifetime mode (Figure 1A).
Time-resolved FRET acquisition and analysis
Fluorescence decay waveforms for lifetime determination, were detected directly as previously described3,5. The 473 nm passively Q-switched microchip laser (Concepts Research Corporation, Belgium, WI) delivers highly reproducible, and high-energy pulses (~1 μJ) at 5 kHz repetition rate. A full fluorescence decay waveform was detected in response to each laser pulse over a 128 ns time window, using a photomultiplier module from Hamamatsu (Cat# H10720-210) and a proprietary transient digitizer from Fluorescence Innovations (Minneapolis, MN). A 488 nm long-pass filter from Semrock (Rochester, NY) and 517/20 bandpass emission filter (lifetime mode), were used, ensuring that only emission from the GFP donor was detected. A 488 nm dichroic mirror directed fluorescence signal toward the PMT (lifetime mode) or spectrograph (spectral mode) using a fiber optic cable (Figure 1A).
The observed donor fluorescence waveform FD(t) was analyzed using least-squares minimization global analysis software5 and fitted ((Eq. 1) by a simulation SD(t), consisting of a n-exponential decay model MD(t) (characterized by pre-exponential factors Ai and lifetimes τDi),convolved with the instrument response function IRF(t), acquired by recording scatter from 0.31 μm latex microsphere suspensions (Thermo Fischer).
| (Eq. 1) |
For initial analysis, a single-exponential model (n = 1) was assumed, and FRET (efficiency) was calculated from
| (Eq. 2) |
where τDA is the lifetime of the intramolecular FRET biosensor and τD is the lifetime of the corresponding donor-only cell line. The fluorescence lifetime of eGFP from a single-exponential fit of the donor-only control was 2.58 ± 0.02 ns (Figure S6), in agreement with reported values 11
Spectral FRET acquisition and analysis
The fluorescence spectra were recorded with a fiber-optic spectrometer. The uncooled linear array detector offers higher data density (pitch) compared to the multimode PMTs that have more commonly been used for spectral unmixing in fluorescence microscopy.12. The linear array detector is also substantially smaller and less costly compared to a TE-cooled 2-dimensional scientific grade CCD camera.
The observed fluorescence emission spectrum F(λ) was fitted by least-squares minimization to a linear combination of component spectra
| (Eq. 3) |
where D is donor, A is acceptor, C is cell autofluorescence, and W is water Raman, and a, b, c, d are the weighting (scalar) coefficients determined from the fit. The fitted spectrum for each well was determined using least squares minimization with Matlab (Mathworks) to solve for the scalar coefficients (Eq. 3). For an intramolecular FRET sensor with a 1:1 ratio of donor D and acceptor A molecules, FRET efficiency (FRET was determined from
| (Eq. 4) |
where QR is the ratio of quantum yields (QD/QA) in the absence of FRET, AR is the ratio of molar absorptivity (εA/εD), both obtained from reported values.13 QR was corrected for spectrometer sensitivity at the appropriate wavelengths (Figure S5). The only experimentally observed variable in (Eq. 4) becomes the fluorescence ratio (FR).
| (Eq. 5) |
A full derivation of (Eq. 4) is in Supplementary Data.
Results
Two one-color stable cell lines expressing GFP-SERCA or RFP-SERCA were developed as controls for analysis of 2-color spectra. Reference (basis) spectra from these cell lines, untransfected cells (water Raman plus cell autofluorescence), and buffer solution (water Raman) were acquired using an detector integration time of 100 ms for each well of a 384-well plate. The GFP-only reference spectrum was corrected for autofluorescence and water Raman contributions (Figures S1,2). The RFP-only spectrum was acquired by excitation at 532 nm (Figure S3), under which conditions the Raman and autofluorescence signals were negligible. Each of the four reference spectra was quite reproducible, so a single set of reference spectra, acquired only one time, was used to decompose or unmix the component spectra from the observed fluorescence emission spectrum in 2CS samples for over six months. Reference spectra are superimposed in Figure 1C, normalized to the peak intensity of the GFP sample. The RFP spectrum shown corresponds to the intensity observed with excitation at 473 nm. Spectral unmixing (Eq. 3) resolved the distinct components of GFP and RFP for quantitative determination of the apparent FRET (Eq. 4, Eq. 5). Figure 1D illustrates the analysis of a sample of cells expressing the 2CS biosensor, showing the observed spectrum (orange) and the best fit (dashed black) to the four components, yielding a FRET value of 13.7% (SD = 0.11%).
Spectral unmixing of GFP and RFP live-cell mixtures
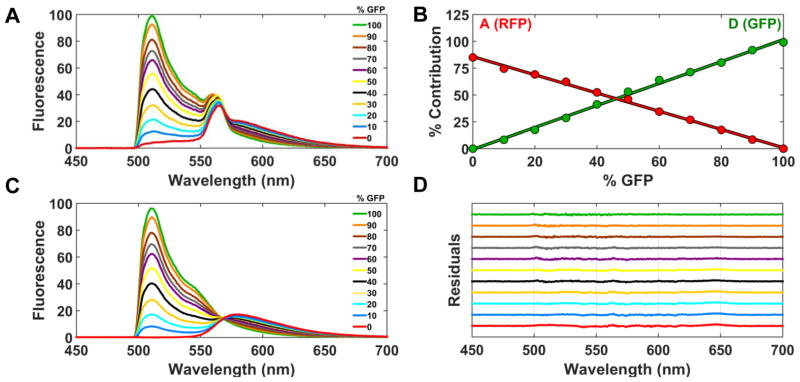
Accuracy and precision of the spectral unmixing method was verified by analyzing known mixtures of GFP- and RFP-expressing HEK293 cell lines, dispensed at 106 cells/mL into a 384-well plate. The GFP-only control cell line was mixed with cells expressing the RFP-only control construct to produce wells with the percentage of GFP increasing in 10% increments. The total volume per well was 50 μL. The expression of the RFP-only cell line was five times that of the GFP-only cell line, as determined by quantitative western blotting. The observed spectra (raw data) show a uniform decrease in the GFP contribution (500–550 nm region) (Figure 2A).
Figure 2.
Analysis of mixtures of GFP- and RFP-expressing stable cell lines. (A) Cells expressing a GFP-only control construct were mixed with cells expressing a RFP-only control construct in a 384-well plate. The % of GFP cells was varied as indicated, but the total volume and number of cells remained constant. The observed spectrum of each GFP mixture is shown (average from 32 wells). Each observed spectrum was fit using linear least-squares minimization to determine the four scalar coefficients (Eq. 3), and the results are plotted in (B), showing that the fluorescence contributions of GFP and RFP to the emission spectrum vary inversely and linearly. (C) shows the results of A after subtracting contributions from autofluorescence and Raman. (D) shows the residuals from fits in B, offset vertically for clarity.
The individual scalar coefficients from the fluorescence emission spectrum of each mixture were assessed as described in (Eq. 3). The scalar coefficients of GFP (D) and RFP (A) were converted to fluorescence signal by multiplying by the total emission from the corresponding reference specta (FRFP and FGFP) using. (Eq. 4,5). The contributions of GFP (D) and RFP (A) increased inversely and linearly as expected. The contribution from cellular autofluorescence and water Raman remained constant across the GFP/RFP live-cell gradient (Figure 2B) and were subtracted from the spectrum of each mixture (Figure 2C). An isoemissive point at 567 nm clearly distinguishes the change in GFP and RFP emission across the gradient of cellular mixtures. The residuals obtained using the four-component model (Eq. 3) are plotted for each %GFP mixture in Figure 2D. The residuals remain flat, demonstrating excellent agreement between the fit and observed spectrum (Eq. 3).
The scalar coefficients were used to determine the contribution of each component to the total fluorescence signal. At the lowest mixture of cells expressing GFP (10% GFP), there are approximately 5,000 GFP-expressing cells and 45,000 RFP-expressing cells per well (50 μL total volume per well). Based on the diameter of the laser beam (500 microns), well dimensions, and diameter of one cell, we estimate approximately 500 cells expressing GFP contribute to the observed spectrum. As such, we have performed preliminary studies with low-volume 1536-well PCR plates, which indicate decreasing the total volume and number of cells by a factor of ten does not significantly degrade the data quality (results not shown).
Concentration-response curve comparison of 2CS FRET change with known SERCA inhibitors
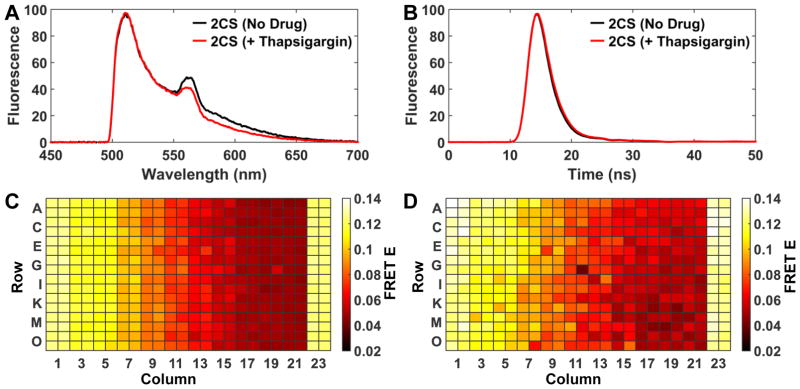
The spectral unmixing method’s capability to resolve 2CS FRET changes via controlled addition of the known SERCA inhibitor thapsigargin, which binds to SERCA2a with subnanmolar affinity (Ki = 2 nM), was evaluated and compared to lifetime mode.14 Ten different concentrations of thapsigargin were dispensed across a 384-well plate (0.8, 1, 2, 3, 4, 5, 6, 8, 10, and 50 nM thapsigargin concentrations, n = 32 wells for each concentration). Matching DMSO controls (0.5 μL DMSO / 50 μL total well volume) were located in the outer columns (1, 2, 23, and 24) of the 384 well plate. The spectra were decomposed using a four-component model (Eq. 3). The 2CS FRET change, acquired in spectral mode, is reflected by a decrease in the RFP-region (550–650 nm) of the fitted spectrum (Figure 3A).
Figure 3.
Spectral and lifetime mode comparison of 2CS concentration-dependent FRET change in response to the known SERCA inhibitor thapsigargin. (A) Fluorescence emission spectra (average of 32 wells) normalized to GFP intensity, showing 8% reduction in FRET (decreased RFP emission) in response to 50 nM thapsigargin. (B) Nanosecond time-resolved fluorescence decay waveforms (average of 32 wells) normalized to GFP-only, showing a 6% reduction in FRET (longer GFP lifetime) in response to 50 nm thapsigargin.(red decay) (C) Heat map of FRET efficiency calculated from spectral mode. 10 different concentrations of thapsigargin (0.8,1,2,3,4,5,6,8,10, and 50 nM, increasing left to right) were dispensed across a 384-well plate, with DMSO controls at columns 1, 2, 23, and 24. (D) Heat map of FRET efficiency calculated from lifetime mode from the same plate.
For direct comparison with spectral recording, nanosecond time-resolved fluorescence decay waveforms were acquired on the same samples (Figure 3B). The fluorescence lifetime for each well was determined as described above (Eq. 1). A saturating concentration of thapsigargin (Tg) (50 nM) induced a 200 picosecond increase in the GFP lifetime, corresponding to a 6% decrease in FRET efficiency, as determined from the lifetime change compared to the donor-only control (Eq. 2), as shown in Figure 3B. Heatmaps of FRET efficiency demonstrate excellent uniformity at each concentration across the plate (Figure 3C, D), with greater precision evident for the spectral data.
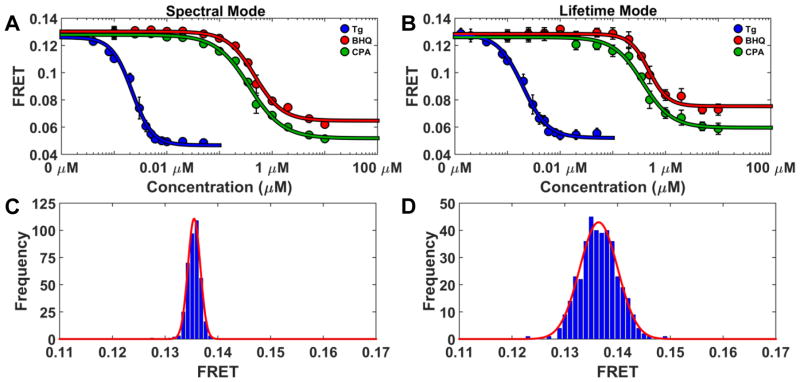
The sensitivity of FRET detection for both lifetime and spectral modes was further investigated by generating 14-point concentration-response curves for addition of thapsigargin and two other well-established SERCA inhibitors 1,4-dihydroxy-2,5-di-tert-butylbenzene (BHQ) and cylcopiazonic acid (CPA). Reported Ki’s fall in the range 2–7 μM for BHQ and 90–2500 nM for CPA. The concentration-dependent FRET change14,15, in response to the three known SERCA effectors, again exhibited excellent agreement between the FRET efficiency acquired from both spectral and lifetime modes (Figure 4C and D.).
Figure 4.
(A and B) show the concentration-dependent 2CS FRET response of three well-established SERCA inhibitors, thapsigargin (Tg), 4-dihydroxy-2, 5-di-tert-butylbenzene (BHQ), and cylcopiazonic acid (CPA), in a 384-well plate (n = 8 wells for each concentration). Fits to the Hill equation (curves) give EC50 values in close agreement for the two modes, and in good agreement with published data on SERCA inhibition. Histograms (C) (Spectral Mode) and (D) (Lifetime Mode) illustrate the precision of FRET determination from 384 wells of 2CS cells without the addition of drug. Mean and standard deviation values for FRET, determined from the Gaussian fits shown in red, were 13.7% ± 0.11 for spectral and 13.6% ± 0.36 for lifetime.
The experimentally determined apparent FRET equilibrium constants of thapsigargin and CPA agree with the previously reported Ki values (Figure 4A and B). Note that error bars (n = 8 wells) represent one standard deviation, not standard error of the mean. A tenfold difference from the FRET EC50 and reported Ki from functional activity assays was found for BHQ. This finding could reflect a difference between live-cell assays and biochemical functional assays performed on purified proteins. However, we have found the Ki of BHQ to be 400 nM, as assessed by measuring the rate of ATP hydrolysis (data not shown).
The precision of the spectral unmixing and lifetime methods was further assessed by evaluating the FRET efficiency determined from 384 wells of 2CS cells, without the addition of drug. Histograms for all 384 wells of the 2CS cells-only control plate are shown in (Figure 4C and D). The average FRET efficiency was 13.7% (spectral mode) and 13.6% (lifetime mode) with standard deviations of 0.11% (spectral mode) and 0.36% (lifetime mode).
Spectral fitting results in high assay precision, even if cellular autofluorescence is high
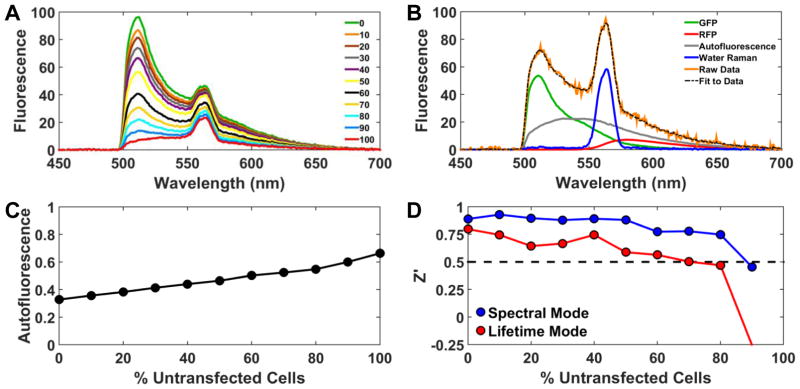
Live-cell fluorescence assays are prone to artifacts from cellular autofluorescence, dispensing error, and other variability in sample preparation.17 Spectral unmixing is a very effective way to resolve the cellular autofluorescence component, maintaining high precision and accuracy of FRET determination. To simulate increasing autofluorescence (e.g., due to low biosensor expression or transient transfection), cells expressing the 2CS FRET biosensor were mixed with known amounts of untransfected cells and dispensed across a column-wise gradient of one 384 well plate (Figure 5A). The well volume (50 μL) and number of cells per well (50,000) were held constant. Spectra were analyzed as in Figure 1D, as illustrated in Figure 5B for 80% untransfected cells, resulting in much greater autofluorescence than that in Figure 1D. The autofluorescence signal from each mixture was assessed by determining c ((Eq. 3), and the expected linear increase was observed (Figure 5C). To assess the effect of autofluorescence on the quality of the HTS assay, a 384-well plate was prepared with half the wells containing 100 nM thapsigargin and half being DMSO control wells (%v/v). These positive and negative controls were used to define the signal window for determination of assay quality factor Z′ 10,16, yielding values of 0.90 (spectral mode) and 0.77 (lifetime mode), indicating that both modes provide an excellent assay for HTS (Z′ > 0.5), until the sample is diluted by 80% (lifetime) or 90% (spectral) with untransfected cells (Figure 5D).
Figure 5.
Spectral fitting increases assay precision by solving for the contribution of cellular autofluorescence. (A) Spectra were obtained from mixtures of transfected cells (expressing 2CS), with the indicated % of untransfected cells. Each spectrum is the average from 16 wells. (B) Example of data analysis using (Eq. 3, showing the fit to the data in A for the case of 80% untransfected cells. (C) Autofluorescence (c in (Eq. 3, normalized to the sum of all four components) from fits. (D) Quality factor Z′ 10,16, using the effect of 100 nM Tg (Figure 4) to define the signal window.
Accurate FRET efficiency determination from cyan and yellow fluorescent proteins
Although GFP and RFP (and other red-shifted FRET pairs) are less susceptible to compound fluorescence artifacts18, the overwhelming majority of genetically-encoded FRET-based biosensors established and studied to date involve cyan (CFP) and yellow (YFP) fluorescent proteins. 19,19,19,19,19 Accordingly, we present an illustration of the spectral plate reader’s performance using this FRET pair.
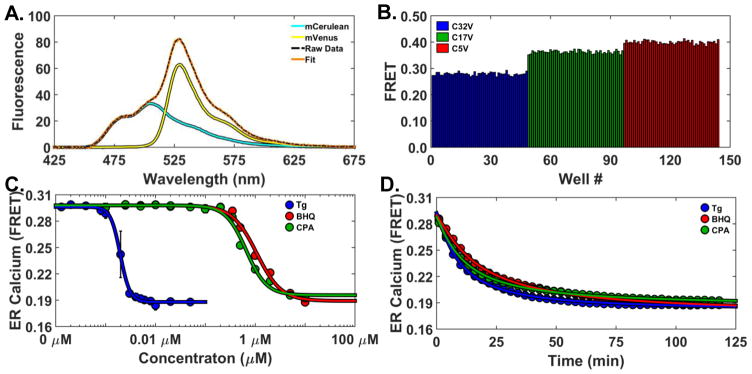
Reference standards consisting of mCerulean (CFP) and mVenus (YFP) tethered by flexible linkers of increasing lengths of 5, 17, and 32 amino acids (designated C5V, C17V, and C32V, respectively)20 have been widely used in FRET calibrations (Koushik et al. 2006). Various means to record the FRET signal, including subsequent lifetime and spectral analysis, have been previously applied. These controls can be used to calibrate and validate new FRET detection technology. The consensus FRET efficiencies for these constructs are 43 ± 2 (C5V), 38 ± 3 (C17V), and 31 ± 2 (C32V) %. Transient transfections of HEK293 cells with these FRET reference standards and the appropriate donor CFP (mCerulean) and acceptor YFP (mVenus) labeled constructs were performed. The cells were harvested and assessed on the plate reader with excitation at 434/17 nm from a laser-driven light source (Energetiq). FRET efficiency was evaluated. Optimized transfection protocols for large-scale transient transfections, were found to obtain sufficiently high expression of the FRET standard constructs so that contributions from autofluorescence and water Raman were negligible. Subsequently, only a two-component fit was required, as shown by the fitted and observed data with component spectra (Figure 6A). The CFP/YFP ratio (FR in (Eq. 4,(Eq. 5) was converted to FRET efficiency as described in Supplemental Material (Derivation), using reported values of extinction coefficients and quantum yields.21. The acceptor/donor fluorescence ratio (FR in (Eq. 5) was then calibrating to the previously reported FRET efficiency of C5V.20.
Figure 6.
Accurate FRET efficiency determination from CFP and YFP biosensors in HEK293 cells. (A). Two-component spectral fit of the C17V FRET standard. (B) FRET data from three CFP-YFP FRET pairs with different lengths. 48 wells for each of the three pairs were studied in a 384-well plate. (C) Concentration-response curves showing the effects of three SERCA inhibitors on FRET (120 min after mixing), using the CFP/YFP-based D1ER cameleon FRET calcium sensor (n = 8 wells for each concentration). Curves show best fits to the Hill equation.(D) Time-dependent effects of SERCA inhibitors on ER Calcium at saturating drug concentrations. DMSO controls have no effect on ER calcium concentrations (data not shown). Thapsigargin (Tg, blue) irreversibly binds SERCA with high-affinity and depletes calcium at a faster rate than BHQ and CPA, which have micromolar binding affinities.
FRET efficiencies determined from spectral mode were in excellent agreement with the reported results 42.3 ± 0.7 (C5V), 38.3 ± 0.7 (C17V), and 29.0 ± 0.6 % (C32V) as shown in Figure 6B. The precision was high; each bar represents the data from a single well. These results validate the capability of accurate FRET efficiency determination from CFP and YFP FRET pairs using the spectral unmixing method.
Investigation of a CFP and YFP FRET pair was evaluated with the well-known cameleon calcium sensor, in which the calmodulin-binding (M13) domain of myosin light chain kinase (MLCK) and calcium-binding domain of calmodulin are fused together and located between two fluorescence proteins (eCFP/mCitrine). These calcium sensors have allowed cellular [Ca2+] to be monitored more directly and reliably than using calcium-sensitive dyes.22 The endoplasmic reticulum-targeted cameleon sensor (D1ER) was selected because it is known to be sensitive to the SERCA inhibitors thapsigargin, CPA, and BHQ. Instead of detecting direct ligand binding, as in the 2CS studies reported above, the D1ER sensor can be used to monitor changes in ER calcium levels.23 Upon SERCA inhibition, calcium is no longer pumped into the ER, leading to calcium depletion through various mechanisms including IP3-gated receptors and ryanodine receptors. D1ER senses these changes as calcium binding leads to a conformational rearrangement of the biosensors, increasing FRET between CFP and YFP. Therefore, calcium depletion due to SERCA inhibition is detected as a decrease in FRET.
A HEK293 stable clone with constitutive expression of the D1ER cameleon calcium sensor was generated using G418 antibiotic selection. The localization of D1ER to the endoplasmic reticulum (ER) lumen was verified by microscopy and expression remained constant over months in culture. Cells were harvested and dispensed into 384-well plates containing the same concentrations of thapsigargin, CPA, and BHQ, as previously evaluated with 2CS (Figure 4). D1ER FRET was monitored only in spectral mode, because the CFP donor cannot be excited effectively by the 473 nm laser used in lifetime mode. ER calcium was monitored by repeatedly scanning the 384 well plate at three-minute intervals over a 120-minute period. The expected concentration and time-dependent decreases in FRET were observed. The high-affinity and selective SERCA inhibitor thapsigargin produced a sigmoidal FRET response with an equilibrium constant of 1.97 nM (ER calcium depletion shown at 120 min time point) (Figure 6C).
The submicromolar SERCA inhibitors BHQ and CPA also produced a concentration-dependent effect. FRET EC50 values were evaluated for each concentration curve and agree with the expected values based on their affinity for SERCA. The time-dependent effect of SERCA inhibitors on endoplasmic reticulum (ER) calcium concentration can be determined by evaluating the rate of calcium depletion (FRET decrease) in response to titration with the inhibitors. Thapsigargin (Tg) irreversibly binds SERCA with high-affinity and depletes calcium at a faster rate than BHQ and CPA, which have micromolar binding affinities (Figure 6D). The time dependence of calcium depletion was evaluated by fitting each curve to the Hill equation to determine the rate of calcium depletion T50. The T50 of thapsigargin, BHQ, and CPA was 9.5, 17.4, and 11.7 minutes; respectively.
Discussion
Accurately recording a fluorescence signal is an essential element of fluorescence spectrometers, microplate readers, fluorescence microscopes, flow cytometers, qPCR machines, chromatography, capillary array electrophoresis detectors, gel scanners, and sequencers. However, scanning a wavelength-selective filter (monochromator) through a range of emission wavelengths is usually employed only for research-grade fluorescence spectrometers, where data quality is more important than measurement speed. Microplate readers equipped with monochromators, now commonly available, could serve as alternatives to research-grade spectrometers. The primary difference between the two classes of instruments lies in the sample holder format and optical geometry, i.e., cuvettes and right angle for spectrometers, plastic plates and epi-illumination for plate readers. It is plausible to assume that a research-grade, cuvette-based fluorescence spectrometer will always provide much higher data quality than could ever be possible in a microplate reader, because the right-angle geometry, large sample volumes, and high-quality optics of the sample container minimize artifacts from light scattering and other sources of interfering background.24 However until now, a comprehensive study that directly compares the data quality obtained with a fluorescence microplate reader to that produced by a fluorescence spectrometer did not exist (Figure S1). The de facto standard approach to performance characterization of fluorescence spectrometers is the water Raman test.25 The corresponding de facto standard approach to performance characterization of microplate readers is a fluorescence limit-of-detection test.26 The data presented here strongly suggest that the quality gap between fluorescence spectrometers and microplate readers is much smaller than is generally assumed.
Genetically encoded FRET sensors are usually studied via imaging in a fluorescence microscope using a rigorous 3-cube technique.1 Corrections for cross-talk or bleed-through between the donor and acceptor excitation/emission26 are determined by assessing images of fluorescence proteins expressed individually at each wavelength of interest. Acquisition at multiple excitation and emission wavelengths is typically required during each microscopy experiment. A major advantage revealed by these studies is that the shape of the full emission spectrum (2048 data points spread across the 300–800 nm wavelength region) can be used to reliably and accurately quantitate fluorescence signal without the need for exhaustive controls on each of day of experiments. Reference spectrum from control cell lines (in suspension) need only to be acquired one time. They were used to calibrate for detector sensitivity and found to produce reproducible results for months on end. This drastically reduced the workload of maintaining and culturing control cell lines, thereby increasing productivity by reducing the number of conditions required for each experiment.
Another source of potential error in imaging experiments is autofluorescence contributions or interfering background emission. Microscopy methods rely on defining regions of interest, designed to isolate the cells of interest (e.g., cells that have the highest fluorescence signal) from the background. These results are averaged over a statistically meaningful number of cells.27 The laborious nature of fluorescence emission correction from cellular autofluorescence and fluorescent protein crosstalk has made its usefulness for high-throughput drug screening impractical.
The spectral unmixing method incorporates essentially the same filter cubes and epi-illumination geometry as fluorescence microscopy. However, no attempt is made to image individual cells, as a simple lens directs the excitation light into a microplate well and collects the emitted fluorescence signal. This cells-in-wells approach treats the cells as a homogeneous solution. Excitation of less than 50,000 cells placed in suspension, yielded enough photons to obtain high-quality spectra and fluorescence decay rates (lifetime) in a fraction of a second.
Spectral unmixing in fluorescence microscopy has previously been limited to low-throughput high-end instruments, typically based on a multi-anode PMT with 32 channels on roughly 10-nm spacing.28 The present approach utilizes a linear array detector and records at intervals of approximately 0.5 nm. This is substantially narrower than the width of the spectral features of interest, but advantageous nonetheless because oversampling improves the robustness of the fitting..
Many methods have been developed to decompose the fluorescence spectrum for the purposes of correcting waveform distortions associated with monochromator-based excitation and emission.27,29 The complexities of these methods are unsuitable for large-scale drug discovery campaigns. The development of a simplified method for multi-component spectral analysis for the determination of FRET efficiency from a live-cell biosensor resulted in a assay suitable for high-throughput screening as shown by the Z′ in Figure 5D. Reference spectra of the known components are used to decompose the spectrum from the sample of interest (Figure 1C). The spectral recording technology allows for robust measurements with inexpensive equipment in comparison to fluorescence microscopes and flow cytometry. The spectral unmixing method can also be used to solve for unknown components such as sample contamination or, as shown in our following companion paper 30, the direct identification of fluorescent compounds (false-positives) during a high-throughput drug screen.
Data acquired by both spectral and lifetime modes were shown to be extremely precise. The focus of this article is to demonstrate the novel spectral recording technology, as the lifetime technology has previously been evaluated. 3,5,6 However, this is the first demonstration of the top-read fluorescence lifetime plate reader. This optical configuration is advantageous as inexpensive black-bottom microplates can be used, instead of glass-bottom microplates. Another benefit is that temperature can be controlled by placing a heat source underneath the microplate. These studies directly compared two novel fluorescence plate reader technologies. A three-fold increase in precision was found for spectral mode over lifetime mode as shown in Figure 4C, D. These studies utilized data analysis methods that can currently be used for large-scale drug discovery efforts as they are not computationally taxing and can be performed in real time. The precision of the lifetime method is likely to be increased by development of more sophisticated analysis methods. For example, in these studies a single-exponential model was used to fit the lifetime data and no attempt was made to correct the lifetime data for artifacts from cellular autofluorescence. Demonstrations of advancements in using more-rigorous global lifetime analysis can be found in the accompanying article reporting high-throughput screening performance.
The increased precision from spectral mode, as compared with lifetime mode, is likely to be obsderved primarily when employing two-color biosensors, in which every donor has an acceptor on the same molecule. In biosensors created by reacting dyes with amino acid side chains, and/or involving donor and acceptor on different proteins, lifetime detection has the advantage of resolving heterogeneous populations of donors. Thes unique structural resolution of the lifetime mode also permits the resolution of multiple structural states of the biosensor1,31,32, providing more detailed structural information about the results of screening.
Two distinct classes of live-cell FRET biosensors were shown, those that are designed specifically for structure determination (Figure 3A; Figure 4A; Figure 6B) and those that exploit a structural change in the biosensor for the purpose of quantitating the concentration of some species, such as [Ca2+], in the cellular milieu (Figure 6C,D). There are many applications in which a better way to detect structural changes of either a cytosolic or membrane protein via FRET would be valuable. Developing and engineering fluorescent protein FRET biosensors can be challenging, as they can be difficult to express (low signal, high cellular autofluorescence), they exhibit a low FRET signal (because the donor and acceptor are too far apart), or the dynamic range of the signal window may be limited. These problems can be overcome, as incredibly small changes in FRET signal can be detected using this spectral and lifetime detection technology. Even more so, detection of weak GFP fluorescence masked by cellular autofluorescence was clearly resolved using the spectral unmixing methods.
Beyond increased sensitivity and precision, the rapid acquisition rates reported here (10 wells or more per s) allow for time-course studies. Previous reports suggest that the resolution and sensitivity of microplate readers is inadequate to directly monitor calcium flux in live-cells.33 To date, high-throughput screening with cameleon sensors, such as D1ER, has been achieved using laborious high-content imaging34 or calcium-sensitive fluorescent dyes with CCD-camera based plate readers (FLIPR, Molecular Devices). To our knowledge, this is the first time changes in live-cell calcium levels were shown to be monitored with high speed and precision in a fluorescence microplate reader using a FRET based biosensor (Figure 6C). Short acquisition times of 200 ms per well were used to repeatedly scan portions of high-density microplates, and monitor calcium flux at rates comparable to standard microscopy techniques; except across a range of chemical perturbations (Figure 6D). Thus, the D1ER FRET-based calcium biosensor may be a powerful tool for high-throughput screening, linking the structural perturbations of 2CS to functional changes, at the level of calcium dysregulation in live-cells.
The spectral and lifetime detection methods presented here are widely applicable across the life sciences beyond high-throughput and high-content assays. Potential assays, which may benefit from this platform are phenotypic screening of libraries of mutant constructs, recombinant antibody development, or protein stability assays, where sample quantities are limited. Future technological developments may also include cell sorting by the addition of microfluidics devices.
In conclusion, a new instrument records fluorescence spectra in a microplate reader at speeds fully compatible with high-throughput screening applications. The resolution of the recorded spectra is improved in comparison to that provided by standard cuvette-based fluorescence spectrometers, even though the acquisition rates are 100 times faster and sample volumes 100 times smaller (Figure S1). The novel spectral and lifetime technologies were thoroughly evaluated and when used together are complementary, creating a new combination of precision and resolution, particularly in applications to living cells expressing genetically encoded FRET biosensors. Accuracy and precision is comparable to or greater than those achieved with much lower throughput instruments, such as cuvette-based spectrofluorometers and fluorescence microscopes. These technical breakthroughs in fluorescence recording enable their use in high-throughput screening applications, as illustrated in the following article.
Supplementary Material
Acknowledgments
We thank Jesse E. McCaffrey, Karl Petersen, Razvan L. Cornea, Benjamin Binder, and J. Michael Autry for helpful discussions, Octavian Cornea for assistance in manuscript preparation, and Simon J. Gruber and Seth L. Robia for development of the reagents and materials used for many of the experiments. Fluorescence microscopy was performed at the UMN Imaging Center, flow cytometry at the UMN Lillehei Heart Institute, and spectroscopy was performed at the UMN Biophysical Technology Center. pcDNA-D1ER was a gift from Amy Palmer & Roger Tsien (Addgene plasmid # 36325). C5, C17V, and C32V was a gift from Steven Vogel (Addgene plasmid # 26394, 26395, and 26396)
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grants R42DA037622 (to G.D.G and D.D.T.), R01HL129814 (to D.D.T.), and R01GM27906 (to D.D.T.). T.M.S. was supported by the NIH Chemistry-Biology Interface Training Grant (5T32GM008700), and by predoctoral fellowships from 3M and Arnold H. Johnson.
Footnotes
Declaration of Conflicting Interests
Dr. Thomas holds equity in and serves as an executive officer for Photonic Pharma LLC. This relationship has been reviewed and managed by the University of Minnesota.
References
- 1.Hou Z, Hu S, Blackwell DJ, et al. Two-color calcium pump reveals closure of the cytoplasmic headpiece with calcium binding. PLoS ONE. 2012 doi: 10.1371/journal.pone.0040369. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pallikkuth S, Blackwell DJ, Hu Z, et al. Phosphorylated phospholamban stabilizes a compact conformation of the cardiac calcium-ATPase. Biophys J. 2013;105:1812–1821. doi: 10.1016/j.bpj.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gruber SJ, Cornea RL, Li J, et al. Discovery of enzyme modulators via high-throughput time-resolved FRET in living cells. J Biomol Screen. 2014;19:215–222. doi: 10.1177/1087057113510740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shcherbo D, Souslova EA, Goedhart J, et al. Practical and reliable FRET/FLIM pair of fluorescent proteins. BMC Biotechnol. 2009;9:24. doi: 10.1186/1472-6750-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen KJ, Peterson KC, Muretta JM, et al. Fluorescence lifetime plate reader: resolution and precision meet high-throughput. Rev Sci Instrum. 2014;85:113101. doi: 10.1063/1.4900727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iram SH, Gruber SJ, Raguimova ON, et al. ATP-Binding Cassette Transporter Structure Changes Detected by Intramolecular Fluorescence Energy Transfer for High-Throughput Screening. Mol Pharmacol. 2015;88:84–94. doi: 10.1124/mol.114.096792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muretta JM, Kyrychenko A, Ladokhin AS, et al. High-performance time-resolved fluorescence by direct waveform recording. Rev Sci Instrum. 2010;81:103101. doi: 10.1063/1.3480647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzman C, Oetken-Lindholm C, Abankwa D. Automated High-Throughput Fluorescence Lifetime Imaging Microscopy to Detect Protein-Protein Interactions. J Lab Autom. 2015 doi: 10.1177/2211068215606048. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki M, Sakata I, Sakai T, et al. A high-throughput direct fluorescence resonance energy transfer-based assay for analyzing apoptotic proteases using flow cytometry and fluorescence lifetime measurements. Anal Biochem. 2015;491:10–17. doi: 10.1016/j.ab.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Cornea RL, Gruber SJ, Lockamy EL, et al. High-throughput FRET assay yields allosteric SERCA activators. J Biomol Screen. 2013;18:97–107. doi: 10.1177/1087057112456878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suhling K, Siegel J, Phillips D, et al. Imaging the environment of green fluorescent protein. Biophys J. 2002;83:3589–3595. doi: 10.1016/S0006-3495(02)75359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leavesley SJ, Britain AL, Cichon LK, et al. Assessing FRET using spectral techniques. Cytometry A. 2013;83:898–912. doi: 10.1002/cyto.a.22340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorn TLaK. Fluorescent protein properties. Retrieved from http://nic.ucsf.edu/FPvisualization/
- 14.Wootton LL, Michelangeli F. The effects of the phenylalanine 256 to valine mutation on the sensitivity of sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) Ca2+ pump isoforms 1, 2, and 3 to thapsigargin and other inhibitors. J Biol Chem. 2006;281:6970–6976. doi: 10.1074/jbc.M510978200. [DOI] [PubMed] [Google Scholar]
- 15.Michelangeli F, East JM. A diversity of SERCA Ca2+ pump inhibitors. Biochem Soc Trans. 2011;39:789–797. doi: 10.1042/BST0390789. [DOI] [PubMed] [Google Scholar]
- 16.Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 17.Palmer AE, Qin Y, Park JG, et al. Design and application of genetically encoded biosensors. Trends Biotechnol. 2011;29:144–152. doi: 10.1016/j.tibtech.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam AJ, St-Pierre F, Gong Y, et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat Methods. 2012;9:1005–1012. doi: 10.1038/nmeth.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Addgene: Fluorescent Protein Guide: Biosensors. Retrieved from https://www.addgene.org/fluorescent-proteins/biosensors/
- 20.Koushik SV, Chen H, Thaler C, et al. Cerulean, Venus, and VenusY67C FRET reference standards. Biophysical journal. 2006;91:L99–L101. doi: 10.1529/biophysj.106.096206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cranfill PJ, Sell BR, Baird MA, et al. Quantitative assessment of fluorescent proteins. Nat Methods. 2016;13:557–562. doi: 10.1038/nmeth.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer AE, Tsien RY. Measuring calcium signaling using genetically targetable fluorescent indicators. Nat Protoc. 2006;1:1057–1065. doi: 10.1038/nprot.2006.172. [DOI] [PubMed] [Google Scholar]
- 23.Palmer AE, Jin C, Reed JC, et al. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci U S A. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakowicz J. Principles of Fluorescence Spectroscopy. 3. 2006. [Google Scholar]
- 25.Scientific H. Signal to Noise Ratio Explained. 2015. [Google Scholar]
- 26.Thomsen V, Schatzlein D, Mercuro D. Limits of detection in spectroscopy. Spectroscopy. 2003;18:112–114. [Google Scholar]
- 27.Zhang L, Qin G, Chai L, et al. Spectral wide-field microscopic fluorescence resonance energy transfer imaging in live cells. J Biomed Opt. 2015;20:86011. doi: 10.1117/1.JBO.20.8.086011. [DOI] [PubMed] [Google Scholar]
- 28.Larson JM. The Nikon C1si combines high spectral resolution, high sensitivity, and high acquisition speed. Cytometry A. 2006;69:825–834. doi: 10.1002/cyto.a.20305. [DOI] [PubMed] [Google Scholar]
- 29.Mustafa S, Hannagan J, Rigby P, et al. Quantitative Forster resonance energy transfer efficiency measurements using simultaneous spectral unmixing of excitation and emission spectra. J Biomed Opt. 2013;18:26024. doi: 10.1117/1.JBO.18.2.026024. [DOI] [PubMed] [Google Scholar]
- 30.Lakowicz JR. Principles of fluorescence spectroscopy. New York: Springer; 2006. [Google Scholar]
- 31.Li J, James ZM, Dong X, et al. Structural and functional dynamics of an integral membrane protein complex modulated by lipid headgroup charge. J Mol Biol. 2012;418:379–389. doi: 10.1016/j.jmb.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong X, Thomas DD. Time-resolved FRET reveals the structural mechanism of SERCA-PLB regulation. Biochem Biophys Res Commun. 2014;449:196–201. doi: 10.1016/j.bbrc.2014.04.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meijer M, Hendriks HS, Heusinkveld HJ, et al. Comparison of plate reader-based methods with fluorescence microscopy for measurements of intracellular calcium levels for the assessment of in vitro neurotoxicity. Neurotoxicology. 2014;45:31–37. doi: 10.1016/j.neuro.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Honarnejad K, Kirsch AK, Daschner A, et al. FRET-based calcium imaging: a tool for high-throughput/content phenotypic drug screening in Alzheimer disease. Journal of biomolecular screening. 2013;18:1309–1320. doi: 10.1177/1087057113502672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.