Abstract
Background
Psoriasis is a common skin disease and moderate to severe psoriasis is associated with a dose-dependent risk for metabolic and cardiovascular morbidity. It has previously been speculated that women have less severe psoriasis, as men are overrepresented in psoriasis registers and consume more care.
Objective
The objective of this study was to investigate, for the first time, the sex differences in the severity of psoriasis using the gold standard of severity measurement, the Psoriasis Area and Severity Index (PASI), and the distinct elements of the PASI score.
Design, Setting and Participants
This was a cross-sectional study based on the national registry for systemic treatment of psoriasis in Sweden (PsoReg), with 5438 patients experiencing moderate to severe psoriasis. Differences in the PASI score and its elements at enrolment were tested by multivariable ordinal logistic regressions.
Main Outcome Measures
The different components of the PASI score were used to analyze the assessment of disease severity. For each body area (head, arms, trunk, and legs), the score of the plaque characteristics and degree of skin involvement were used as outcomes.
Results
Women had statistically significantly lower median PASI scores (5.4) than men (7.3) [p < 0.001], which was consistent across all ages. The difference remained statistically significant in a multivariable linear regression. The itemized PASI analyses from the Mann–Whitney–Wilcoxon tests and the adjusted ordinal logistic regressions confirmed that women had significantly lower scores than men in all areas of the body, except for the head. No differences in the use of medications prior to enrolment could be found that may cause this difference between the sexes.
Conclusions
As the PsoReg contains the detailed disease measurement PASI, which was traditionally used for selected participants in clinical studies only, a nationwide unselected population could be investigated. The fact that women have less severe psoriasis can explain the dominance of males in the systemic treatment of psoriasis. These findings motivate a gender perspective in the management of psoriasis and in the prevention and management of its comorbidities.
Key Points
| This register-based study revealed that the severity of psoriasis differs between men and women when analyzing the Psoriasis Area and Severity Index (PASI), the most widely used instrument to assess disease severity. |
| The distinct elements of the PASI score were analyzed separately and it was found that men had more severe psoriasis than women in all areas of the body (trunk, arms and legs), except for the head. |
Introduction
Psoriasis is one of the most common skin diseases, with a prevalence of approximately 2–4% in the Western world [1]. It is known that moderate to severe psoriasis is associated with a dose-dependent risk of cardiovascular morbidity, metabolic syndrome, and depression [2–4]. The prevalence of psoriasis is considered to be balanced between the sexes [5, 6]; however, several studies have indicated sex differences in the treatment of psoriasis. In 1945, Romanus showed that a population of 550 Swedish patients had statistically significant differences in the severity of psoriasis (p < 0.001) depending on their sex. The highest severity in his categorization “continues symptoms and recommended hospitalization” was fulfilled by 38.4% of men but only 28% of women [7]. It has also been shown that women receive topical treatments to a greater extent than men [8], while men are more likely to receive systemic treatment [9, 10]. Furthermore, men are more often treated by dermatologists, whereas women are treated by general practitioners or treat themselves at home [11–13], which has led to the hypothesis that systemic psoriasis treatment for women of fertile age may be avoided [13, 14]. However, we have previously shown that when adjusting for disease severity, among other factors, sex was not a decisive factor in the initiation of biological treatments, which have high acquisition costs [15].
The gold standard for assessing the severity of psoriasis is the Psoriasis Area and Severity Index (PASI), which combines the assessment of the severity of lesions and the extent of the affected area in a single index score [16]. We have previously observed that the PASI score was a more important factor than the Dermatology Life Quality Index (DLQI) in the decision to initiate biologic treatment, and that women tended to have lower PASI scores than men [17]. Therefore, the observed treatment differences between the sexes might be due to less severe disease in women. Consequently, we performed this study to test the hypothesis that, at enrolment in the PsoReg, severe psoriasis is less common in women. By analyzing the distinct elements of the PASI score, we also wanted to investigate whether any pattern could be discerned between men and women in the different body parts and/or dimensions of PASI measures.
Methods
Study Population
Data were retrieved in May 2016, and, in total, 5438 patients with moderate to severe psoriasis were registered in the PsoReg at this time. PsoReg, initiated in 2006, is the national registry for systemic treatment of psoriasis in Sweden. Patients are registered at local, regional, and university hospitals, as well as in private practices and at treatment centers initiated by the Swedish Psoriasis Patient Organization (PSO) [18]. To be included in the PsoReg, patients need to be diagnosed with moderate to severe psoriasis and treated with, or considered for, systemic treatment by a specialist in dermatology. Thus, patients with mild psoriasis who are treated in primary care are not eligible for inclusion in the register. Nationwide, 65% of all biologically treated psoriasis patients and approximately 45% of all systemically treated psoriasis patients are estimated to be included in the PsoReg.
Definition of Variables
PASI is a combined score consisting of several dimensions of psoriasis, and is based on four areas: head, arms, trunk, and legs. Furthermore, for each body area, three plaque characteristics are assessed by the degree of erythema (redness), induration (thickness), and desquamation (scaling). The scores of the clinical signs in each area are summed and are finally weighted according to the area’s proportion of the body, before being converted to the final score, which ranged from 0 to a theoretical maximum of 72.
For each patient, information on sex, age, body mass index (BMI), disease duration, diagnosis of psoriatic arthritis (PsA), smoking status, PASI score, and the season in which assessment of the PASI score was carried out was retrieved from the PsoReg.
Statistical Analysis
Patient characteristics were analyzed at enrolment to examine differences between women and men. Continuous variables (age, disease duration, and BMI) were analyzed using the Student’s t test, while categorical variables (smoking, PsA, obesity, and season of the PASI evaluation) were tested using the Chi square test. The difference in the aggregated PASI score was analyzed using a Mann–Whitney–Wilcoxon test and a multivariable linear regression. At enrolment, a kernel-smoothing estimation was used to plot the difference in PASI score for both men and women, according to age at enrolment.
The differences in the independent PASI components between women and men were first investigated using the Mann–Whitney–Wilcoxon test separately for the different assessments: degree of involvement, erythema, induration and desquamation, and within each body region (head, trunk, arms, and legs). To be able to adjust for potential confounders and effect modifiers, a multiple linear regression was used to analyze the weighted aggregated PASI score, while multiple ordinal logistic regressions were used to analyze the different components of the PASI assessment. For each body area, four different regressions were fitted, where the score of the plaque characteristics (erythema, induration, and desquamation) and degree of skin involvement were outcomes. Age (continuous), sex (dichotomous), BMI (continuous), disease duration (continuous), PsA (dichotomous), smoking status (dichotomous), and season (categorical) were included as independent variables. The season variable was categorized into four different periods; winter (December, January, February), spring (March, April and May), summer (June, July, August), and autumn (September, October, November). The assumption of proportionality in the ordinal logistic regressions was tested by estimating the odds ratios in logistic regressions to make sure that the estimates did not vary between the two methods. Furthermore, different thresholds were also tested for the various categories to ensure that the results were stable.
To illustrate differences between sexes in the treatment of psoriasis on a national level, independent from the PsoReg, aggregated information on all dispensed prescriptions for (biologic treatment) ustekinumab (Anatomical Therapeutic Chemical [ATC] code L04AC05) and (topical treatment) calcipotriol (± corticosteroid; ATC codes D05AX02, D05AX52) was collected from the Prescribed Drug Register (PDR). Both substances are indicated for psoriasis and PsA, but not for other indications.
All statistical analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA). A p value ≤0.05 was considered to be statistically significant.
Ethics
This research was approved by the Umeå Ethical Review Board, and patients were recruited into the study after informed consent had been obtained.
Results
This study included 3252 (59.8%) men and 2186 (40.2%) women. For a subset of subjects (n = 3125; 57.5%), information on dispensed medications was available for analysis. At enrolment, women had significantly lower (p < 0.001) median PASI (5.4, interquartile range [IQR] 2.7–9.9) than men (7.5, IQR 3.6–12.2). Furthermore, women were older (p < 0.001), consisted of a higher proportion of smokers (p = 0.003), and had a higher extent of PsA (p = 0.015) compared with men. BMI was higher in the male group (p = 0.003), but there was no significant difference in the proportion of patients with obesity (BMI >30) [p = 0.896]. Disease duration was longer for women compared with men (p = 0.002), and no difference was found between men and women when comparing the time of year the PASI measurements were conducted (p = 0.315) (Table 1).
Table 1.
Patient characteristics
| All patients [n = 5438] | |||||
|---|---|---|---|---|---|
| No. of evaluable patients | Men [n = 3252] | No. of evaluable patients | Women [n = 2186] | p value | |
| Median PASI (IQR) | 2988 | 7.3 (3.6–12.2) | 1982 | 5.4 (2.7–9.9) | <0.001a |
| Median age (IQR), years | 3252 | 50.0 (39.0–61.0) | 2186 | 54.0 (42.0–65.0) | <0.001b |
| Median BMI (IQR) | 3109 | 27.5 (24.7–30.8) | 2064 | 26.4 (23.2–31.1) | 0.003b |
| Obese | 3252 | 28.6% | 2186 | 28.0% | 0.896c |
| Median disease duration, years (IQR) | 3094 | 18.0 (9.0–28.0) | 2083 | 20.0 (10.0–31.0) | 0.002b |
| Ongoing PsA | 2413 | 29.3% | 1601 | 36.1% | 0.015c |
| Current smokers | 3252 | 21.8% | 2186 | 31.3% | <0.001c |
| Season of PASI determination | 3252 | 2186 | |||
| Winter (December, January, February) | 30.0% | 28.0% | 0.296c | ||
| Spring (March, April, May) | 28.1% | 28.7% | |||
| Summer (June, July, August) | 13.4% | 14.9% | |||
| Autumn (September, October, November) | 28.6% | 28.4% | |||
IQR interquartile range, PASI Psoriasis Area and Severity Index, BMI body mass index, PsA psoriatic arthritis
aWilcoxon two-sample test
b t test
cChi square test
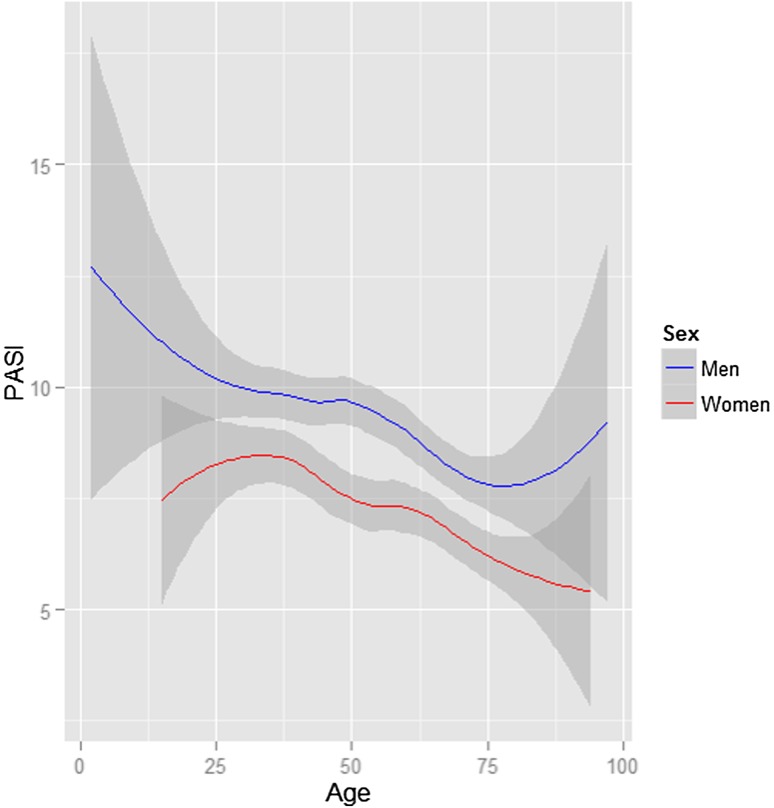
The kernel-smoothing plot shows that, in the PsoReg, women had a lower PASI score at enrolment compared with men, irrespective of age at registration. Both men and women had a declining trend in PASI throughout the age ranges (Fig. 1).
Fig. 1.
Age and PASI (95 % CI) score at enrolment in PsoReg stratified by sex. CI confidence interval, PASI Psoriasis Area and Severity Index
In the multiple linear regression analysis with PASI as the dependent variable, after adjusting for age, BMI, disease duration, PsA, smoking status, and season, sex was a significant explanatory variable (p < 0.001), with women having a lower PASI score than men.
Dimensions in Each Body Area
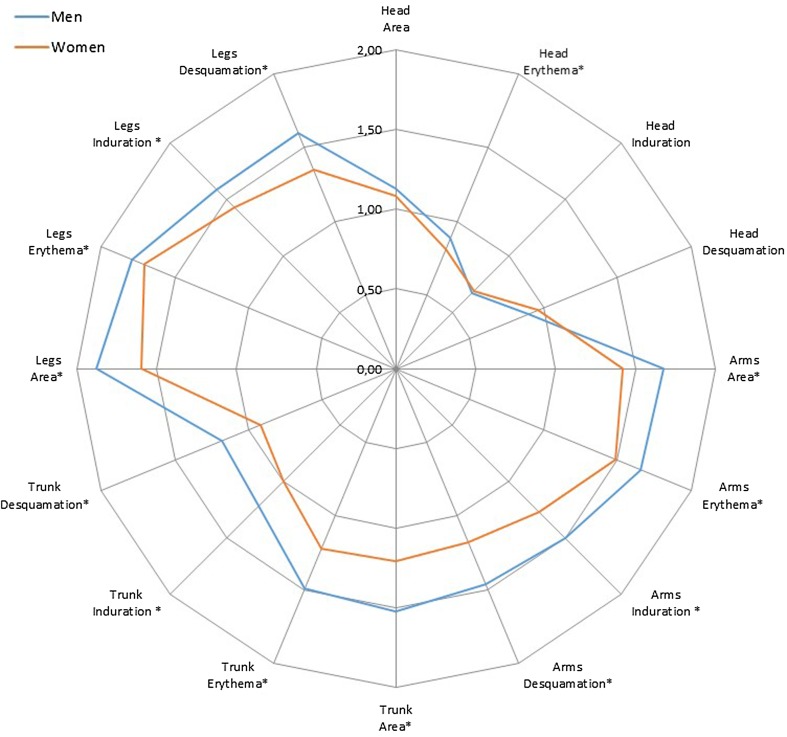
The difference between men and women’s PASI score elements at enrolment was tested using the Mann–Whitney–Wilcoxon test, and using the plaque characteristics (erythema, induration, and desquamation) and degree of skin involvement in each body region. Results showed that women had a significantly lower PASI score in all components in each body region, except for the characteristics ‘head area’, ‘head induration’, and ‘head desquamation’, where the scores were almost identical for women and men (Fig. 2).
Fig. 2.
The unweighted mean PASI are shown in the spider chart. * indicates statistical significance in Mann- Whitney-Wilcoxon test at 5%-significance level, PASI Psoriasis Area and Severity Index
The aggregated weighted PASI sums for each area (head, trunk, arms, and legs) were used as outcomes in the multiple ordinal regressions, with the same result as in the analysis of each element for the body regions described above.
Ordinal Logistic Regressions
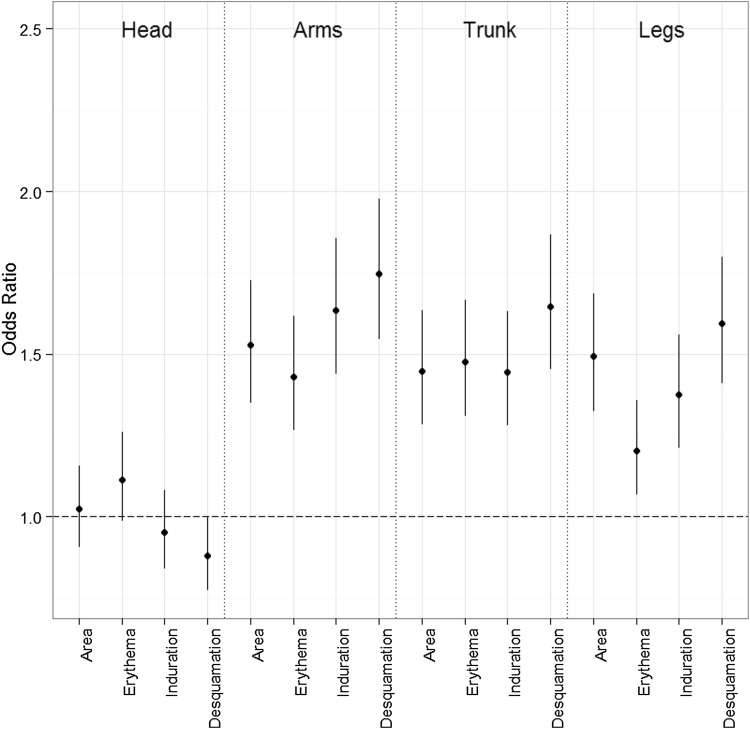
The assessment of plaque characteristics and the degree of skin involvement for each body region were used as the outcome in the multiple ordinal logistic regressions. The models were adjusted by age, BMI, smoking, season, and PsA, and the results were consistent with those of the Mann–Whitney–Wilcoxon tests. Significant differences were found for all plaque characteristics and the degree of skin involvement in the arms, legs and trunk body regions, where women had lower scores. No differences could be identified with respect to the head. The largest differences between men and women were found in desquamation [odds ratio men vs. women: arms 1.75 (CI 95% 1.55–1.98), trunk 1.65 (CI 95% 1.45–1.87), and legs 1.59 (CI 95% 1.41–1.80)] and induration at the arms [odds ratio men vs. women 1.63 (CI 95% 1.43–1.27)] (see Fig. 3).
Fig. 3.
The odds ratios from the ordinal logistic regressions adjusted by age, body mass index, disease duration, psoriatic arthritis, smoking status and season), with women as reference (= 1.0)
To compare the use of specific antipsoriatic drugs, independent from the PsoReg, the number of patients nationwide prescribed one of the two commonly used psoriasis-specific treatments—ustekinumab or calcipotriol (with or without corticosteroid)—was investigated in the PDR. Of the patients prescribed calcipotriol between 2006 and 2014, on average 43.3% (range 41.9–44.1%) were women. Similarly, 39.6% (range 34.8–43.8%) of patients prescribed ustekinumab between 2010 and 2014 were women.
Discussion
We observed that women have less severe psoriasis compared with men, after controlling for several possible confounders. By analyzing the separate PASI elements, we further confirmed that the lower PASI score in women is consistent and is not a result of chance variations. In a multivariable linear regression, controlling for several other factors, this difference remained statistically significant. The difference in all but one of the distinct elements of the PASI score was also significant, both in unadjusted comparisons of medians and in the ordinal logistic regression models adjusted by age, BMI, disease duration, concomitant PsA, smoking status, and season. However, with respect to the head, women’s and men’s PASI scores were equal (Figs. 2, 3). This is probably a consequence of sex- and gender-specific differences in hair growth, care, and styling, with women both (1) ‘shielding’ their scalp from the beneficial effect of sunlight on psoriasis [19, 20], and (2) provoking their head psoriasis via the Koebner reaction [21], to such an extent that it falls into a similar range as males.
Previous studies have shown that specialist treatment in psoriasis care is unbalanced, with men being more likely to undergo specialist treatment than women [9, 13]. In the PsoReg, approximately 60% of registered patients are men. Most other European Registries for systemic psoriasis treatment show an even larger dominance of registered men: Denmark 66%, Germany 60%, Italy 67%, The Netherlands 68%, and Spain 63% [22].
We employed the PDR to evaluate the nationwide prescription of two psoriasis-specific treatments, independent from the PsoReg. Both the biologic ustekinumab and topical treatments with calcipotriol were, to a larger extent, prescribed to men (55–60%). The PASI score, at enrolment in the PsoReg, shows that women have significantly lower disease activity compared with men across all ages, including women of both fertile- and non-fertile age (Fig. 1), making it unlikely that a restrictive prescription of systemic psoriasis treatment for women of fertile age is a relevant factor. Other differences between men and women that were shown at enrolment are merely a reflection of demographic factors that can be observed in the general population, and indicate a representative enrolment in the PsoReg. On average, men have a higher BMI than women, while a higher proportion of women smoke and suffer from joint disease.
In a descriptive study from Ireland, it was observed that twice as many men (n = 93) received systemic treatment compared with women (n = 53) [p = 0.015], and that women had less severe psoriasis (experienced by the clinicians) compared with men [10]. In a retrospective study from Japan, Sakai et al. explored the prognostic factors for the long-term outcome of plaque psoriasis patients (109 men and 60 women) in a logistic regression, and found that men developed more severe psoriasis according to the Dermatology Index of Disease Severity during follow-up compared with women (p = 0.046), after adjusting for age and BMI [23]. Furthermore, in a questionnaire survey based on 5739 patients, Zachariae et al. showed that men received more systemic treatment in the form of retinoids, calcipotriol, and psoralen plus ultraviolet A (PUVA) as well as non-PUVA phototherapy than women, after adjusting for a self-assessed disease severity measurement [11]. These findings correspond with our results as a majority of patients registered in the national registry for systemic treatment of psoriasis in Sweden (PsoReg) were men (59.1%) and had more severe psoriasis than women.
In several other studies, it has been shown that women received less (systemic) psoriasis treatment compared with men; however, as these studies did not consider disease severity [9, 13], different interpretations were made. Previous attempts to take disease severity into consideration when analyzing the consumption of care have been carried out by either questionnaires or the critical assessment tool Dermatology Index of Disease Severity [24]. The objective disease measurement PASI is traditionally used in randomized clinical trials only [25]. The registry-based integration of the objective disease measurement PASI enabled a detailed analysis of disease activity in a nationwide unselected population. As such, this study showed sex differences in the severity of psoriasis with a high level of detail. PsoReg-independent analyses of the PDR corroborated these findings.
Limitations
The generalizability of this study is limited to patients with moderate to severe psoriasis in need of systemic treatment managed by dermatology specialists. The PASI is the gold standard for assessing the severity of psoriasis. The assessment is an objective description of disease severity, and PASI has good inter- and intra-rater reliability [26, 27]. There is a theoretical possibility that the observed differences between the sexes with regard to disease severity are due to the selective recruitment of patients to PsoReg. For this to be true, dermatologists would either be more inclined to register men with high disease activity than women with high disease activity, or, conversely, women with a low PASI score would be more likely to be registered in PsoReg than men with a low PASI score. However, we have not looked at other dimensions of psoriasis severity, such as the DLQI. Considering this, if women are more inclined to be enrolled in the PsoReg due to a high DLQI score, while having a lower PASI score, this could theoretically explain some of the discrepancies between the severity of sexes shown in this study. However, one would still wonder why women with a high PASI score did not find their way into the register. Taken together, these scenarios are not likely, especially when considering (1) the dominance of men registered in the registries for systemic psoriasis treatment all over Europe; (2) previous descriptions of higher consumption of systemic psoriasis treatment by men [9, 10]; and, finally, (3) the PsoReg-independent dominance of men in the PDR for both psoriasis- specific treatments (calcipotriol and ustekinumab) demonstrated here.
Conclusions
For more than 70 years it has been speculated that women have less severe psoriasis compared with men. By investigating, for the first time, the sex differences in the severity of psoriasis using the gold standard of severity measurement—the PASI score—and the distinct elements of the PASI score, we were able to corroborate this thesis in a nationwide population. Further research is needed to substantiate this finding in different populations.
Acknowledgements
The authors would like to thank all patients and health professionals for their efforts to progress PsoReg.
Compliance with ethical standards
Conflict of interest
Marcus Schmitt-Egenolf is responsible for dermatology in the project management of the national guidelines for psoriasis at the Swedish Board of Health and Welfare. Anders Sundström receives and has received grants from several drug companies for performing post-approval safety studies and comparative effectiveness studies; however, the present work is not related to any of these studies. David Hägg and Marie Eriksson declare that no competing interests exist.
Funding
The PsoReg receives financial support from the Swedish Board of Health and Welfare and the Swedish Association of Local Authorities and Regions. This study was funded in part by Umeå University’s Industrial Doctoral School for Research and Innovation (IDS). The sponsor had no access to the data, and data collection, study design, interpretation, and analyses were carried out independently by the authors.
References
- 1.Parisi R, Symmons DP, Griffiths CE, Ashcroft DM, Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) Project Team Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 2.Horreau C, Pouplard C, Brenaut E, Barnetche T, Misery L, Cribier B, et al. Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(Suppl 3):12–29. doi: 10.1111/jdv.12163. [DOI] [PubMed] [Google Scholar]
- 3.Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Investig Dermatol. 2012;132(3 Pt 1):556–562. doi: 10.1038/jid.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146(8):891–895. doi: 10.1001/archdermatol.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrandiz C, Bordas X, Garcia-Patos V, Puig S, Pujol R, Smandia A. Prevalence of psoriasis in Spain (Epiderma Project: phase I) J Eur Acad Dermatol Venereol. 2001;15(1):20–23. doi: 10.1046/j.1468-3083.2001.00191.x. [DOI] [PubMed] [Google Scholar]
- 6.Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141(12):1537–1541. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
- 7.Romanus T. Psoriasis from a prognostic and hereditary point of view. Acta Derm Venereol. 1945;26:3–138. [Google Scholar]
- 8.Zachariae H, Zachariae R, Blomqvist K, Davidsson S, Molin L, Mork C, et al. Quality of life and prevalence of arthritis reported by 5,795 members of the Nordic Psoriasis Associations. Data from the Nordic Quality of Life Study. Acta Derm Venereol. 2002;82(2):108–113. doi: 10.1080/00015550252948130. [DOI] [PubMed] [Google Scholar]
- 9.Hotard RS, Feldman SR, Fleischer AB., Jr Sex-specific differences in the treatment of severe psoriasis. J Am Acad Dermatol. 2000;42(4):620–623. doi: 10.1067/mjd.2000.101596. [DOI] [PubMed] [Google Scholar]
- 10.White D, O’Shea SJ, Rogers S. Do men have more severe psoriasis than women? J Eur Acad Dermatol Venereol. 2012;26(1):126–127. doi: 10.1111/j.1468-3083.2011.04026.x. [DOI] [PubMed] [Google Scholar]
- 11.Zachariae H, Zachariae R, Blomqvist K, Davidsson S, Molin L, Mork C, et al. Treatment of psoriasis in the Nordic countries: a questionnaire survey from 5739 members of the psoriasis associations data from the Nordic Quality of Life Study. Acta Derm Venereol. 2001;81(2):116–121. doi: 10.1080/00015550152384254. [DOI] [PubMed] [Google Scholar]
- 12.Uttjek M, Dufaker M, Nygren L, Stenberg B. Psoriasis care consumption and expectations from a gender perspective in a psoriasis population in northern Sweden. Acta Derm Venereol. 2005;85(6):503–508. doi: 10.1080/00015550510036667. [DOI] [PubMed] [Google Scholar]
- 13.Nyberg F, Osika I, Evengard B. “The Laundry Bag Project”: unequal distribution of dermatological healthcare resources for male and female psoriatic patients in Sweden. Int J Dermatol. 2008;47(2):144–149. doi: 10.1111/j.1365-4632.2008.03485.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman MB, Farhangian M, Feldman SR. Psoriasis during pregnancy: characteristics and important management recommendations. Expert Rev Clin Immunol. 2015;11(6):709–720. doi: 10.1586/1744666X.2015.1037742. [DOI] [PubMed] [Google Scholar]
- 15.Hagg D, Eriksson M, Sundstrom A, Schmitt-Egenolf M. The higher proportion of men with psoriasis treated with biologics may be explained by more severe disease in men. PLoS One. 2013;8(5):e63619. doi: 10.1371/journal.pone.0063619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredriksson T, Pettersson U. Severe psoriasis: oral therapy with a new retinoid. Dermatologica. 1978;157(4):238–244. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 17.Hagg D, Sundstrom A, Eriksson M, Schmitt-Egenolf M. Decision for biological treatment in real life is more strongly associated with the Psoriasis Area and Severity Index (PASI) than with the Dermatology Life Quality Index (DLQI) J Eur Acad Dermatol Venereol. 2015;29(3):452–456. doi: 10.1111/jdv.12576. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt-Egenolf M. PsoReg: the Swedish registry for systemic psoriasis treatment. The registry’s design and objectives. Dermatology. 2007;214(2):112–117. doi: 10.1159/000098568. [DOI] [PubMed] [Google Scholar]
- 19.Lebwohl M, Ali S. Treatment of psoriasis: part 1. Topical therapy and phototherapy. J Am Acad Dermatol. 2001;45(4):487–498. doi: 10.1067/mjd.2001.117046. [DOI] [PubMed] [Google Scholar]
- 20.Naldi L, Griffiths CE. Traditional therapies in the management of moderate to severe chronic plaque psoriasis: an assessment of the benefits and risks. Br J Dermatol. 2005;152(4):597–615. doi: 10.1111/j.1365-2133.2005.06563.x. [DOI] [PubMed] [Google Scholar]
- 21.Weiss G, Shemer A, Trau H. The Koebner phenomenon: review of the literature. J Eur Acad Dermatol Venereol. 2002;16(3):241–248. doi: 10.1046/j.1473-2165.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- 22.Ormerod AD, Augustin M, Baker C, Chosidow O, Cohen AD, Dam TN, et al. Challenges for synthesising data in a network of registries for systemic psoriasis therapies. Dermatology. 2012;224(3):236–243. doi: 10.1159/000338572. [DOI] [PubMed] [Google Scholar]
- 23.Sakai R, Matsui S, Fukushima M, Yasuda H, Miyauchi H, Miyachi Y. Prognostic factor analysis for plaque psoriasis. Dermatology. 2005;211(2):103–106. doi: 10.1159/000086437. [DOI] [PubMed] [Google Scholar]
- 24.Williams HC. Is a simple generic index of dermatologic disease severity an attainable goal? Arch Dermatol. 1997;133(11):1451–1452. doi: 10.1001/archderm.1997.03890470129020. [DOI] [PubMed] [Google Scholar]
- 25.Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64(Suppl 2):ii65–ii68. doi: 10.1136/ard.2004.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berth-Jones J, Grotzinger K, Rainville C, Pham B, Huang J, Daly S, et al. A study examining inter- and intrarater reliability of three scales for measuring severity of psoriasis: Psoriasis Area and Severity Index, Physician’s Global Assessment and Lattice System Physician’s Global Assessment. Br J Dermatol. 2006;155(4):707–713. doi: 10.1111/j.1365-2133.2006.07389.x. [DOI] [PubMed] [Google Scholar]
- 27.Puzenat E, Bronsard V, Prey S, Gourraud PA, Aractingi S, Bagot M, et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24(Suppl 2):10–16. doi: 10.1111/j.1468-3083.2009.03562.x. [DOI] [PubMed] [Google Scholar]